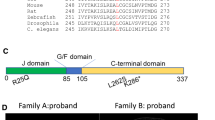
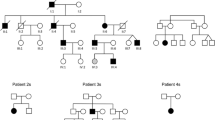
Abstract
DnaJ homolog, subfamily B, member 4, a member of the heat shock protein 40 chaperones encoded by DNAJB4, is highly expressed in myofibers. We identified a heterozygous c.270 T > A (p.F90L) variant in DNAJB4 in a family with a dominantly inherited distal myopathy, in which affected members have specific features on muscle pathology represented by the presence of cytoplasmic inclusions and the accumulation of desmin, p62, HSP70, and DNAJB4 predominantly in type 1 fibers. Both Dnajb4F90L knockin and knockout mice developed muscle weakness and recapitulated the patient muscle pathology in the soleus muscle, where DNAJB4 has the highest expression. These data indicate that the identified variant is causative, resulting in defective chaperone function and selective muscle degeneration in specific muscle fibers. This study demonstrates the importance of DNAJB4 in skeletal muscle proteostasis by identifying the associated chaperonopathy.










Similar content being viewed by others
References
Agbulut O, Destombes J, Thiesson D, Butler-Browne G (2000) Age-related appearance of tubular aggregates in the skeletal muscle of almost all male inbred mice. Histochem Cell Biol 114:477–481. https://doi.org/10.1007/s004180000211
Andley UP, Hamilton PD, Ravi N, Weihl CC (2011) A knock-in mouse model for the R120G mutation of alphaB-crystallin recapitulates human hereditary myopathy and cataracts. PLoS One 6:e17671. https://doi.org/10.1371/journal.pone.0017671
Anttonen A-K, Mahjneh I, Hämäläinen RH, Lagier-Tourenne C, Kopra O, Waris L et al (2005) The gene disrupted in Marinesco-Sjögren syndrome encodes SIL1, an HSPA5 cochaperone. Nat Genet 37:1309–1311. https://doi.org/10.1038/ng1677
Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M et al (2010) Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr Biol 20:143–148. https://doi.org/10.1016/j.cub.2009.11.022
Augusto V, Padovani CR, Campos GER (2004) Skeletal muscle fiber types in C57BL6J mice. J Morphol Sci 21:89–94
Bengoechea R, Findlay AR, Bhadra AK, Shao H, Stein KC, Pittman SK et al (2020) Inhibition of DNAJ-HSP70 interaction improves strength in muscular dystrophy. J Clin Invest 130:4470–4485. https://doi.org/10.1172/JCI136167
Blumen SC, Astord S, Robin V, Vignaud L, Toumi N, Cieslik A et al (2012) A rare recessive distal hereditary motor neuropathy with HSJ1 chaperone mutation. Ann Neurol 71:509–519. https://doi.org/10.1002/ana.22684
Bugiardini E, Rossor AM, Lynch DS, Swash M, Pittman AM, Blake JC et al (2017) Homozygous mutation in HSPB1 causing distal vacuolar myopathy and motor neuropathy. Neurol Genet 3:e168. https://doi.org/10.1212/nxg.0000000000000168
Caplan AJ (2003) What is a co-chaperone? Cell Stress Chaperones 8:105–107. https://doi.org/10.1379/1466-1268(2003)008%3c0105:wiac%3e2.0.co;2
Celichowski J, Grottel K (1993) Twitch/tetanus ratio and its relation to other properties of motor units. NeuroReport 5:201–204. https://doi.org/10.1097/00001756-199312000-00003
Cohen E, Bonne G, Rivier F, Hamroun D (2021) The 2022 version of the gene table of neuromuscular disorders (nuclear genome). Neuromuscul Disord 31:1313–1357. https://doi.org/10.1016/j.nmd.2021.11.004
Dirk KP, Zheng M, Pinkert S, Vecchi G, Ciryam P et al (2015) Widespread proteome remodeling and aggregation in aging C. elegans. Cell 161:919–932. https://doi.org/10.1016/j.cell.2015.03.032
Ederle H, Funk C, Abou-Ajram C, Hutten S, Funk EBE, Kehlenbach RH et al (2018) Nuclear egress of TDP-43 and FUS occurs independently of Exportin-1/CRM1. Sci Rep. https://doi.org/10.1038/s41598-018-25007-5
French RL, Grese ZR, Aligireddy H, Dhavale DD, Reeb AN, Kedia N et al (2019) Detection of TAR DNA-binding protein 43 (TDP-43) oligomers as initial intermediate species during aggregate formation. J Biol Chem 294:6696–6709. https://doi.org/10.1074/jbc.ra118.005889
Fuentealba RA, Udan M, Bell S, Wegorzewska I, Shao J, Diamond MI et al (2010) Interaction with polyglutamine aggregates reveals a Q/N-rich domain in TDP-43. J Biol Chem 285:26304–26314. https://doi.org/10.1074/jbc.m110.125039
Ghaoui R, Palmio J, Brewer J, Lek M, Needham M, Evilä A et al (2016) Mutations in HSPB8 causing a new phenotype of distal myopathy and motor neuropathy. Neurology 86:391–398. https://doi.org/10.1212/wnl.0000000000002324
Gonzaga-Jauregui C, Harel T, Gambin T, Kousi M, Griffin LB, Francescatto L et al (2015) Exome sequence analysis suggests that genetic burden contributes to phenotypic variability and complex neuropathy. Cell Rep 12:1169–1183. https://doi.org/10.1016/j.celrep.2015.07.023
Hackman P, Sarparanta J, Lehtinen S, Vihola A, Evilä A, Jonson PH et al (2013) Welander distal myopathy is caused by a mutation in the RNA-binding protein TIA1. Ann Neurol 73:500–509. https://doi.org/10.1002/ana.23831
Harms MB, Sommerville RB, Allred P, Bell S, Ma D, Cooper P et al (2012) Exome sequencing reveals DNAJB6 mutations in dominantly-inherited myopathy. Ann Neurol 71:407–416. https://doi.org/10.1002/ana.22683
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475:324–332. https://doi.org/10.1038/nature10317
Head SI, Arber MB (2013) An active learning mammalian skeletal muscle lab demonstrating contractile and kinetic properties of fast- and slow-twitch muscle. Adv Physiol Educ 37:405–414. https://doi.org/10.1152/advan.00155.2012
Hiraga K, Inoue YU, Asami J, Hotta M, Morimoto Y, Tatsumoto S et al (2020) Redundant type II cadherins define neuroepithelial cell states for cytoarchitectonic robustness. Commun Biol 3:574. https://doi.org/10.1038/s42003-020-01297-2
Iida A, Takano K, Takeshita E, Abe-Hatano C, Hirabayashi S, Inaba Y et al (2019) A novel PAK3 pathogenic variant identified in two siblings from a Japanese family with X-linked intellectual disability: case report and review of the literature. Cold Spring Harb Mol Case Stud 5:a003988. https://doi.org/10.1101/mcs.a003988
Inoue M, Iida A, Noguchi S, Nonaka I, Nishino I (2017) Comprehensive genome analysis of Japanese patients with myofibrillar myopathy. Neuromuscul Disord 27:S120. https://doi.org/10.1016/j.nmd.2017.06.103
Inoue M, Noguchi S, Sonehara K, Nakamura-Shindo K, Taniguchi A, Kajikawa H et al (2021) A recurrent homozygous ACTN2 variant associated with core myopathy. Acta Neuropathol 142:785–788. https://doi.org/10.1007/s00401-021-02363-7
Inoue M, Uchino S, Iida A, Noguchi S, Hayashi S, Takahashi T et al (2019) COX6A2 variants cause a muscle-specific cytochrome c oxidase deficiency. Ann Neurol 86:193–202. https://doi.org/10.1002/ana.25517
Johnson MA, Polgar J, Weightman D, Appleton D (1973) Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18:111–129. https://doi.org/10.1016/0022-510x(73)90023-3
Kampinga HH, Craig EA (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11:579–592. https://doi.org/10.1038/nrm2941
Kedia N, Arhzaouy K, Pittman SK, Sun Y, Batchelor M, Weihl CC et al (2019) Desmin forms toxic, seeding-competent amyloid aggregates that persist in muscle fibers. Proc Natl Acad Sci USA 116:16835–16840. https://doi.org/10.1073/pnas.1908263116
Klaips CL, Jayaraj GG, Hartl FU (2018) Pathways of cellular proteostasis in aging and disease. J Cell Biol 217:51–63. https://doi.org/10.1083/jcb.201709072
Koga H, Kaushik S, Cuervo AM (2011) Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res Rev 10:205–215. https://doi.org/10.1016/j.arr.2010.02.001
Küsters B, Van Hoeve BJA, Schelhaas HJ, Ter Laak H, Van Engelen BGM, Lammens M (2009) TDP-43 accumulation is common in myopathies with rimmed vacuoles. Acta Neuropathol 117:209–211. https://doi.org/10.1007/s00401-008-0471-2
Lee Y, Jonson PH, Sarparanta J, Palmio J, Sarkar M, Vihola A et al (2018) TIA1 variant drives myodegeneration in multisystem proteinopathy with SQSTM1 mutations. J Clin Invest 128:1164–1177. https://doi.org/10.1172/jci97103
Lualdi M, Alberio T, Fasano M (2020) Proteostasis and proteotoxicity in the network medicine era. Int J Mol Sci 21:6405. https://doi.org/10.3390/ijms21176405
Lupo V, Aguado C, Knecht E, Espinos C (2016) Chaperonopathies: spotlight on hereditary motor neuropathies. Front Mol Biosci 3:81. https://doi.org/10.3389/fmolb.2016.00081
Mackenzie IR, Nicholson AM, Sarkar M, Messing J, Purice MD, Pottier C et al (2017) TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron 95:808-816.e809. https://doi.org/10.1016/j.neuron.2017.07.025
Malicdan MC, Noguchi S, Hayashi YK, Nonaka I, Nishino I (2009) Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat Med 15:690–695. https://doi.org/10.1038/nm.1956
Malicdan MC, Noguchi S, Nishino I (2009) Monitoring autophagy in muscle diseases. Methods Enzymol 453:379–396. https://doi.org/10.1016/S0076-6879(08)04019-6
MéJat A, Decostre VR, Li J, Renou L, Kesari A, Hantaï D et al (2009) Lamin A/C–mediated neuromuscular junction defects in Emery-Dreifuss muscular dystrophy. J Cell Biol 184:31–44. https://doi.org/10.1083/jcb.200811035
Mercuri E, Jungbluth H, Muntoni F (2005) Muscle imaging in clinical practice: diagnostic value of muscle magnetic resonance imaging in inherited neuromuscular disorders. Curr Opin Neurol 18:526–537. https://doi.org/10.1097/01.wco.0000183947.01362.fe
Mercuri E, Pichiecchio A, Allsop J, Messina S, Pane M, Muntoni F (2007) Muscle MRI in inherited neuromuscular disorders: past, present, and future. J Magn Reson Imaging 25:433–440. https://doi.org/10.1002/jmri.20804
Milone M, Liewluck T (2019) The unfolding spectrum of inherited distal myopathies. Muscle Nerve 59:283–294. https://doi.org/10.1002/mus.26332
Moore CW, Beveridge TS, Rice CL (2017) Fiber type composition of the palmaris brevis muscle: implications for palmar function. J Anat 231:626–633. https://doi.org/10.1111/joa.12652
Morelli FF, Verbeek DS, Bertacchini J, Vinet J, Mediani L, Marmiroli S et al (2017) Aberrant compartment formation by HSPB2 Mislocalizes Lamin A and compromises nuclear integrity and function. Cell Rep 20:2100–2115. https://doi.org/10.1016/j.celrep.2017.08.018
Noguchi S, Ogawa M, Malicdan MC, Nonaka I, Nishino I (2017) Muscle weakness and fibrosis due to cell autonomous and non-cell autonomous events in collagen VI deficient congenital muscular dystrophy. EBioMedicine 15:193–202. https://doi.org/10.1016/j.ebiom.2016.12.011
Olivé M, Winter L, Fürst DO, Schröder R (2020) 246th ENMC International Workshop: protein aggregate myopathies 24–26 May 2019, Hoofddorp, The Netherlands. Neuromuscul Disord. https://doi.org/10.1016/j.nmd.2020.11.003
Palmio J, Jonson PH, Inoue M, Sarparanta J, Bengoechea R, Savarese M et al (2019) Mutations in the J domain of DNAJB6 cause dominant distal myopathy. Neuromuscul Disord. https://doi.org/10.1016/j.nmd.2019.11.005
Polla B (2004) Respiratory muscle fibres: specialisation and plasticity. Thorax 59:808–817. https://doi.org/10.1136/thx.2003.009894
Quijano-Roy S, Carlier RY, Fischer D (2011) Muscle imaging in congenital myopathies. Semin Pediatr Neurol 18:221–229. https://doi.org/10.1016/j.spen.2011.10.003
Round JM, Jones DA, Chapman SJ, Edwards RHT, Ward PS, Fodden DL (1984) The anatomy and fibre type composition of the human adductor pollicis in relation to its contractile properties. J Neurol Sci 66:263–272. https://doi.org/10.1016/0022-510X(84)90015-7
Sandell S, Huovinen S, Palmio J, Raheem O, Lindfors M, Zhao F et al (2016) Diagnostically important muscle pathology in DNAJB6 mutated LGMD1D. Acta Neuropathol Commun 4:9. https://doi.org/10.1186/s40478-016-0276-9
Sandell SM, Mahjneh I, Palmio J, Tasca G, Ricci E, Udd BA (2013) ‘Pathognomonic’ muscle imaging findings in DNAJB6 mutated LGMD1D. Eur J Neurol 20:1553–1559. https://doi.org/10.1111/ene.12239
Sarparanta J, Jonson PH, Golzio C, Sandell S, Luque H, Screen M et al (2012) Mutations affecting the cytoplasmic functions of the co-chaperone DNAJB6 cause limb-girdle muscular dystrophy. Nat Genet 44(450–455):S451-452. https://doi.org/10.1038/ng.1103
Sarparanta J, Jonson PH, Kawan S, Udd B (2020) Neuromuscular diseases due to chaperone mutations: a review and some new results. Int J Mol Sci 21:1409. https://doi.org/10.3390/ijms21041409
Sato T, Hayashi YK, Oya Y, Kondo T, Sugie K, Kaneda D et al (2013) DNAJB6 myopathy in an Asian cohort and cytoplasmic/nuclear inclusions. Neuromuscul Disord 23:269–276. https://doi.org/10.1016/j.nmd.2012.12.010
Savarese M, Sarparanta J, Vihola A, Jonson PH, Johari M, Rusanen S et al (2020) Panorama of the distal myopathies. Acta Myol 39:245–265. https://doi.org/10.36185/2532-1900-028
Selcen D (2011) Myofibrillar myopathies. Neuromuscul Disord 21:161–171. https://doi.org/10.1016/j.nmd.2010.12.007
Selcen D, Muntoni F, Burton BK, Pegoraro E, Sewry C, Bite AV et al (2009) Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann Neurol 65:83–89. https://doi.org/10.1002/ana.21553
Senderek J, Krieger M, Stendel C, Bergmann C, Moser M, Breitbach-Faller N et al (2005) Mutations in SIL1 cause Marinesco-Sjögren syndrome, a cerebellar ataxia with cataract and myopathy. Nat Genet 37:1312–1314. https://doi.org/10.1038/ng1678
Smith DA, Carland CR, Guo Y, Bernstein SI (2014) Getting folded: chaperone proteins in muscle development, maintenance and disease. Anat Rec (Hoboken) 297:1637–1649. https://doi.org/10.1002/ar.22980
Soto C, Pritzkow S (2018) Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci 21:1332–1340. https://doi.org/10.1038/s41593-018-0235-9
Stefani M (2004) Protein misfolding and aggregation: new examples in medicine and biology of the dark side of the protein world. Biochim Biophys Acta 1739:5–25. https://doi.org/10.1016/j.bbadis.2004.08.004
Stein KC, Bengoechea R, Harms MB, Weihl CC, True HL (2014) Myopathy-causing mutations in an HSP40 chaperone disrupt processing of specific client conformers. J Biol Chem 289:21120–21130. https://doi.org/10.1074/jbc.M114.572461
Terry EE, Zhang X, Hoffmann C, Hughes LD, Lewis SA, Li J et al (2018) Transcriptional profiling reveals extraordinary diversity among skeletal muscle tissues. Elife. https://doi.org/10.7554/elife.34613
Toigo M, Boutellier U (2006) New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur J Appl Physiol 97:643–663. https://doi.org/10.1007/s00421-006-0238-1
van Oosten-Hawle P, Porter RS, Morimoto RI (2013) Regulation of organismal proteostasis by transcellular chaperone signaling. Cell 153:1366–1378. https://doi.org/10.1016/j.cell.2013.05.015
Vicart P, Caron A, Guicheney P, Li Z, Prévost M-C, Faure A et al (1998) A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet 20:92–95. https://doi.org/10.1038/1765
Wattjes MP, Kley RA, Fischer D (2010) Neuromuscular imaging in inherited muscle diseases. Eur Radiol 20:2447–2460. https://doi.org/10.1007/s00330-010-1799-2
Weihl CC, Töpf A, Bengoechea R, Duff J, Charlton R, Garcia SK et al (2022) Loss of function variants in DNAJB4 cause a myopathy with early respiratory failure. Acta Neuropathol. https://doi.org/10.1007/s00401-022-02510-8
Weihl CC, Udd B, Hanna M, ENMC workshop study group (2018) 234th ENMC International Workshop: chaperone dysfunction in muscle disease Naarden, The Netherlands, 8–10 December 2017. Neuromuscul Disord 28:1022–1030. https://doi.org/10.1016/j.nmd.2018.09.004
Williams DR, Reardon K, Roberts L, Dennet X, Duff R, Laing NG et al (2005) A new dominant distal myopathy affecting posterior leg and anterior upper limb muscles. Neurology 64:1245–1254. https://doi.org/10.1212/01.wnl.0000156524.95261.b9
Yamazawa T, Kobayashi T, Kurebayashi N, Konishi M, Noguchi S, Inoue T et al (2021) A novel RyR1-selective inhibitor prevents and rescues sudden death in mouse models of malignant hyperthermia and heat stroke. Nat Commun. https://doi.org/10.1038/s41467-021-24644-1
Yonekawa T, Malicdan MC, Cho A, Hayashi YK, Nonaka I, Mine T et al (2014) Sialyllactose ameliorates myopathic phenotypes in symptomatic GNE myopathy model mice. Brain 137:2670–2679. https://doi.org/10.1093/brain/awu210
Zia A, Pourbagher-Shahri AM, Farkhondeh T, Samarghandian S (2021) Molecular and cellular pathways contributing to brain aging. Behav Brain Funct 17:6. https://doi.org/10.1186/s12993-021-00179-9
Acknowledgements
We thank the patients and their families for their cooperation. We also thank Hisayoshi Nakamura, Keiko Hiraki-Kamon, and Keiko Ishikawa for technical assistance. This study was supported by the Intramural Research Grant for Neurological and Psychiatric Disorders of the National Center of Neurology and Psychiatry under Grant Numbers 2-5 (S.H., I.N.), 2-6 (S.N.), 3-8 (M.T.), and 3-9 (S.N.); KAKENHI (19K17021) from the Japan Society for the Promotion of Science (M.I.); Japan Agency for Medical Research and Development under Grant Numbers 22ek0109490h0003 (A.I., S.H., S.N., I.N.); and NIH under Grant Numbers, NIH R01AR068797 (C.C.W.) and K24AR073317 (C.C.W.).
Author information
Authors and Affiliations
Contributions
MI, SN, and IN designed and oversaw this study. MI, SN, YUI, AI, MO, RB, SKP, and TI performed the experiments. MI, SN, AI, and CCW analyzed the data. MI, KW, YH, TS, MT, YO, YT, and HM provided the samples and clinical data. MI, SN, and CCW wrote the manuscript. All the authors contributed to and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Inoue, M., Noguchi, S., Inoue, Y.U. et al. Distinctive chaperonopathy in skeletal muscle associated with the dominant variant in DNAJB4. Acta Neuropathol 145, 235–255 (2023). https://doi.org/10.1007/s00401-022-02530-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-022-02530-4