Abstract
Research question
Does antioestrogen effect of clomiphene citrate (CC) on the endometrium reduce implantation and thereby decrease pregnancy and live birth rate per transferred embryo?
Methods
In this cohort, unstimulated IVF cycles modified with clomiphene citrate (CC-NC-IVF) and unstimulated, natural IVF cycles (NC-IVF) conducted between 2011 and 2016 were included. CC was applied in a dosage of 25mcg per day, starting on cycle day 7 until ovulation trigger day. Primary outcomes were clinical pregnancy rate, defined as amniotic sac visible in ultrasound, and live birth rate per transferred embryo. Miscarriage rate calculated as amniotic sac not ending in a live birth was secondary outcome. A modified mixed-effect Poisson regression model was applied, and adjustments were made for female age, parity, type and cause of infertility. Additionally, stratification by parity and age was performed.
Results
Four hundred and ninety-nine couples underwent a total of 1042 IVF cycles, 453 being NC-IVF and 589 being CC-NC-IVF cycles. Baseline characteristics of both groups did not differ. Addition of CC did neither decrease clinical pregnancy rate (aRR 0.86; 95% CI 0.67–1.12) nor live birth rate per transferred embryo (aRR 0.84; 95% CI 0.62–1.13) in comparison with NC-IVF. Miscarriage rate did not differ between CC-NC-IVF and NC-IVF (aRR 0.95; 95% CI 0.57–1.57).
Conclusion
Low-dose CC does not reduce pregnancy or live birth rate per transferred embryo. It can be used in infertility treatment without negatively affecting the endometrium and implantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
It is unclear if the antiestrogen effect of Clomiphen citrate reduces implantation after fresh embryo transfer. Low-dose CC (25 mcg) added to natural cycle IVF does not interfere with implantation and therefore not reduce pregnancy or live birth rate per transferred embryo. |
Introduction
Clomiphene citrate (CC) is a selective oestrogen receptor modulator (SERM) that blocks the hypothalamic oestrogen receptors. This mimics a low serum concentration of oestrogen, which in turn induces negative feedback at the hypothalamic and pituitary glands, leading to increased secretion of gonadotropin-releasing hormone (GnRH) at the hypothalamus and of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from anterior pituitary. This induces follicular maturation in the ovaries, and the consecutive LH surge thereby eventually induces ovulation [1, 2].
In medically assisted reproduction (MAR), CC has been used for ovulation induction in case of dysovulatory cycles with the aim to increase the chance for natural conception—if necessary in combination with intrauterine insemination [3]. CC has also been added in doses of 25–100 mcg to unstimulated (CC-NC-IVF) and minimally stimulated in vitro fertilization (IVF) cycles in assisted reproductive technology (ART) treatments to increase the chance of successful oocyte gain at oocyte pick-up (OPU). It also reduces the risk for premature ovulation by impeding LH surge [4, 5]. CC is especially recommended for low-responders [6, 7] or is a low-cost alternative to regular IVF often performed in low-income countries [8].
Despite its benefits regarding successful OPU outcomes, CC has been suggested to interfere with successful embryo implantation. It reduces endometrial thickness due to its antiestrogenic activity [9], and it leads to a number of morphological irregularities in the endometrium such as increased stromal oedema and decreased glandular expression [10,11,12]. Reduced endometrial thickness is associated with lower chances for implantation and successful pregnancy outcome [13]. In CC-NC-IVF, CC is applied to improve oocyte gain but only a few studies assessed the impact on the endometrium and implantation by addressing clinical pregnancy rates and live births in comparison with unstimulated natural cycle IVF (NC-IVF). Those studies find mixed results with one study showing reduced implantation and pregnancy rates with CC [14], one not finding any differences [15] and one suggesting increased pregnancy rates with the addition of CC [16]. Results remain inconclusive and the studies differ in design, dosages of CC, and treatment schemes. It is further suspected that CC affects the function of fallopian tubes, increasing the risk for ectopic pregnancies [11, 17].
The aim of the present study is to assess the effect on endometrial receptivity of low-dose CC (25 mcg) used in CC-NC-IVF. Clinical pregnancy rates proving successful implantation, livebirth and miscarriage rates of embryos derived from CC-NC-IVF are compared to rates of embryos of NC-IVF.
Materials and methods
Data sources
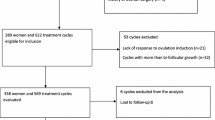
Data for the present study were extracted from two different sources, the Swiss ART registry FIVNAT [18] and the Bern IVF Cohort [19]. FIVNAT provided data on all cycles performed at the University Hospital’s infertility centre between 2011 and 2016 (n = 3456). Thawing cycles (n = 910) and cycles without embryo transfer (n = 542) were excluded. The Bern IVF Cohort delivered data concerning all transfers of fresh embryos that led to a pregnancy (n = 311). Cycles with embryo transfer of FIVNAT (n = 2004) and Bern IVF Cohort (n = 311) were linked. Inconsistencies were clarified using medical records to increase data quality and to eliminate duplicates (Fig. 1).
Study population
All NC-CC-IVF and all NC-IVF cycles performed between 2011 and 2016 at the Bern University Hospital were considered for the study if at least one fresh cleavage stage embryo was transferred (n = 1997). The following cycles were excluded: cycles with missing or inconsistent information (n = 6), maternal age over 42 years (n = 62) and cycles using additional gonadotropins for stimulation (n = 887). Finally, 1042 cycles were included, 453 NC-IVF cycles and 589 NC-CC-IVF cycles (Fig. 1). The ethics commission of the canton of Bern approved the study on February 26, 2020 (BASEC 2020-11800021).
Treatment protocols
Treatment regime was a mutual decision between patient and physician. The decision was based on personal preferences regarding the different characteristics of NC-IVF and NC-CC-IVF and feasibility [20]. Both treatments required a regular menstrual cycle. CC was more likely to be administered in case of premature ovulation in a previous treatment cycle. In NC-IVF aspiration was scheduled when the diameter of the leading follicle was ≥ 16 mm and E2 concentration was ≥ 700 pmol/L. In CC-NC-IVF, patients took 25 mg CC in the morning, beginning on cycle day 7 until trigger day [15]. OPU was scheduled when diameter of the leading follicle reached ≥ 16 mm and E2 concentrations were ≥ 700L depending on the number of follicles. Ovulation was triggered with subcutaneous injection of 5000 IU of hCG 36–36.5 h before aspiration. OPU was performed without anaesthesia, using 19G monoluminal needles. Follicles were flushed 5 times as described elsewhere [21]. Oocytes were fertilized by intracytoplasmic sperm injection (ICSI) or rarely by IVF. All procedures were carried out in the same laboratory under standardized conditions. Embryo transfer of the available embryos (1–2) was performed at cleavage stage, 2–3 days after OPU. At that time, the vitrification of embryos was not allowed in Switzerland, which prohibited embryo selection. If both embryos kept in culture-reached cleavage stage, double embryo transfer was performed.
Luteal phase supplementation using intravaginal micronized progesterone 200 mg once a day was applied if necessary, oestrogen was never added [22]. At 14 days after OPU, beta-HCG was tested in serum, if positive an ultrasound scan was performed two weeks later. Clinical pregnancy was confirmed by sonographic detection of an amniotic sac.
Statistical analysis
Primary outcomes were defined as number of amniotic sacs detected by ultrasound and the number of live births per embryo transferred. The secondary outcome was defined as the number of sonographically detected amniotic sacs not resulting in a live birth, therefore being classified as a miscarriage.
Baseline characteristics for each cycle of both treatment groups were analysed using linear regression for the comparison of continuous variables and logistic regression for the comparison of binary variables. For the comparison of the main indications for IVF treatment, a Chi-square test was applied. A modified multilevel Poisson regression model was used to calculate rate ratios of the primary outcomes [23]. This model accounts for the dependency of treatment cycles as the couples and the cycles define the levels. The number of embryos transferred within the same cycle is considered the count to start with and the amniotic sacs or the live births are the count outcome [24]. The same model was applied to assess the miscarriage rate where the number of amniotic sacs detected by ultrasound was the start count and the number of amniotic sacs lost was considered the count outcome [25].
The model was adjusted for the following co-variates: female age at the time of oocyte retrieval (continuous), parity (yes or no), type of infertility (primary or secondary) and main cause of infertility categorized as: reduced ovarian reserve, tubal factor, endometriosis rASRM stage I/II, endometriosis rASRM stage III/IV, anovulation, male factor, idiopathic or other reasons. A p value of < 0.05 was considered statistically significant.
All analyses were conducted in STATA Version 16 (StataCorp, College Station, Texas, USA).
Sensitivity analysis
Several sensitivity analyses were conducted. The first including only one cycle per included women to avoid oversampling of couples with many cycles and potentially lower chances for treatment success. The second subanalysis included only couples, which did not switch treatment between NC-IVF and CC-NC-IVF during the inclusion period (n = 385). In the third analysis, the women were stratified by age (≤ 35; > 35) to assess the effect of age-related fertility decline [26]. Finally, the analysis was stratified by parity (nulliparous; parous) to assess the impact of parity as a positive predictive factor.
Results
Main analysis
In this study, 499 couples underwent a total of 1042 IVF cycles, 589 of them as CC-NC-IVF and 453 as NC-IVF. Of all couples, 239 (47.9%) contributed only one cycle to the sample and 260 (52.1%) contributed between 2 and 18 cycles. In CC-NC-IVF in 35.3% of cycles, more than one oocyte was collected, whereas in NC-IVF this was only the case in 4% of the cycles, leading to a higher proportion of double embryo transfers in CC-NC-IVF. In total, 1173 embryos were transferred. 711 embryos in CC-NC-IVF cycles with 467 (79.3%) in single and 244 (20.7% of cycles) in double transfers. 462 embryos in NC-IVF cycle with 444 (98%) being single and 18 (2% of cycles) being double embryo transfers (Table 1).
The baseline characteristics for both groups were not different regarding female age and infertility characteristics. However, the number of oocytes retrieved, and the number of double embryo transfers was higher in CC-NC-IVF cycles (Table 1).
The results per cycle while ignoring the number of embryos transferred were the following:
In CC-NC-IVF, 96 (16.3%) cycles resulted in a clinical pregnancy of which 73 (76%) (70 singletons and 3 twins; 12.39%) ended in a live birth and 23 (24.0%) in a miscarriage, 19 of those miscarried (19.8%) in the first trimester, one in the second and three cycles ended with ectopic pregnancies. In NC-IVF, 80 (17.6%) cycles resulted in a clinical pregnancy of which 60 (75%) ended in a livebirth of a singleton and 20 (25%) in a miscarriage; 18 (22.5%) within the first trimester and 2 in the second.
The unadjusted analysis revealed that the addition of CC did not decrease the clinical pregnancy rate (rate ratio (RR) 0.84; 95% CI 0.63–1.11; p = 0.21) or live birth rate per transferred embryo (RR 0.82; 95% CI 0.59–1.14; p = 0.24). Miscarriage rate was not different in CC-NC-IVF compared to NC-IVF (RR 1.14; 95% CI 0.66–1.98; p = 0.64) either. The adjusted analysis confirmed these results showing an aRR of 0.86 for clinical pregnancy (95% CI 0.67–1.12; p = 0.27) and 0.84 for live birth per transferred embryo (95% CI 0.62–1.13; p = 0.25). Adjustments did not reveal any differences in misscariage rates between CC-NC-IVF compared to NC-IVF either (aRR 0.95; 95% CI 0.57–1.60; p = 0.86) (Tables 2, 3, 4).
Sensitivity analyses
If exclusively the first or only cycle of each couple was included in the analysis, 210 had CC-NC-IVF and 289 had NC-IVF treatment. The analysis of this subsample revealed no differences between the two treatment types. Of the couples that contributed more than one cycle (n = 260), most couples had the same treatment (n = 146; 56.2%) in every included cycle, whereas 114 (43.8%) did switch and had cycles of both treatments. In total, 385 couples contributed one or all cycles of the same treatment, which resulted in 654 cycles analysed in this subanalysis. Results of modified Poisson regression including only couples continuing with the same treatment did not show differences between CC-NC-IVF and NC-IVF either. Finally, age did not reveal any differences between CC-NC-IVF and NC-IVF, whereas parous women seemed to have a better clinical pregnancy rate in NC-IVF treatment, but this was not seen for live births (Online Resource 1).
Discussion
This study suggests that low-dose CC, such as 25mcg in modified CC-NC-IVF, does not have a clinically relevant impact on endometrial receptivity and consecutively on implantation when compared to unstimulated NC-IVF. This result was robust in different sensitivity analyses.
In employing a woman’s physiological menstrual cycle, NC-IVF offers an ideal model to analyse the clinical impact of the known unfavourable effects of CC on endometrium and reproductive function. Because of the prohibition of embryo selection in Switzerland, the potential bias introduced by embryo selection is not present. An additional strength of the present study is the focus on one IVF centre with large experience and standardized laboratory protocols. To increase power, all available cycles and thus more than one cycle per couple were included. The hierarchical count model accounts for the dependency of the cycles within the same couple and for the embryos transferred in the same cycle, and several sensitivity analyses were conducted to assess potential influence of further factors. Limitations are found in the study’s observational design and the non-randomized allocation of IVF treatment according to patients and physicians’ preference, which can lead to selection bias. Additionally, data might be skewed by the fact that women who got pregnant in their first treatment cycle dropped out faster and contributed fewer, but successful cycles. The study does not provide information on earlier ART cycle failure or OPU outcome, where CC has been shown to have a positive influence, mainly in avoiding preterm ovulation [15].
To our knowledge, only few previous studies assessed pregnancy outcome after CC-NC-IVF in comparison with NC-IVF and it is not possible to draw clear conclusions. In a prospective trial by Ingerslev et al. 132 women with no previous IVF attempts were randomized to either 100 mg CC on cycle days 3–7 (n = 68 with 111 cycles) or to no stimulation at all (n = 64 with 114 cycles). More cycles with successful embryo transfers were seen in the CC group (n = 59 vs n = 29). In addition, a higher proportion of clinical pregnancies per transfer was achieved in the CC group (33.9% vs 13.8%, p = 0.047), and the difference was even larger as per cycle started (18.0% vs 3.5%, p < 0.001). The higher embryo transfer rate and success by cycle is believed to be related to the positive impact of CC on OPU success. Comparison to the present study is, however, limited by the fact that a dose of 100 mcg CC would have a different effect on the endometrium than the minimal dose of 25 mcg applied. No results for miscarriages or livebirths were presented, and the results were not corrected for dependency of cycles in the same women nor for the number of embryos transferred [16].
Abe et al. assessed pregnancy rates of NC-CC-IVF in 834 women following different types of embryo transfers (fresh cleavage, blastocyst or a frozen cleavage or blastocyst). Following the transfer of a single fresh cleavage embryo, the only setting comparable to the one in the present study, in 24.0% of cycles, a clinical pregnancy was detected and in 18.7% of the cycles, a livebirth was achieved. These results and the miscarriage risk were highly dependent on age of the women [5].
In a cohort study by Kato et al. including only one cycle per women, pregnancy success following CC-NC-IVF (n = 24) was compared to NC-IVF (n = 157). For stimulation, 50–100 mg CC on day 3 until the day before ovulation trigger was applied. In contrast to Ingerslev et al. clinical pregnancy (45.8% vs 69.4%) and livebirth rates (29.2% vs 56.1%) were reported to be significantly lower per single blastocyst transfer in CC-NC-IVF. The adverse effect of CC was not seen after the transfer of a vitrified embryo, suggesting frozen embryo transfer within natural cycle being a better option following CC stimulation, a fact confirming the hypothesis of a negative effect of CC on the endometrium and not on follicular maturation. However, the number of fresh transfers following CC-NC-IVF was very low [14].
Von Wolff et al. compared NC-IVF and CC-NC-IVF cycles stimulated with 25 mg CC on day 7 until the day of ovulation trigger in 112 women having both treatments each. In contrast to the previously discussed studies, they did not find any differences in pregnancy and implantation rates compared to NC-IVF, but the primary outcome of the study was preterm ovulation and the sample size was limited [15]. The present study could confirm these results with a larger sample size, including the cycles from the previous study, referring to 20.2% of all cycles.
A recent study found a CC-induced delay of endometrial maturation in women treated with 100mcg CC for 5 days [27]. Endometrial thickness can be reduced by CC by around 1–2 mm [9, 28, 29]. Thin endometrium has been shown to decrease pregnancy success in gonadotropin-stimulated [13] as well as in gonadotropin-unstimulated cycles [30]. Additionally, an association of endometrial thickness at trigger day with lower ongoing pregnancy rates has been explicitly shown for minimal stimulated IVF cycles with added CC [31]. However, it remains unclear whether the endometrium thinning effect is the reason for lower pregnancy rates in CC treatments compared to other ART [32]. Further negative effects of CC on the reproductive system have previously been postulated: In rats, CC treatment induced cell type specific apoptosis in the uterus and in the fallopian tube, changed the uterine morphologic conditions and decreased the expression of oestrogen receptor-alpha through its activation. All these changes may interfere with implantation and pregnancy success and the exact biological mechanisms, and their clinical relevance in humans has not been entirely clarified [11, 33]. A higher ectopic pregnancy rate as a result of CC cycles in humans has already been shown by several studies. A total of three ectopic pregnancies out of 96 clinical pregnancies (3.1%) were observed in the present study, which is high compared to a presented 1.4% in pregnancies following ART overall [17, 34].
The heterogenic outcomes of previous studies can hardly be explained. It is possible that the unfavourable molecular impacts of CC on endometrial function are dose dependent or mainly associated with long-term or chronic use of CC. However, an older trial did not find dose-dependent effects of CC [35].
In the present study, differences in implantation, miscarriage and live birth rates between NC-IVF and CC-NC-IVF were not identified. It suggests that CC at the dose of 25mcg does not have a clinically relevant impact on endometrial function affecting implantation, miscarriage or live birth rates. Low-dose CC is a low-cost alternative that can be given until the day of ovulation trigger in fresh IVF cycles. However, more evidence of randomized clinical trials comparing unstimulated and CC stimulated IVF cycles involving different CC dosages are required to define cut-offs of save CC dosages.
Data availability statement
The participants did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available. Data of Swiss IVF Registry is subject to third party restrictions. More information on FIVNAT can be found on www.sgrm.org.
References
Kerin JF, Liu JH, Phillipou G, Yen SSC (1985) Evidence for a hypothalamic site of action of clomiphene citrate in women. J Clin Endocrinol Metab. https://doi.org/10.1210/jcem-61-2-265
Rebar R, Judd HL, Yen SSC et al (1976) Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest. https://doi.org/10.1172/JCI108400
Johnson MH, Elder K (2015) The Oldham notebooks: an analysis of the development of IVF 1969–1978. IV. Ethical aspects. Reprod Biomed Soc Online. https://doi.org/10.1016/j.rbms.2015.04.002
Zarei A, Alborzi S, Askary E et al (2018) Effects of clomiphene citrate for prevention of premature luteinizing hormone surge in those undergoing intrauterine insemination outcome: a randomized, double-blind, placebo-controlled trial. J Adv Pharm Technol Res. https://doi.org/10.4103/japtr.JAPTR_293_18
Abe T, Yabuuchi A, Ezoe K et al (2020) Success rates in minimal stimulation cycle IVF with clomiphene citrate only. J Assist Reprod Genet 37:297–304. https://doi.org/10.1007/s10815-019-01662-z
Ochin H, Ma X, Wang L et al (2018) Low dose clomiphene citrate as a mild stimulation protocol in women with unsuspected poor in vitro fertilization result can generate more oocytes with optimal cumulative pregnancy rate. J Ovarian Res. https://doi.org/10.1186/s13048-018-0408-x
Teramoto S, Kato O (2007) Minimal ovarian stimulation with clomiphene citrate: a large-scale retrospective study. Reprod Biomed Online 15:134–148. https://doi.org/10.1016/S1472-6483(10)60701-8
Sophonsritsuk A, Choktanasiri W, Weerakiet S, Rojanasakul A (2005) Comparison of outcomes and direct cost between minimal stimulation and conventional protocols on ovarian stimulation in in vitro fertilization. J Obstet Gynaecol Res 31:459–463. https://doi.org/10.1111/j.1447-0756.2005.00320.x
Dehbashi S, Parsanezhad ME, Alborzi S, Zarei A (2003) Effect of clomiphene citrate on endometrium thickness and echogenic patterns. Int J Gynecol Obstet. https://doi.org/10.1016/S0020-7292(02)00341-7
Bonhoff AJ, Naether OGJ, Johannisson E (1996) Effects of clomiphene citrate stimulation on endometrial structure in infertile women. Hum Reprod. https://doi.org/10.1093/oxfordjournals.humrep.a019264
Nutu M, Feng Y, Egecioglu E et al (2010) Stromal cell-specific apoptotic and antiestrogenic mechanisms may explain uterine defects in humans after clomiphene citrate therapy. Am J Obstet Gynecol 203:65.e1-65.e10. https://doi.org/10.1016/j.ajog.2010.03.039
Sereepapong W, Suwajanakorn S, Triratanachat S et al (2000) Effects of clomiphene citrate on the endometrium of regularly cycling women. Fertil Steril. https://doi.org/10.1016/S0015-0282(99)00509-9
Kasius A, Smit JG, Torrance HL et al (2014) Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update 20:530–541. https://doi.org/10.1093/humupd/dmu011
Kato K, Ezoe K, Yabuuchi A et al (2018) Comparison of pregnancy outcomes following fresh and electively frozen single blastocyst transfer in natural cycle and clomiphene-stimulated IVF cycles. Hum Reprod Open. https://doi.org/10.1093/hropen/hoy006
von Wolff M, Nitzschke M, Stute P et al (2014) Low-dosage clomiphene reduces premature ovulation rates and increases transfer rates in natural-cycle IVF. Reprod Biomed Online 29:209–215. https://doi.org/10.1016/j.rbmo.2014.04.013
Ingerslev HJ, Højgaard A, Hindkjær J, Kesmodel U (2001) A randomized study comparing IVF in the unstimulated cycle with IVF following clomiphene citrate. Hum Reprod 16:696–702. https://doi.org/10.1093/humrep/16.4.696
Jwa SC, Seto S, Takamura M et al (2020) Ovarian stimulation increases the risk of ectopic pregnancy for fresh embryo transfers: an analysis of 68,851 clinical pregnancies from the Japanese assisted reproductive technology registry. Fertil Steril 114:1198–1206. https://doi.org/10.1016/j.fertnstert.2020.06.032
De Geyter C, Fehr P, Moffat R et al (2015) Twenty years’ experience with the Swiss data registry for assisted reproductive medicine: outcomes, key trends and recommendations for improved practice. Swiss Med Wkly. https://doi.org/10.4414/smw.2015.14087
Mitter VR, Fasel P, Berlin C et al (2022) Perinatal outcomes in singletons after fresh IVF/ICSI: results of two cohorts and the birth registry. Reprod Biomed Online. https://doi.org/10.1016/j.rbmo.2021.12.007
von Wolff M (2019) The role of natural cycle IVF in assisted reproduction. Best Pract Res Clin Endocrinol Metab. https://doi.org/10.1016/j.beem.2018.10.005
Schwartz ASK, Calzaferri I, Roumet M et al (2020) Follicular flushing leads to higher oocyte yield in monofollicular IVF: a randomized controlled trial. Hum Reprod. https://doi.org/10.1093/humrep/deaa165
von Wolff M, Kohl Schwartz A, Stute P et al (2017) Follicular flushing in natural cycle IVF does not affect the luteal phase–a prospective controlled study. Reprod Biomed Online. https://doi.org/10.1016/j.rbmo.2017.04.003
Zou G (2004) A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 159:702–706. https://doi.org/10.1093/aje/kwh090
Dodge LE, Farland LV, Correia KFB et al (2020) Choice of statistical model in observational studies of ART. Hum Reprod 35:1499–1504
Mitter V, Grädel F, Koh. Schwartz AS, Vo. Wolff M (2021) P–693 gonadotropin stimulation reduces the implantation and live birth but not the miscarriage rate–a study based on the comparison of stimulated and unstimulated IVF. Hum Reprod. https://doi.org/10.1093/humrep/deab130.692
González-Foruria I, Peñarrubia J, Casals G et al (2016) Age, independent from ovarian reserve status, is the main prognostic factor in natural cycle in vitro fertilization. Fertil Steril. https://doi.org/10.1016/j.fertnstert.2016.04.007
Montenegro IS, Kuhl CP, de Almeida Schneider R et al (2021) Use of clomiphene citrate protocol for controlled ovarian stimulation impairs endometrial maturity. J Bras Reprod Assist. https://doi.org/10.5935/1518-0557.20200056
Aanesen A, Nygren KG, Nylund L (2010) Modified natural cycle IVF and mild IVF: A 10 year Swedish experience. Reprod Biomed Online. https://doi.org/10.1016/j.rbmo.2009.10.017
Takasaki A, Tamura H, Taketani T et al (2013) A pilot study to prevent a thin endometrium in patients undergoing clomiphene citrate treatment. J Ovarian Res 6:1–5. https://doi.org/10.1186/1757-2215-6-94/TABLES/2
von Wolff M, Fäh M, Roumet M et al (2018) Thin Endometrium is also associated with lower clinical pregnancy rate in unstimulated menstrual cycles: a study based on natural cycle IVF. Front Endocrinol (Lausanne) 9:1–6. https://doi.org/10.3389/fendo.2018.00776
Nishihara S, Fukuda J, Ezoe K et al (2020) Does the endometrial thickness on the day of the trigger affect the pregnancy outcomes after fresh cleaved embryo transfer in the clomiphene citrate-based minimal stimulation cycle? Reprod Med Biol 19:151–157. https://doi.org/10.1002/rmb2.12315
Gadalla MA, Huang S, Wang R et al (2018) Effect of clomiphene citrate on endometrial thickness, ovulation, pregnancy and live birth in anovulatory women: systematic review and meta-analysis. Ultrasound Obstet Gynecol 51:64–76
Shao R, Nutu M, Weijdegård B et al (2009) Clomiphene citrate causes aberrant tubal apoptosis and estrogen receptor activation in rat fallopian tube: implications for tubal ectopic pregnancy. Biol Reprod 80:1262–1271. https://doi.org/10.1095/biolreprod.108.074237
Santos-Ribeiro S, Tournaye H, Polyzos NP (2016) Trends in ectopic pregnancy rates following assisted reproductive technologies in the UK: a 12-year nationwide analysis including 160 000 pregnancies. Hum Reprod 31:393–402. https://doi.org/10.1093/HUMREP/DEV315
Jain JK, Kuo J (2004) Pregnancy outcomes with increased clomiphene citrate dose. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol 19:141–145. https://doi.org/10.1080/09513590400007234
Acknowledgements
We thank Costanzo Limoni for the extraction of data from FIVNAT. For data collection in the Bern IVF Cohort, we thank Marlene Berchtold, Lena Bommer, Pascale Fasel, Ursina Küng, Mirja Minger, Maria Paulsson, Livia Purtschert and Daniela Steiner.
Funding
Open access funding provided by University of Bern. Study administration was supported by IBSA Institut Biochimique, Lugano, Switzerland. VRM received funding from the Swiss National Science Foundation (Early Postdoc Mobility Grant P2BEP3-191798), Foundation Fridericus and partly by the Research Council of Norway through its Centre’s of Excellence funding scheme (project nb 262700). Data collection of the Bern IVF Cohort was supported by Merck (Switzerland) AG. The funding bodies had no role in the design, analyses or interpretation of the results.
Author information
Authors and Affiliations
Contributions
FG contributed to protocol development, study administration, data curation, manuscript writing and editing. VRM conducted protocol and project development, study administration, data curation, analysis, interpretation of results, manuscript writing and critical review. AKS contributed to project development, data collection, interpretation of results and critical review. MW performed conception of study, data collection, interpretation of results, manuscript writing and critical review.
Corresponding author
Ethics declarations
Conflict of interest
Flavia Grädel has no financial or non-financial interests to declare. Michael von Wolff received a research grant from IBSA Institut Biochimique, Lugano, Switzerland. Alexandra S Kohl Schwartz has received funding for the set-up of the Bern IVF cohort from Merck, Switzerland SA. Vera R Mitter received congress and travel support from Theramex.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. The Ethics Commission of the Canton of Bern approved the study on February 26, 2020 (BASEC 2020-00021).
Consent to participate
Not applicable. This study is a further use of health-related personal data and the need for informed consent was waived by the Ethics Commission of the Canton of Bern.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grädel, F., von Wolff, M., Kohl Schwartz, A.S. et al. Low-dose clomiphene citrate does not reduce implantation and live birth rates in otherwise unstimulated modified natural cycle IVF—retrospective cohort study. Arch Gynecol Obstet 307, 1073–1081 (2023). https://doi.org/10.1007/s00404-022-06878-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06878-6