Abstract
Purpose
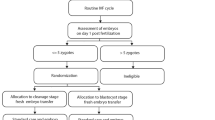
This retrospective cohort study determined the relative efficacy of blastocyst and cleavage-stage transfers in patients with differing numbers of zygotes.
Methods
A total of 1116 women whose embryo transfers were planned independently of patient characteristics were included. Cleavage-stage (D3) and blastocyst-stage (D5) transfer outcomes were analyzed per number of zygotes. The D5 group included transfer cancellations as the intention-to-treat population. The effect of the embryo transfer date on the clinical outcomes (clinical pregnancy and implantation rates) was analyzed using multivariate logistic regression.
Results
Among the patients, 584 and 532 underwent D3 and D5 embryo transfers, respectively. The clinical pregnancy rates were significantly higher in D5 patients with ≥ 6 zygotes (25.7% vs 48.3%). The multivariate logistic regression analysis for clinical pregnancy did not show significant differences between the blastocyst and cleavage-stage transfers in patients with ≤ 5 zygotes (0.874 [0.635-1.204]). Compared to the cleavage-stage, blastocyst-stage transfers for patients with ≥ 6 zygotes resulted in a three-fold increase in clinical pregnancy rates (3.122 [1.797-5.425]).
Conclusion
Blastocyst transfers were not inferior to cleavage-stage embryo transfers among patients with few zygotes and were preferable for patients with several zygotes.

Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Cutting R (2018) Single embryo transfer for all. Best Pract Res Clin Obstet Gynaecol 53:30–37. https://doi.org/10.1016/j.bpobgyn.2018.07.001
Wang X, Du M, Guan Y, Wang B, Zhang J, Liu Z (2017) Comparative neonatal outcomes in singleton births from blastocyst transfers or cleavage-stage embryo transfers: a systematic review and meta-analysis. Reprod Biol Endocrinol 15(1):36. https://doi.org/10.1186/s12958-017-0255-4
De Vos A, Dos Santos-Ribeiro S, Tournaye H, Verheyen G (2020) Birthweight of singletons born after blastocyst-stage or cleavage-stage transfer: analysis of a data set from three randomized controlled trials. J Assist Reprod Genet 37(1):127–132. https://doi.org/10.1007/s10815-019-01641-4
Li W, Xue X, Zhao W, Ren A, Zhuo W, Shi J (2017) Blastocyst transfer is not associated with increased unfavorable obstetric and perinatal outcomes compared with cleavage-stage embryo transfer. Gynecol Endocrinol 33(11):857–860. https://doi.org/10.1080/09513590.2017.1332175
Marconi N, Raja EA, Bhattacharya S, Maheshwari A (2019) Perinatal outcomes in singleton live births after fresh blastocyst-stage embryo transfer: a retrospective analysis of 67 147 IVF/ICSI cycles. Hum Reprod 34(9):1716–1725. https://doi.org/10.1093/humrep/dez133
Shi W, Zhang W, Li N, Xue X, Liu C, Qu P, Shi J, Huang C (2019) Comparison of perinatal outcomes following blastocyst and cleavage-stage embryo transfer: analysis of 10 years’ data from a single centre. Reprod Biomed Online 38(6):967–978. https://doi.org/10.1016/j.rbmo.2018.12.031
Krishnamoorthy K, Perlman BE, Morelli SS, Greenberg P, Jindal SK, Mcgovern P (2019) Ectopic/heterotopic pregnancy outcomes after blastocyst-stage frozen-thawed embryo transfers compared with cleavage stage: a SART-CORS study. Fertil Steril 112(3):E178. https://doi.org/10.1016/j.fertnstert.2019.07.582
Yin Y, Chen G, Li K, Liao Q, Zhang S, Ma N, Chen J, Zhang Y, Ai J (2017) Propensity score-matched study and meta-analysis of cumulative outcomes of day 2/3 versus day 5/6 embryo transfers. Front Med 11(4):563–569. https://doi.org/10.1007/s11684-017-0535-6
ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine. Electronic address: coticchio.biogenesi@grupposandonato.it (2017) The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod Biomed Online 35(5):494–510. https://doi.org/10.1016/j.rbmo.2017.06.015
Practice Committee of the American Society for Reproductive Medicine, & Practice Committee of the Society for Assisted Reproductive Technology (2018) Blastocyst culture and transfer in clinically assisted reproduction: a committee opinion. Fertil Steril 110(7):1246–1252. https://doi.org/10.1016/j.fertnstert.2018.09.011
ALPHA Scientists in Reproductive Medicine, ESHRE Special Interest Group of Embryology (2011) Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod Biomed Online 22(6):632–646. https://doi.org/10.1016/j.rbmo.2011.02.001
Haas J, Meriano J, Bassil R, Barzilay E, Casper RF (2019) What is the optimal timing of embryo transfer when there are only one or two embryos at cleavage stage? Gynecol Endocrinol 35(8):665–668. https://doi.org/10.1080/09513590.2019.1580259
Levi-Setti PE, Cirillo F, Smeraldi A, Morenghi E, Mulazzani G, Albani E (2018) No advantage of fresh blastocyst versus cleavage stage embryo transfer in women under the age of 39: a randomized controlled study. J Assist Reprod Genet 35(3):457–465. https://doi.org/10.1007/s10815-017-1092-2
Yang L, Cai S, Zhang S, Kong X, Gu Y, Lu C, Dai J, Gong F, Lu G, Lin G (2018) Single embryo transfer by Day 3 time-lapse selection versus Day 5 conventional morphological selection: a randomized, open-label, non-inferiority trial. Hum Reprod 33(5):869–876. https://doi.org/10.1093/humrep/dey047
Magli MC, Jones GM, Gras L, Gianaroli L, Korman I, Trounson AO (2000) Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Hum Reprod 15(8):1781–1786. https://doi.org/10.1093/humrep/15.8.1781
Braude P, Bolton V, Moore S (1988) Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 332(6163):459–461. https://doi.org/10.1038/332459a0
Xiao JS, Healey M, Talmor A, Vollenhoven B (2019) When only one embryo is available, is it better to transfer on Day 3 or to grow on? Reprod Biomed Online 39(6):916–923. https://doi.org/10.1016/j.rbmo.2019.08.003
De Croo I, De Sutter P, Tilleman K (2020) A stepwise approach to move from a cleavage-stage to a blastocyst-stage transfer policy for all patients in the IVF clinic. Human Reproduction Open 2020(3):hoaa34. https://doi.org/10.1093/hropen/hoaa034
Milki AA, Hinckley MD, Gebhardt J, Dasig D, Westphal LM, Behr B (2002) Accuracy of day 3 criteria for selecting the best embryos. Fertil Steril 77(6):1191–1195. https://doi.org/10.1016/s0015-0282(02)03104-7
Keefe D, Kumar M, Kalmbach K (2015) Oocyte competency is the key to embryo potential. Fertil Steril 103(2):317–322. https://doi.org/10.1016/j.fertnstert.2014.12.115
Karlıkaya G, Boynukalin FK, Gultomruk M, Kavrut M, Abalı R, Demir B, Ecemis S, Yarkiner Z, Bahceci M (2021) Euploidy rates of embryos in young patients with good and low prognosis according to the POSEIDON criteria. Reprod Biomed Online 42(4):733–741. https://doi.org/10.1016/j.rbmo.2021.01.001
Kahraman S, Çil AP, Oğur C, Semiz A, Yilanlioglu C (2016) Probability of finding at least one euploid embryo and the euploidy rate according to the number of retrieved oocytes and female age using FISH and array CGH. J Reprod Biotechnol Fertil 5:2058915816653277. https://doi.org/10.1177/2058915816653277
Tulay P, Gultomruk M, Findikli N, Bahceci M (2016) Number of embryos biopsied as a predictive indicator for the outcome of preimplantation genetic diagnosis by fluorescence in situ hybridisation in translocation cases. Zygote 24(1):107–114. https://doi.org/10.1017/S0967199414000793
Esteves SC, Carvalho JF, Bento FC, Santos J (2019) A novel predictive model to estimate the number of mature oocytes required for obtaining at least one euploid blastocyst for transfer in couples undergoing in vitro fertilization/intracytoplasmic sperm injection: the ART calculator. Front Endocrinol 10:99. https://doi.org/10.3389/fendo.2019.00099
Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A (2011) Association between the number of eggs and live birth in IVF treatment: an analysis of 400,135 treatment cycles. Hum Reprod 26(7):1768–1774. https://doi.org/10.1093/humrep/der106
Smeltzer S, Acharya K, Truong T, Pieper C, Muasher S (2019) Clinical pregnancy (CP) and live birth (LB) increase significantly with each additional fertilized oocyte up to nine, and CP and LB decline after that: an analysis of 15,803 first fresh in vitro fertilization cycles from the Society for Assisted Reproductive Technology registry. Fertil Steril 112(3):520-526.e1. https://doi.org/10.1016/j.fertnstert.2019.04.023
Zhu Q, Zhu J, Wang Y, Wang B, Wang N, Yin M, Zhang S, Lyu Q, Kuang Y (2019) Live birth rate and neonatal outcome following cleavage-stage embryo transfer versus blastocyst transfer using the freeze-all strategy. Reprod Biomed Online 38(6):892–900. https://doi.org/10.1016/j.rbmo.2018.12.034
Harlev A, Pariente M, Har-Vardi I, Friger M, Levitas E (2020) Pregnancy outcomes of fresh IVF conceived pregnancies after embryo transfer at different stages of early embryonic development. J Matern Fetal Neonatal Med. https://doi.org/10.1080/14767058.2020.1716215
Martins WP, Nastri CO, Rienzi L, van der Poel SZ, Racowsky GC (2017) Blastocyst vs cleavage-stage embryo transfer: systematic review and meta-analysis of reproductive outcomes. Ultrasound Obstet Gynecol 49(5):583–591. https://doi.org/10.1002/uog.17327
National Collaborating Centre for Women’s and Children’s Health (UK) (2013) Fertility: assessment and treatment for people with fertility problems. Royal College of Obstetricians & Gynaecologists, London
Adriaenssens T, Van Vaerenbergh I, Coucke W, Segers I, Verheyen G, Anckaert E, De Vos M, Smitz J (2019) Cumulus–corona gene expression analysis combined with morphological embryo scoring in single embryo transfer cycles increases live birth after fresh transfer and decreases time to pregnancy. J Assist Reprod Genet 36(3):433–443. https://doi.org/10.1007/s10815-018-01398-2
Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D (2016) Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD002118.pub5
Irani M, Zaninovic N, Canon C, O’Neill C, Gunnala V, Zhan Q, Palermo G, Reichman D, Rosenwaks Z (2018) A rationale for biopsying embryos reaching the morula stage on Day 6 in women undergoing preimplantation genetic testing for aneuploidy. Hum Reprod 33(5):935–941. https://doi.org/10.1093/humrep/dey053
Desai N, Ploskonka S, Goodman L, Attaran M, Goldberg JM, Austin C, Falcone T (2016) Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil Steril 106(6):1370–1378. https://doi.org/10.1016/j.fertnstert.2016.07.1095
Neal SA, Morin SJ, Franasiak JM, Goodman LR, Juneau CR, Forman EJ, Werner MD, Scott RT Jr (2018) Preimplantation genetic testing for aneuploidy is cost-effective, shortens treatment time, and reduces the risk of failed embryo transfer and clinical miscarriage. Fertil Steril 110(5):896–904. https://doi.org/10.1016/j.fertnstert.2018.06.021
Drakopoulos P, Blockeel C, Stoop D, Camus M, deVos M, Tournaye H, Polyzos NP (2016) Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod 31:370–376. https://doi.org/10.1093/humre3p/dev316
Patrizio P, Vaiarelli A, Levi Setti PE, Tobler KJ, Shoham G, Leong M, Shoham Z (2015) How to define, diagnose and treat poor responders? Responses from a worldwide survey of IVF clinics. Reprod Biomed Online 30:581–592. https://doi.org/10.1016/j.rbmo.2015.03.002
Vaiarelli A, Cimadomo D, Ubaldi N, Rienzi L, Ubaldi FM (2018) What is new in the management of poor ovarian response in IVF? Curr Opin Obstet Gynecol 30(3):155–162. https://doi.org/10.1097/GCO.0000000000000452
Kuang Y, Chen Q, Hong Q, Lyu Q, Ai A, Fu Y, Shoham Z (2014) Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod Biomed Online 29:684–691. https://doi.org/10.1016/j.rbmo.2014.08.009
Ding J, Yin T, Zhang Y, Zhou D, Yang J (2018) The effect of blastocyst transfer on newborn sex ratio and monozygotic twinning rate: an updated systematic review and meta-analysis. Reprod Biomed Online 37(3):292–303. https://doi.org/10.1016/j.rbmo.2018.05.015
Hattori H, Kitamura A, Takahashi F, Kobayashi N, Sato A, Miyauchi N, Nishigori H, Mizuno S, Sakurai K, Ishikuro M, Obara T, Tatsuta N, Nishijima I, Fujiwara I, Kuriyama S, Metoki H, Yaegashi N, Nakai K, Arima T, Japan Environment and Children’s Study Group (2019) The risk of secondary sex ratio imbalance and increased monozygotic twinning after blastocyst transfer: data from the Japan Environment and Children’s Study. Reprod Biol Endocrinol 17(1):27. https://doi.org/10.1186/s12958-019-0471-1
Wang S, Chen L, Fang J, Jiang W, Zhang N (2019) Comparison of the pregnancy and obstetric outcomes between single cleavage-stage embryo transfer and single blastocyst transfer by time-lapse selection of embryos. Gynecol Endocrinol 35(9):792–795. https://doi.org/10.1080/09513590.2019.1594762
Litzky JF, Boulet SL, Esfandiari N, Zhang Y, Kissin DM, Theiler RN, Marsit CJ (2018) Birthweight in infants conceived through in vitro fertilization following blastocyst or cleavage-stage embryo transfer: a national registry study. J Assist Reprod Genet 35(6):1027–1037. https://doi.org/10.1007/s10815-018-1168-7
Zhu Q, Wang N, Wang B, Wang Y, Kuang Y (2018) The risk of birth defects among children born after vitrified blastocyst transfers and those born after fresh and vitrified cleavage-stage embryo transfers. Arch Gynecol Obstet 298(4):833–840. https://doi.org/10.1007/s00404-018-4870-x
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SO: Conceptualization, formal analysis, methodology, writing. MS: Formal analysis, supervision, writing. MC: Methodology, supervision, visualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Approved by the Institutional Review Board of Akdeniz University, Faculty of Medicine (Approval Number: 27012021/71).
Consent to participate
Approved.
Consent for publication
Approved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dirican, E.K., Olgan, S., Sakinci, M. et al. Blastocyst versus cleavage transfers: who benefits?. Arch Gynecol Obstet 305, 749–756 (2022). https://doi.org/10.1007/s00404-021-06224-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06224-2