Abstract
Purpose
Endometriosis (EMT) is a chronic benign disease with high prevalence. This study investigated the diagnostic value of serum miR-17-5p, miR-424-5p, and their combined expressions for EMT.
Methods
Total 80 EMT patients of reproductive age who underwent laparoscopy or laparotomy and were confirmed by pathological examination were included as the study subjects, and another 80 healthy women of reproductive age receiving gynecological examination and ultrasonography with no pelvic abnormalities were selected as the control group. The whole blood samples of enrolled subjects were collected and clinical characteristics were recorded. The miR-17-5p, miR-424-5p, VEGFA, IL-4, and IL-6 levels in the serum were measured. ROC curve was used to evaluate the diagnostic efficacy of miR-17-5p and miR-424-5p expressions for EMT. Pearson correlation was performed to analyze the correlation of miR-17-5p and miR-424-5p with clinical indexes in EMT patients.
Results
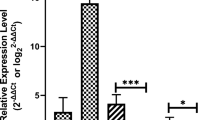
miR-17-5p and miR-424-5p were downregulated in EMT patients. For diagnosing EMT, the AUC of miR-17-5p was 0.865 and cutoff value was 0.890 (91.3% sensitivity and 85% specificity), the AUC of miR-424-5p was 0.737, and cutoff value was 0.915 (98.8% sensitivity and 61.2% specificity), and the AUC of miR-424-5p combined with miR-17-5p was 0.938 and cutoff value was 2.205 (93.8% sensitivity and 88.7% specificity), with the diagnostic efficacy higher than miR-424-5p or miR-17-5p alone. miR-17-5p and miR-424-5p expressions were negatively correlated with dysmenorrhea, infertility, pelvic pain, and rASRM stage, but not with age, BMI, menstrual disorder, and nulliparity. VEGFA, IL-4, IL-6, and CA-125 were increased in EMT patients and were inversely associated with miR-17-5p and miR-424-5p.
Conclusion
miR-424-5p combined with miR-17-5p has high diagnostic efficacy for EMT.



Similar content being viewed by others
Availability of data and materials
All the data generated or analyzed during this study are included in this published article.
References
Garcia-Ibanez P, Yepes-Molina L, Ruiz-Alcaraz AJ, Martinez-Esparza M, Moreno DA, Carvajal M, Garcia-Penarrubia P (2020) Brassica bioactives could ameliorate the chronic inflammatory condition of endometriosis. Int J Mol Sci. https://doi.org/10.3390/ijms21249397
Golabek A, Kowalska K, Olejnik A (2021) Polyphenols as a diet therapy concept for endometriosis-current opinion and future perspectives. Nutrients. https://doi.org/10.3390/nu13041347
Anastasiu CV, Moga MA, Elena Neculau A, Balan A, Scarneciu I, Dragomir RM, Dull AM, Chicea LM (2020) Biomarkers for the noninvasive diagnosis of endometriosis: state of the art and future perspectives. Int J Mol Sci. https://doi.org/10.3390/ijms21051750
Wang D, Yang Q, Wang H, Liu C (2021) Malignant transformation of hepatic endometriosis: a case report and literature review. BMC Womens Health 21:249. https://doi.org/10.1186/s12905-021-01366-6
Hur C, Falcone T (2021) Robotic treatment of bowel endometriosis. Best Pract Res Clin Obstet Gynaecol 71:129–143. https://doi.org/10.1016/j.bpobgyn.2020.05.012
Greenbaum H, Galper BL, Decter DH, Eisenberg VH (2021) Endometriosis and autoimmunity: can autoantibodies be used as a non-invasive early diagnostic tool? Autoimmun Rev 20:102795. https://doi.org/10.1016/j.autrev.2021.102795
Peng C, Huang Y, Zhou Y (2021) Dydrogesterone in the treatment of endometriosis: evidence mapping and meta-analysis. Arch Gynecol Obstet 304:231–252. https://doi.org/10.1007/s00404-020-05900-z
Samimi M, Pourhanifeh MH, Mehdizadehkashi A, Eftekhar T, Asemi Z (2019) The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: basic science and new insights based on gene expression. J Cell Physiol 234:19384–19392. https://doi.org/10.1002/jcp.28666
Bjorkman S, Taylor HS (2019) MicroRNAs in endometriosis: biological function and emerging biomarker candidatesdagger. Biol Reprod 100:1135–1146. https://doi.org/10.1093/biolre/ioz014
Agrawal S, Tapmeier T, Rahmioglu N, Kirtley S, Zondervan K, Becker C (2018) The miRNA Mirage: how close are we to finding a non-invasive diagnostic biomarker in endometriosis? A systematic review. Int J Mol Sci. https://doi.org/10.3390/ijms19020599
Fridrich A, Hazan Y, Moran Y (2019) Too many false targets for MicroRNAs: challenges and pitfalls in prediction of miRNA targets and their gene ontology in model and non-model organisms. BioEssays 41:e1800169. https://doi.org/10.1002/bies.201800169
Mirna M, Paar V, Rezar R, Topf A, Eber M, Hoppe UC, Lichtenauer M, Jung C (2019) MicroRNAs in inflammatory heart diseases and sepsis-induced cardiac dysfunction: a potential scope for the future? Cells. https://doi.org/10.3390/cells8111352
Cho S, Mutlu L, Grechukhina O, Taylor HS (2015) Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril 103(1252–60):e1. https://doi.org/10.1016/j.fertnstert.2015.02.013
Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, Taylor HS (2016) Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril 106:402–409. https://doi.org/10.1016/j.fertnstert.2016.04.013
Huan Q, Cheng SC, Du ZH, Ma HF, Li C (2021) LncRNA AFAP1-AS1 regulates proliferation and apoptosis of endometriosis through activating STAT3/TGF-beta/Smad signaling via miR-424-5p. J Obstet Gynaecol Res 47:2394–2405. https://doi.org/10.1111/jog.14801
Papari E, Noruzinia M, Kashani L, Foster WG (2020) Identification of candidate microRNA markers of endometriosis with the use of next-generation sequencing and quantitative real-time polymerase chain reaction. Fertil Steril 113:1232–1241. https://doi.org/10.1016/j.fertnstert.2020.01.026
Hsu CY, Hsieh TH, Tsai CF et al (2014) miRNA-199a-5p regulates VEGFA in endometrial mesenchymal stem cells and contributes to the pathogenesis of endometriosis. J Pathol 232:330–343. https://doi.org/10.1002/path.4295
Bourhis M, Palle J, Galy-Fauroux I, Terme M (2021) Direct and indirect modulation of T Cells by VEGF-A counteracted by anti-angiogenic treatment. Front Immunol 12:616837. https://doi.org/10.3389/fimmu.2021.616837
Ma Y, Huang YX, Chen YY (2017) miRNA34a5p downregulation of VEGFA in endometrial stem cells contributes to the pathogenesis of endometriosis. Mol Med Rep 16:8259–8264. https://doi.org/10.3892/mmr.2017.7677
Braza-Boils A, Gilabert-Estelles J, Ramon LA, Gilabert J, Mari-Alexandre J, Chirivella M, Espana F, Estelles A (2013) Peritoneal fluid reduces angiogenesis-related microRNA expression in cell cultures of endometrial and endometriotic tissues from women with endometriosis. PLoS ONE 8:e62370. https://doi.org/10.1371/journal.pone.0062370
Pang QX, Liu Z (2020) miR-17–5p mitigates endometriosis by directly regulating VEGFA. J Biosci 45. https://www.ncbi.nlm.nih.gov/pubmed/32515360
Braza-Boils A, Mari-Alexandre J, Gilabert J, Sanchez-Izquierdo D, Espana F, Estelles A, Gilabert-Estelles J (2014) MicroRNA expression profile in endometriosis: its relation to angiogenesis and fibrinolytic factors. Hum Reprod 29:978–988. https://doi.org/10.1093/humrep/deu019
Wang F, Wang H, Jin D, Zhang Y (2018) Serum miR-17, IL-4, and IL-6 levels for diagnosis of endometriosis. Medicine (Baltimore) 97:e10853. https://doi.org/10.1097/MD.0000000000010853
Acien P, Shaw RW, Irvine L, Burford G, Gardner R (1989) CA 125 levels in endometriosis patients before, during and after treatment with danazol or LHRH agonists. Eur J Obstet Gynecol Reprod Biol 32:241–246. https://doi.org/10.1016/0028-2243(89)90042-7
Knific T, Vouk K, Vogler A, Osredkar J, Gstottner M, Wenzl R, Rizner TL (2018) Models including serum CA-125, BMI, cyst pathology, dysmenorrhea or dyspareunia for diagnosis of endometriosis. Biomark Med 12:737–747. https://doi.org/10.2217/bmm-2017-0426
Bazot M, Kermarrec E, Bendifallah S, Darai E (2021) MRI of intestinal endometriosis. Best Pract Res Clin Obstet Gynaecol 71:51–63. https://doi.org/10.1016/j.bpobgyn.2020.05.013
Shan J, Ni Z, Cheng W, Zhou L, Zhai D, Sun S, Yu C (2021) Gut microbiota imbalance and its correlations with hormone and inflammatory factors in patients with stage 3/4 endometriosis. Arch Gynecol Obstet 304:1363–1373. https://doi.org/10.1007/s00404-021-06057-z
Wu Y, Yuan W, Ding H, Wu X (2022) Serum exosomal miRNA from endometriosis patients correlates with disease severity. Arch Gynecol Obstet 305:117–127. https://doi.org/10.1007/s00404-021-06227-z
Zhao L, Gu C, Ye M, Zhang Z, Li L, Fan W, Meng Y (2018) Integration analysis of microRNA and mRNA paired expression profiling identifies deregulated microRNA-transcription factor-gene regulatory networks in ovarian endometriosis. Reprod Biol Endocrinol 16:4. https://doi.org/10.1186/s12958-017-0319-5
Pateisky P, Pils D, Szabo L, Kuessel L, Husslein H, Schmitz A, Wenzl R, Yotova I (2018) hsa-miRNA-154-5p expression in plasma of endometriosis patients is a potential diagnostic marker for the disease. Reprod Biomed Online 37:449–466. https://doi.org/10.1016/j.rbmo.2018.05.007
Jia SZ, Yang Y, Lang J, Sun P, Leng J (2013) Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum Reprod 28:322–330. https://doi.org/10.1093/humrep/des413
Wu J, Cui SH, Li HZ, Li QH, Yuan R, Zhang YP, Zhao TW (2016) Ultrasound diagnosis in gynecological acute abdomen. J Biol Regul Homeost Agents 30: 211–7. https://www.ncbi.nlm.nih.gov/pubmed/27049094
Wang S, Yi M, Zhang X, Zhang T, Jiang L, Cao L, Zhou Y, Fang X (2021) Effects of CDKN2B-AS1 on cellular proliferation, invasion and AKT3 expression are attenuated by miR-424-5p in a model of ovarian endometriosis. Reprod Biomed Online 42:1057–1066. https://doi.org/10.1016/j.rbmo.2021.02.004
Xu SL, Tian YY, Zhou Y, Liu LQ (2020) Diagnostic value of circulating microRNAs in thyroid carcinoma: A systematic review and meta-analysis. Clin Endocrinol (Oxf) 93:489–498. https://doi.org/10.1111/cen.14217
Wu XG, Chen JJ, Zhou HL, Wu Y, Lin F, Shi J, Wu HZ, Xiao HQ, Wang W (2021) Identification and validation of the signatures of infiltrating immune cells in the eutopic endometrium endometria of women with endometriosis. Front Immunol 12:671201. https://doi.org/10.3389/fimmu.2021.671201
Knox B, Ong YC, Bakar MA, Grover SR (2019) A longitudinal study of adolescent dysmenorrhoea into adulthood. Eur J Pediatr 178:1325–1332. https://doi.org/10.1007/s00431-019-03419-3
Esfandiari F, Chitsazian F, Jahromi MG, Favaedi R, Bazrgar M, Aflatoonian R, Afsharian P, Aflatoonian A, Shahhoseini M (2021) HOX cluster and their cofactors showed an altered expression pattern in eutopic and ectopic endometriosis tissues. Reprod Biol Endocrinol 19:132. https://doi.org/10.1186/s12958-021-00816-y
Li J, Ren L, Li M, Yang C, Chen J, Chen Q (2021) Screening of potential key genes related to tubal factor infertility based on competitive endogenous RNA network. Genet Test Mol Biomarkers 25:325–333. https://doi.org/10.1089/gtmb.2020.0083
Feng Y, Zou S, Weijdegard B, Chen J, Cong Q, Fernandez-Rodriguez J, Wang L, Billig H, Shao R (2014) The onset of human ectopic pregnancy demonstrates a differential expression of miRNAs and their cognate targets in the Fallopian tube. Int J Clin Exp Pathol 7: 64–79. https://www.ncbi.nlm.nih.gov/pubmed/24427327
Karadadas E, Hortu I, Ak H, Ergenoglu AM, Karadadas N, Aydin HH (2020) Evaluation of complement system proteins C3a, C5a and C6 in patients of endometriosis. Clin Biochem 81:15–19. https://doi.org/10.1016/j.clinbiochem.2020.04.005
Wei Y, Liang Y, Lin H, Dai Y, Yao S (2020) Autonomic nervous system and inflammation interaction in endometriosis-associated pain. J Neuroinflammation 17:80. https://doi.org/10.1186/s12974-020-01752-1
Zhou WJ, Yang HL, Shao J, Mei J, Chang KK, Zhu R, Li MQ (2019) Anti-inflammatory cytokines in endometriosis. Cell Mol Life Sci 76:2111–2132. https://doi.org/10.1007/s00018-019-03056-x
Mosbah A, Nabiel Y, Khashaba E (2016) Interleukin-6, intracellular adhesion molecule-1, and glycodelin A levels in serum and peritoneal fluid as biomarkers for endometriosis. Int J Gynaecol Obstet 134:247–251. https://doi.org/10.1016/j.ijgo.2016.01.018
Korbel C, Gerstner MD, Menger MD, Laschke MW (2018) Notch signaling controls sprouting angiogenesis of endometriotic lesions. Angiogenesis 21:37–46. https://doi.org/10.1007/s10456-017-9580-7
Delbandi AA, Mahmoudi M, Shervin A, Heidari S, Kolahdouz-Mohammadi R, Zarnani AH (2020) Evaluation of apoptosis and angiogenesis in ectopic and eutopic stromal cells of patients with endometriosis compared to non-endometriotic controls. BMC Womens Health 20:3. https://doi.org/10.1186/s12905-019-0865-4
Hortu I, Ozceltik G, Karadadas E, Erbas O, Yigitturk G, Ulukus M (2020) The role of ankaferd blood stopper and oxytocin as potential therapeutic agents in endometriosis: a rat model. Curr Med Sci 40:556–562. https://doi.org/10.1007/s11596-020-2213-1
Kokot I, Piwowar A, Jedryka M, Solkiewicz K, Kratz EM (2021) Diagnostic significance of selected serum inflammatory markers in women with advanced endometriosis. Int J Mol Sci. https://doi.org/10.3390/ijms22052295
Malutan AM, Drugan C, Drugan T, Ciortea R, Mihu D (2016) The association between interleukin-4 -590C/T genetic polymorphism, IL-4 serum level, and advanced endometriosis. Cent Eur J Immunol 41:176–181. https://doi.org/10.5114/ceji.2016.60992
Volpato LK, Horewicz VV, Bobinski F, Martins DF, Piovezan AP (2018) Annexin A1, FPR2/ALX, and inflammatory cytokine expression in peritoneal endometriosis. J Reprod Immunol 129:30–35. https://doi.org/10.1016/j.jri.2018.08.002
Jiang J, Jiang Z, Xue M (2019) Serum and peritoneal fluid levels of interleukin-6 and interleukin-37 as biomarkers for endometriosis. Gynecol Endocrinol 35:571–575. https://doi.org/10.1080/09513590.2018.1554034
Li Y, Guo W, Cai Y (2020) NEAT1 promotes LPS-induced inflammatory injury in macrophages by regulating MiR-17-5p/TLR4. Open Med (Wars) 15:38–49. https://doi.org/10.1515/med-2020-0007
Li C, Zhang M, Dai Y, Xu Z (2020) MicroRNA-424-5p regulates aortic smooth muscle cell function in atherosclerosis by blocking APOC3-mediated nuclear factor-kappaB signalling pathway. Exp Physiol 105:1035–1049. https://doi.org/10.1113/EP088088
Zhang YZ, Wang J, Xu F (2017) Circulating miR-29b and miR-424 as prognostic markers in patients with acute cerebral infarction. Clin Lab 63:1667–1674. https://doi.org/10.7754/Clin.Lab.2017.170420
Braza-Boils A, Salloum-Asfar S, Mari-Alexandre J et al (2015) Peritoneal fluid modifies the microRNA expression profile in endometrial and endometriotic cells from women with endometriosis. Hum Reprod 30:2292–2302. https://doi.org/10.1093/humrep/dev204
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
CLL is the guarantor of integrity of the entire study; CLL contributed to the study concepts, study design, definition of intellectual content, literature research, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing and manuscript review; SLZ contributed to the study concepts, study design, definition of intellectual content, literature research, clinical studies, data acquisition, data analysis, manuscript preparation, manuscript editing and manuscript review; MJL contributed to the study design, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing and manuscript review; all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no conflicts of interest.
Ethics statement
This study was approved by the academic ethics committee of Hunan Province Maternal and Child Health care Hospital and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All participants were fully informed and voluntarily signed the informed consent before sampling.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, C., Zeng, S. & Li, M. miR-424-5p combined with miR-17-5p has high diagnostic efficacy for endometriosis. Arch Gynecol Obstet 307, 169–177 (2023). https://doi.org/10.1007/s00404-022-06492-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06492-6