Abstract
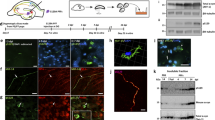
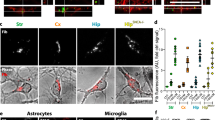
Alpha-synuclein (αSyn) preformed fibrils (PFF) induce endogenous αSyn aggregation leading to reduced synaptic transmission. Neuronal activity modulates release of αSyn; however, whether neuronal activity regulates the spreading of αSyn pathology remains elusive. Here, we established a hippocampal slice culture system from wild-type (WT) mice and found that both Ca2+ influx and the uptake of αSyn PFF were higher in the CA3 than in the CA1 sub-region. Pharmacologically enhancing neuronal activity substantially increased αSyn pathology in αSyn PFF-treated hippocampal or midbrain slice cultures and accelerated dopaminergic neuron degeneration. Consistently, neuronal hyperactivity promoted PFF trafficking along axons/dendrites within microfluidic chambers. Unexpectedly, enhancing neuronal activity in LRRK2 G2019S mutant slice cultures further increased αSyn pathology, especially with more Lewy body (LB) forming than in WT slice cultures. Finally, following injection of αSyn PFF and chemogenetic modulators into the dorsal striatum of WT mice, both motor behavior and αSyn pathology were exacerbated likely by enhancing neuronal activity, since they were ameliorated by reducing neuronal activity. Thus, a greater understanding of the impact of neuronal activity on αSyn aggregation and spreading, as well as dopaminergic neuronal vulnerability, may provide new therapeutic strategies for patients with LB disease (LBD).






Similar content being viewed by others
References
Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE et al (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239–252. https://doi.org/10.1016/s0896-6273(00)80886-7
Arber C, Lovejoy C, Wray S (2017) Stem cell models of Alzheimer's disease: progress and challenges. Alzheimers Res Ther 9:42. https://doi.org/10.1186/s13195-017-0268-4
Bassil F, Brown HJ, Pattabhiraman S, Iwasyk JE, Maghames CM, Meymand ES et al (2020) Amyloid-beta (Abeta) plaques promote seeding and spreading of alpha-synuclein and tau in a mouse model of lewy body disorders with abeta pathology. Neuron 105(260–275):e266. https://doi.org/10.1016/j.neuron.2019.10.010
Bove J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson's disease. NeuroRx 2:484–494. https://doi.org/10.1602/neurorx.2.3.484
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24:197–211. https://doi.org/10.1016/s0197-4580(02)00065-9
Busche MA, Konnerth A (2016) Impairments of neural circuit function in Alzheimer's disease. Philos Trans R Soc Lond B Biol Sci. https://doi.org/10.1098/rstb.2015.0429
Caputo A, Liang Y, Raabe TD, Lo A, Horvath M, Zhang B et a. (2020) Snca-GFP knock-in mice reflect patterns of endogenous expression and pathological seeding. ENeuro. https://doi.org/10.1523/ENEURO.0007-20.2020
Chen H, Ritz B (2018) The search for environmental causes of Parkinson's disease: moving forward. J Parkinsons Dis 8:S9–S17. https://doi.org/10.3233/JPD-181493
Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D'Avanzo C et al (2014) A three-dimensional human neural cell culture model of Alzheimer's disease. Nature 515:274–278. https://doi.org/10.1038/nature13800
Croft CL, Cruz PE, Ryu DH, Ceballos-Diaz C, Strang KH, Woody BM et al (2019) rAAV-based brain slice culture models of Alzheimer's and Parkinson's disease inclusion pathologies. J Exp Med 216:539–555. https://doi.org/10.1084/jem.20182184
Croft CL, Futch HS, Moore BD, Golde TE (2019) Organotypic brain slice cultures to model neurodegenerative proteinopathies. Mol Neurodegener 14:45. https://doi.org/10.1186/s13024-019-0346-0
Croft CL, Kurbatskaya K, Hanger DP, Noble W (2017) Inhibition of glycogen synthase kinase-3 by BTA-EG4 reduces tau abnormalities in an organotypic brain slice culture model of Alzheimer's disease. Sci Rep 7:7434. https://doi.org/10.1038/s41598-017-07906-1
Dong XX, Wang Y, Qin ZH (2009) Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin 30:379–387. https://doi.org/10.1038/aps.2009.24
Duff K, Noble W, Gaynor K, Matsuoka Y (2002) Organotypic slice cultures from transgenic mice as disease model systems. J Mol Neurosci 19:317–320. https://doi.org/10.1385/JMN:19:3:317
Elfarrash S, Jensen NM, Ferreira N, Betzer C, Thevathasan JV, Diekmann R et al (2019) Organotypic slice culture model demonstrates inter-neuronal spreading of alpha-synuclein aggregates. Acta Neuropathol Commun 7:213. https://doi.org/10.1186/s40478-019-0865-5
Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH et al (2010) Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30:6838–6851. https://doi.org/10.1523/JNEUROSCI.5699-09.2010
Emmanouilidou E, Minakaki G, Keramioti MV, Xylaki M, Balafas E, Chrysanthou-Piterou M et al (2016) GABA transmission via ATP-dependent K+ channels regulates alpha-synuclein secretion in mouse striatum. Brain 139:871–890. https://doi.org/10.1093/brain/awv403
Fahn S (2003) Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci 991:1–14. https://doi.org/10.1111/j.1749-6632.2003.tb07458.x
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS et al (2002) alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4:160–164. https://doi.org/10.1038/ncb748
Giasson BI, Murray IV, Trojanowski JQ, Lee VM (2001) A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. The Journal of biological chemistry 276:2380–2386. https://doi.org/10.1074/jbc.M008919200
Gogolla N, Galimberti I, DePaola V, Caroni P (2006) Preparation of organotypic hippocampal slice cultures for long-term live imaging. Nat Protoc 1:1165–1171. https://doi.org/10.1038/nprot.2006.168
Gong W, Sencar J, Bakkum DJ, Jackel D, Obien ME, Radivojevic M et al (2016) Multiple single-unit long-term tracking on organotypic hippocampal slices using high-density microelectrode arrays. Front Neurosci 10:537. https://doi.org/10.3389/fnins.2016.00537
Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, Zhang B et al (2013) Distinct alpha-synuclein strains differentially promote tau inclusions in neurons. Cell 154:103–117. https://doi.org/10.1016/j.cell.2013.05.057
Healy DG, Falchi M, O'Sullivan SS, Bonifati V, Durr A, Bressman Set al (2008) Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol 7:583–590. https://doi.org/10.1016/S1474-4422(08)70117-0
Henderson MX, Cornblath EJ, Darwich A, Zhang B, Brown H, Gathagan RJ et al (2019) Spread of alpha-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nat Neurosci 22:1248–1257. https://doi.org/10.1038/s41593-019-0457-5
Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE et al (2019) The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363:880–884. https://doi.org/10.1126/science.aav2546
Humpel C (2015) Organotypic brain slice cultures: a review. Neuroscience 305:86–98. https://doi.org/10.1016/j.neuroscience.2015.07.086
Irwin DJ, Lee VM, Trojanowski JQ (2013) Parkinson's disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat Rev Neurosci 14:626–636. https://doi.org/10.1038/nrn3549
Karpowicz RJ Jr, Haney CM, Mihaila TS, Sandler RM, Petersson EJ, Lee VM (2017) Selective imaging of internalized proteopathic alpha-synuclein seeds in primary neurons reveals mechanistic insight into transmission of synucleinopathies. J Biol Chem 292:13482–13497. https://doi.org/10.1074/jbc.M117.780296
Lesage S, Drouet V, Majounie E, Deramecourt V, Jacoupy M, Nicolas A et al (2016) Loss of VPS13C function in autosomal-recessive parkinsonism causes mitochondrial dysfunction and increases PINK1/parkin-dependent mitophagy. Am J Hum Genet 98:500–513. https://doi.org/10.1016/j.ajhg.2016.01.014
Loria F, Vargas JY, Bousset L, Syan S, Salles A, Melki R et al (2017) alpha-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol 134:789–808. https://doi.org/10.1007/s00401-017-1746-2
Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ et al (2012) Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338:949–953. https://doi.org/10.1126/science.1227157
Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, Lee VM (2012) Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med 209:975–986. https://doi.org/10.1084/jem.20112457
Luna E, Decker SC, Riddle DM, Caputo A, Zhang B, Cole T et al (2018) Differential alpha-synuclein expression contributes to selective vulnerability of hippocampal neuron subpopulations to fibril-induced toxicity. Acta Neuropathol 135:855–875. https://doi.org/10.1007/s00401-018-1829-8
Matikainen-Ankney BA, Kezunovic N, Mesias RE, Tian Y, Williams FM, Huntley GW et al (2016) Altered development of synapse structure and function in striatum caused by parkinson's disease-linked LRRK2-G2019S mutation. J Neurosci 36:7128–7141. https://doi.org/10.1523/JNEUROSCI.3314-15.2016
Messing L, Decker JM, Joseph M, Mandelkow E, Mandelkow EM (2013) Cascade of tau toxicity in inducible hippocampal brain slices and prevention by aggregation inhibitors. Neurobiol Aging 34:1343–1354. https://doi.org/10.1016/j.neurobiolaging.2012.10.024
Novotny R, Langer F, Mahler J, Skodras A, Vlachos A, Wegenast-Braun BM et al (2016) Conversion of synthetic abeta to in vivo active seeds and amyloid plaque formation in a hippocampal slice culture model. J Neurosci 36:5084–5093. https://doi.org/10.1523/JNEUROSCI.0258-16.2016
Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J et al (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci 26:10129–10140. https://doi.org/10.1523/JNEUROSCI.1202-06.2006
Opitz-Araya X, Barria A (2011) Organotypic hippocampal slice cultures. J Vis Exp. https://doi.org/10.3791/2462
Pang SY, Ho PW, Liu HF, Leung CT, Li L, Chang EES et al (2019) The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson's disease. Transl Neurodegener 8:23. https://doi.org/10.1186/s40035-019-0165-9
Pankratz N, Foroud T (2007) Genetics of Parkinson disease. Genet Med 9:801–811. https://doi.org/10.1097/gim.0b013e31815bf97c
Perier C, Vila M (2012) Mitochondrial biology and Parkinson's disease. Cold Spring Harb Perspect Med 2:a009332. https://doi.org/10.1101/cshperspect.a009332
Remple MS, Bradenham CH, Kao CC, Charles PD, Neimat JS, Konrad PE (2011) Subthalamic nucleus neuronal firing rate increases with Parkinson's disease progression. Mov Disord 26:1657–1662. https://doi.org/10.1002/mds.23708
Ren C, Ding Y, Wei S, Guan L, Zhang C, Ji Y et al (2019) G2019S variation in LRRK2: an ideal model for the study of Parkinson's disease? Front Hum Neurosci 13:306. https://doi.org/10.3389/fnhum.2019.00306
Rodriguez MC, Obeso JA, Olanow CW (1998) Subthalamic nucleus-mediated excitotoxicity in Parkinson's disease: a target for neuroprotection. Ann Neurol 44:S175–188. https://doi.org/10.1002/ana.410440726
Rodriguez PC, Pereira DB, Borgkvist A, Wong MY, Barnard C, Sonders MS et al (2013) Fluorescent dopamine tracer resolves individual dopaminergic synapses and their activity in the brain. Proc Natl Acad Sci USA 110:870–875. https://doi.org/10.1073/pnas.1213569110
Sampathu DM, Giasson BI, Pawlyk AC, Trojanowski JQ, Lee VM (2003) Ubiquitination of alpha-synuclein is not required for formation of pathological inclusions in alpha-synucleinopathies. Am J Pathol 163:91–100. https://doi.org/10.1016/s0002-9440(10)63633-4
Santa-Maria I, Diaz-Ruiz C, Ksiezak-Reding H, Chen A, Ho L, Wang J et al (2012) GSPE interferes with tau aggregation in vivo: implication for treating tauopathy. Neurobiol Aging 33:2072–2081. https://doi.org/10.1016/j.neurobiolaging.2011.09.027
Schatzle P, Kapitein LC, Hoogenraad CC (2016) Live imaging of microtubule dynamics in organotypic hippocampal slice cultures. Methods Cell Biol 131:107–126. https://doi.org/10.1016/bs.mcb.2015.06.006
Son AY, Biagioni MC, Kaminski D, Gurevich A, Stone B, Di Rocco A (2016) Parkinson's disease and cryptogenic epilepsy. Case Rep Neurol Med 2016:3745631. https://doi.org/10.1155/2016/3745631
Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M (1998) Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett 251:205–208. https://doi.org/10.1016/s0304-3940(98)00504-7
Spillantini MG, Goedert M (2016) Synucleinopathies: past, present and future. Neuropathol Appl Neurobiol 42:3–5. https://doi.org/10.1111/nan.12311
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840. https://doi.org/10.1038/42166
Stoppini L, Buchs PA, Muller D (1991) A simple method for organotypic cultures of nervous tissue. J Neurosci Methods 37:173–182. https://doi.org/10.1016/0165-0270(91)90128-m
Su X, Fischer DL, Li X, Bankiewicz K, Sortwell CE, Federoff HJ (2017) Alpha-synuclein mRNA is not increased in sporadic PD and alpha-synuclein accumulation does not block GDNF signaling in Parkinson's disease and disease models. Mol Ther 25:2231–2235. https://doi.org/10.1016/j.ymthe.2017.04.018
Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18:101–113. https://doi.org/10.1038/nrn.2016.178
Thakur P, Breger LS, Lundblad M, Wan OW, Mattsson B, Luk KC et al (2017) Modeling Parkinson's disease pathology by combination of fibril seeds and alpha-synuclein overexpression in the rat brain. Proc Natl Acad Sci USA 114:E8284–E8293. https://doi.org/10.1073/pnas.1710442114
Uemura N, Uemura MT, Lo A, Bassil F, Zhang B, Luk KC et al (2019) Slow progressive accumulation of oligodendroglial alpha-synuclein (alpha-Syn) pathology in synthetic alpha-syn fibril-induced mouse models of synucleinopathy. J Neuropathol Exp Neurol 78:877–890. https://doi.org/10.1093/jnen/nlz070
Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G et al (2019) Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570:523–527. https://doi.org/10.1038/s41586-019-1289-x
Volpicelli-Daley LA, Luk KC, Lee VM (2014) Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat Protoc 9:2135–2146. https://doi.org/10.1038/nprot.2014.143
Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A et al (2011) Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72:57–71. https://doi.org/10.1016/j.neuron.2011.08.033
Wang C, Kang X, Zhou L, Chai Z, Wu Q, Huang R et al (2018) Synaptotagmin-11 is a critical mediator of parkin-linked neurotoxicity and Parkinson's disease-like pathology. Nat Commun 9:81. https://doi.org/10.1038/s41467-017-02593-y
Wang Y, Wu Q, Hu M, Liu B, Chai Z, Huang R et al (2017) Ligand- and voltage-gated Ca(2+) channels differentially regulate the mode of vesicular neuropeptide release in mammalian sensory neurons. Sci Signal 10:484. https://doi.org/10.1126/scisignal.aal1683
Waxman EA, Giasson BI (2008) Specificity and regulation of casein kinase-mediated phosphorylation of alpha-synuclein. J Neuropathol Exp Neurol 67:402–416. https://doi.org/10.1097/NEN.0b013e31816fc995
Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K et al (2016) Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 19:1085–1092. https://doi.org/10.1038/nn.4328
Wu Q, Takano H, Riddle DM, Trojanowski JQ, Coulter DA, Lee VM (2019) alpha-Synuclein (alphaSyn) preformed fibrils induce endogenous alphaSyn aggregation, compromise synaptic activity and enhance synapse loss in cultured excitatory hippocampal neurons. J Neurosci 39:5080–5094. https://doi.org/10.1523/JNEUROSCI.0060-19.2019
Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H et al (2014) Neuronal activity regulates extracellular tau in vivo. J Exp Med 211:387–393. https://doi.org/10.1084/jem.20131685
Yamada K, Iwatsubo T (2018) Extracellular alpha-synuclein levels are regulated by neuronal activity. Mol Neurodegen 13:9. https://doi.org/10.1186/s13024-018-0241-0
Yu H, Sternad D, Corcos DM, Vaillancourt DE (2007) Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage 35:222–233. https://doi.org/10.1016/j.neuroimage.2006.11.047
Yuan P, Grutzendler J (2016) Attenuation of beta-amyloid deposition and neurotoxicity by chemogenetic modulation of neural activity. J Neurosci 36:632–641. https://doi.org/10.1523/JNEUROSCI.2531-15.2016
Acknowledgements
We thank Dr. Kurt R. Brunden for reading and editing of this manuscript. We thank Drs. Douglas A Coulter (Children’s hospital of Philadelphia) and Hajime Takano (Children’s hospital of Philadelphia) for valuable discussion and technical support. We thank S. Leight and T. Schuck for technical assistance. We thank Drs. A. Caputo for providing SGKI mice and M. X. Henderson for providing LRRK2 G2019S KI mice and discussion. We thank D. M. Riddle for primary hippocampal neuron cultures. We thank Dr. S. Xie for help with statistical analysis.
Funding
This work was supported by NIH/NIA U19 Center grant (AG062418), the Jeff and Anne Keefer Fund, and the Neurodegenerative Disease Research Fund.
Author information
Authors and Affiliations
Contributions
QW designed the studies and generated the data along with MAS. QW analyzed and interpreted all the results. BZ and ESM performed mouse brain injection surgeries. KCL, VM-YL, and JQT participated in discussion of results and design of some experiments, as well as in editing of the manuscript. QW and VM-YL wrote the manuscript, and all coauthors read and approved the manuscript. VM-YL supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (AVI 17973 kb)
Supplementary file2 (AVI 21315 kb)
Rights and permissions
About this article
Cite this article
Wu, Q., Shaikh, M.A., Meymand, E.S. et al. Neuronal activity modulates alpha-synuclein aggregation and spreading in organotypic brain slice cultures and in vivo. Acta Neuropathol 140, 831–849 (2020). https://doi.org/10.1007/s00401-020-02227-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-020-02227-6