Abstract
Introduction
Vancomycin powder (VP) is a well-established topical antibiotic used in spinal surgery to prevent surgical site infections. More recently its extension to hip and knee arthroplasty was introduced. The aim of this study was to examine toxic effects of VP on the viability of human chondrocytes. Our hypothesis was that VP damages human chondrocytes in vitro with increasing concentration and length of exposure.
Material and methods
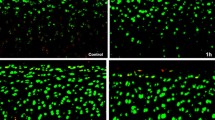
Primary human chondrocytes were isolated and cultured from donated human knee joints. VP was added to these cultures with increasing concentrations (0–50 mg/ml) and length of exposure (0–336 h). Toxicity and viability were analyzed using LDH und XTT Elisa assays. Cell structure and determination of vital versus dead cells were visualized using light microscopy and fluorescence microscopy.
Results
Light microscopy and fluorescence microscopy visualized defect cell structures and cell death proportional to increasing dose and length of exposure to VP. The analysis of LDH activity data showed toxic effects on chondrocytes as early as 2,5 min after exposure to VP. XTT activity data revealed a significant toxic threshold of a VP concentration above 12.5 mg/ml.
Conclusions
These results show that exposure to high VP concentrations yields to a damage of human chondrocytes in vitro. Chondrotoxicity is an immediate effect that is proportional to VP concentration. Therefore, the intraarticular use of high concentrations of vancomycin powder in the presence of native cartilage tissue must be considered critically.





Similar content being viewed by others
References
Almustafa MA, Ewen AM, Deakin AH, Picard F, Clarke JV, Mahmood FF (2018) Risk factors for surgical site infection following lower limb arthroplasty: a retrospective cohort analysis of 3932 lower limb arthroplasty procedures in a high volume arthroplasty unit. J Arthroplasty 33(6):1861–1867
Bullock MW, Brown ML, Bracey DN, Langfitt MK, Shields JS, Lang JE (2017) A bundle protocol to reduce the incidence of periprosthetic joint infections after total joint arthroplasty: a single-center experience. J Arthroplasty 32(4):1067–1073. https://doi.org/10.1016/j.arth.2016.11.028
Hijas-Gómez AI, Lucas WC, Checa-García A, Martínez-Martín J, Fahandezh-Saddi H, Gil-de-Miguel Á, Durán-Poveda M, Rodríguez-Caravaca G (2018) Surgical site infection incidence and risk factors in knee arthroplasty: a 9-year prospective cohort study at a university teaching hospital in Spain. Am J Infect Control 46(12):1335–1340
Lamplot JD, Luther G, Mawdsley EL, Luu HH, Manning D (2015) Modified protocol decreases surgical site infections after total knee arthroplasty. J Knee Surg 28(5):395–403
Lindeque B, Hartman Z, Noshchenko A, Cruse M (2014) Infection after primary total hip arthroplasty. Orthopedics 37(4):257–265
Saku SA, Madanat R, Mäkinen TJ (2018) Reasons and risk factors for ninety day re-admission following primary total knee arthroplasty in a high-volume centre. Int Orthop 42(1):95–99
Shohat N, Bauer T, Buttaro M et al (2019) Hip and knee section, what is the definition of a periprosthetic joint infection (PJI) of the knee and the hip? can the same criteria be used for both joints?: of international consensus on orthopedic infections. J Arthroplasty 34(2S):S325–S327
Bakhsheshian J, Dahdaleh NS, Lam SK, Savage JW, Smith ZA (2015) The use of vancomycin powder in modern spine surgery: systematic review and meta-analysis of the clinical evidence. World Neurosurg 83(5):816–823
He X, Sun T, Wang J, Li G, Fei Q (2019) Application of vancomycin powder to reduce surgical infection and deep surgical infection in spinal surgery: a meta-analysis. Clin Spine Surg 32(4):150–163
Kang DG, Holekamp TF, Wagner SC, Lehman RA (2015) Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: a systematic literature review. Spine J 15(4):762–770
Thompson GH, Poe-Kochert C, Hardesty CK, Son-Hing J, Mistovich RJ (2018) Does vancomycin powder decrease surgical site infections in growing spine surgery?: a preliminary study. J Bone Jt Surg Am 100(6):466–471
Xie L, Zhu J, Luo S, Xie Y, Pu D (2018) Do dose-dependent microbial changes occur during spine surgery as a result of applying intrawound vancomycin powder? a systematic literature review. Asian Spine J 12(1):162–170
Patel NN, Guild GN, Kumar AR (2018) Intrawound vancomycin in primary hip and knee arthroplasty: a safe and cost-effective means to decrease early periprosthetic joint infection. Arthroplast Today 4(4):479–483
Chin SJ, Moore GA, Zhang M, Clarke HD, Spangehl MJ, Young SW (2018) The AAHKS clinical research award: intraosseous regional prophylaxis provides higher tissue concentrations in high BMI patients in total knee arthroplasty: a randomized trial. J Arthroplasty 33(7S):S13–S18
Cohen EM, Marcaccio S, Goodman AD, Lemme NJ, Limbird R (2019) Efficacy and cost-effectiveness of topical vancomycin powder in primary cementless total hip arthroplasty. Orthopedics 26:1–7
Heckmann ND, Mayfield CK, Culvern CN, Oakes DA, Lieberman JR, Valle Della CJ (2019) Systematic review and meta-analysis of intrawound vancomycin in total hip and total knee arthroplasty: a call for a prospective randomized trial. J Arthroplasty 34(8):1815–1822
Qadir R, Ochsner JL, Chimento GF, Meyer MS, Waddell B, Zavatsky JM (2014) Establishing a role for vancomycin powder application for prosthetic joint infection prevention-results of a wear simulation study. J Arthroplasty 29(7):1449–1456
Otte JE, Politi JR, Chambers B, Smith CA (2017) Intrawound vancomycin powder reduces early prosthetic joint infections in revision hip and knee arthroplasty. Surg Technol Int 30:284–289
Young SW, Zhang M, Moore GA, Pitto RP, Clarke HD, Spangehl MJ (2018) The John N. insall award: higher tissue concentrations of vancomycin achieved with intraosseous regional prophylaxis in revision TKA: a randomized controlled trial. Clin Orthop Relat Res 476(1):66–74
Röhner E, Kolar P, Seeger JB, Arnholdt J, Thiele K, Perka C, Matziolis G (2011) Toxicity of antiseptics against chondrocytes: what is best for the cartilage in septic joint surgery? Int Orthop 35(11):1719–1723
Johnson JD, Nessler JM, Horazdovsky RD, Vang S, Thomas AJ, Marston SB (2017) Serum and wound vancomycin levels after intrawound administration in primary total joint arthroplasty. J Arthroplasty 32(3):924–928
Hanada M, Nishikino S, Hotta K, Furuhashi H, Hoshino H, Matsuyama Y (2019) Intrawound vancomycin powder increases post-operative wound complications and does not decrease periprosthetic joint infection in primary total and unicompartmental knee arthroplasties. Knee Surg Sports Traumatol Arthrosc 27(7):2322–2327
Antoci V Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J (2007) Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin Orthop Relat Res 462:200–206
Shaw KA, Eichinger JK, Nadig N, Parada SA (2018) In vitro effect of vancomycin on the viability of articular chondrocytes. J Orthop Trauma 32(3):148–153
Dogan M, Isyar M, Yilmaz I, Bilir B, Sirin DY, Cakmak S, Mahirogullari M (2016) Are the leading drugs against Staphylococcus aureus really toxic to cartilage? J Infect Public Health 9(3):251–258
Schüttler KF, Scharm A, Stein T, Heyse TJ, Lohoff M, Sommer F, Spiess-Naumann A, Efe T (2019) Biomechanical and microbiological effects of local vancomycin in anterior cruciate ligament (ACL) reconstruction: a porcine tendon model. Arch Orthop Trauma Surg 139(1):73–78
Acknowledgements
We would like to thank Mrs. B. Ukena for her assistance and collaboration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors have no proprietary, financial, professional or other personal interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Röhner, E., Zippelius, T., Böhle, S. et al. Vancomycin is toxic to human chondrocytes in vitro. Arch Orthop Trauma Surg 141, 375–381 (2021). https://doi.org/10.1007/s00402-020-03431-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-020-03431-5