Abstract
Purpose
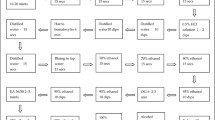
To evaluate the agreement of wet smear microscopy with Gram stain microscopy and to assess whether it is possible to predict Mycoplasmas/Ureaplasmas when analysing vaginal secretion with Gram stain and wet smear microscopy.
Methods
Women with complaints of the abnormal vaginal discharge were invited to participate. A sample of vaginal secretion was taken for wet smear microscopy and for Gram staining analysis. A sample from the endocervical canal was taken for DNA detection of seven infections: Mycoplasma hominis, Mycoplasma genitalium, Ureaplasma parvum, Ureaplasma urealyticum, Chlamydia trachomatis, Neisseria gonorrhoeae and Trichomonas vaginalis. The percentage agreement between wet smear and Gram stain was determined and the Cohen’s Kappa values were calculated.
Results
Of 158 consecutive women included, one (or a few) of the infections were detected in 54% of them and the most frequent infection was Ureaplasma parvum (79% of all the cases with infections). The percentage agreement between vaginal wet smear and Gram stain was 73% (Cohen’s Kappa value 0.63). A statistically significant association between the DNA detected Mycoplasmas/Ureaplasmas and bacterial vaginosis was found (positive amine test p = 0.046, wet smear p = 0.005 and Gram stain p = 0.03).
Conclusions
There was a statistically significant association between bacterial vaginosis and the DNA detected Mycoplasmas/Ureaplasmas. The agreement of vaginal wet smear with Gram stain was good.




Similar content being viewed by others
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Jonsson M, Karlsson R, Rylander E, Boden E, Edlund K et al (1995) The silent suffering women–a population based study on the association between reported symptoms and past and present infections of the lower genital tract. Genitourin Med 71:158–162
Hainer BL, Gibson MV (2011) Vaginitis. Am Fam Physician 83:807–815
Westrom L (1999) Genital infections. In: Gottlieb C, Von Schaultz B (eds) Outpatient gynecology, 1st edn. Elanders Gummessons, Falkoping, pp 120–148
Mylonas I, Bergauer F (2011) Diagnosis of vaginal discharge by wet mount microscopy: a simple and underrated method. Obstet Gynecol Surv 66:359–368
Nardis C, Mosca L, Mastromarino P (2013) Vaginal microbiota and viral sexually transmitted diseases. Ann Ig 25:443–456
Weinstock H, Berman S, Cates W Jr (2004) Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health 36:6–10
Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL (2003) Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis 36:663–668
Brotman RM, Klebanoff MA, Nansel TR, Yu KF, Andrews WW et al (2010) Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis 202:1907–1915
Steinhandler L, Peipert JF, Heber W, Montagno A, Cruickshank C (2002) Combination of bacterial vaginosis and leukorrhea as a predictor of cervical chlamydial or gonococcal infection. Obstet Gynecol 99:603–607
Bjartling C, Osser S, Persson K (2012) Mycoplasma genitalium in cervicitis and pelvic inflammatory disease among women at a gynecologic outpatient service. Am J Obstet Gynecol 206(476):e1-8
Donders GG, Ruban K, Bellen G, Petricevic L (2017) Mycoplasma/ureaplasma infection in pregnancy:to screem or not to screen. J Perinat Med 45(5):505–515
Petersen CS, Danielsen AG, Renneberg J (1999) Direct or referral microscopy of vaginal wet smear for bacterial vaginosis: experience from an STD clinic. Acta Derm Venereol 79:473–474
Kundel HL, Polansky M (2003) Measurement of observer agreement. Radiology 228:303–308
Donders GG, Marconi C, Bellen G, Donders F, Michiels T (2015) Effect of short training on vaginal fluid microscopy (wet mount) learning. J Lower Gen Tract Dis 19(2):165–169
Rumyantseva T, Khayrullina G, Guschin A, Donders G (2019) Prevalence of Ureaplasma spp. and Mycoplasma hominis in healthy women and patients with flora alterations. Diagn Microbiol Infect Dis 93(3):227–231
Donders GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B (2002) Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG 109(1):34–43
Brotman RM (2011) Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest 121:4610–4617
Marrazzo JM, Wiesenfeld HC, Murray PJ, Busse B, Meyn L et al (2006) Risk factors for cervicitis among women with bacterial vaginosis. J Infect Dis 193:617–624
Taylor-Robinson D (2017) Mollicutes in vaginal microbiology: Mycoplasma hominis, Ureaplasma urealyticum, Ureaplasma parvum and Mycoplasma genitalium. Res Microbiol 168(9–10):875–881
Dando SJ, Nitsos I, Kallapur SG, Newnham JP, Polglase GR et al (2012) The role of the multiple banded antigen of Ureaplasma parvum in intra-amniotic infection: major virulence factor or decoy? PLoS ONE 7:e29856
Knox CL, Allan JA, Allan JM, Edirisinghe WR, Stenzel D et al (2003) Ureaplasma parvum and Ureaplasma urealyticum are detected in semen after washing before assisted reproductive technology procedures. Fertil Steril 80:921–929
Rittenschober-Böhm J, Waldhoer T, Schulz SM, Pimpel B, Goeral K, Kasper DC, Witt A, Berger A (2019) Vaginal Ureaplasma parvum serovars and spontaneus preterm birth. Am J Obstet Gynecol 220(6):594.e1-594.e9
Capoccia R, Greub G, Baud D (2013) Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis 26(3):231–240
Horner P, Donders G, Cusini M, Gomberg M, Jensen JS, Unemo M (2018) Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women?—a position statement from the European STI Guidelines Editorial Board. J Eur Acad Dermatol Venereol 32(11):1845–1851
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
GO: project development, data collection and management, manuscript writing and editing; ZB: data collection, manuscript editing; SK: data analysis; AB: data collection, manuscript writing; DB: project development, data collection and management, manuscript writing and editing; DR: project development, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Ethical approval was obtained from the Vilnius Regional Biomedical Research Ethics Committee, reference number No. 158200-13-574-169 of 11 January 2013.
Consent to participate
All the patients gave an informed consent after the procedure had been fully explained.
Consent for publication
All the patients gave an informed consent after the procedure had been fully explained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Opolskiene, G., Bumbuliene, Z., Kiveryte, S. et al. The use of vaginal wet smear: can we predict Mycoplasmas/Ureaplasmas?. Arch Gynecol Obstet 304, 157–162 (2021). https://doi.org/10.1007/s00404-021-05976-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-05976-1