Abstract
We analyze the swelling behavior of N-isopropylacrylamide (NIPAM)-based microgels incorporating the non-thermoresponsive comonomer N-tert-butylacrylamide (NtBAM) using photon correlation spectroscopy (PCS) and atomic force microscopy (AFM). Previous thermodynamic analysis of PNIPAM-co-NtBAM microgel swelling relied on the classical Flory-Rehner theory. However, this approach struggled to accurately describe swelling curves at higher NtBAM content. Our present work combines the original expression for the Flory-Huggins interaction parameter \(\chi _{FH}\) for NtBAM with a recently adapted Hill-like model for the interaction parameter \(\chi _{Hill}\) that accounts for cooperative effects in the volume phase transition in poly(NIPAM) microgels. This approach outperforms other methods in fitting quality. The observed results are revealing an exponential decrease in hydrodynamic radius upon increasing NtBAM content for swollen microgels. In addition, an exponential decay of the number of water molecules leaving the polymer chain during the volume phase transition is found, which can be attributed to the steric influence of one NtBAM monomer on the hydration of neighboring NIPAM monomers. The molar interaction enthalpy \(\Delta H_{SP}= \text {-49 kJ / mol}\) and entropy \(\Delta S_{SP} = \text {-177 J /( mol K)}\) were obtained from the fits of the swelling curves.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microgels are colloidal polymer networks swollen by water. Their swelling behavior exhibits responsiveness to various stimuli such as changes in temperature, pH value, ionic strength or electromagnetic fields [1,2,3,4,5,6,7]. Due to their responses, microgels have a broad spectrum of potential applications [8]. They can serve as carrier systems for nanoparticles in catalysis [9, 10] or as drug delivery systems [11, 12]. Moreover, they can form smart responding membranes [13,14,15], or are utilized for controlling enzyme activity [16]. In addition, microgels can be used to prepare smart surface coatings, i.e., for for vertebrate cell culture applications [17] or for sensors [18,19,20,21]. Moreover, they can accommodate and stabilize metal nanocrystals [10, 22,23,24] which, e.g., allows to regulate inter-nanoparticle separation and thus impacting their optical properties. Furthermore, in catalysis, microgels can serve as smart carriers for enzymes [25] or nanoparticles [26]. The most studied base for microgels is N-isopropylacrylamide (NIPAM) with a volume phase transition temperature (VPTT) of 34 C [27]. The thermoresponsivity of microgels can be enhanced or adjusted by copolymerization with different comonomers [28]. Other neutral comonomers, like N-n-propylacrylamide (NnPAM) or N-isopropylmethacrylamide (NIPMAM), are introduced to tune the VPTT in these microgels [28, 29]. Some of these comonomers like 2-hydroxy-4-(methacryloyloxy)-benzophenone (HMABP) used for UV crosslinking [14] are non-thermoresponsive themselves. From existing literature [14, 30,31,32], it is established that the addition of non-thermoresponsive comonomers leads to a broadening of the volume phase transition (VPT) of the microgels and a decrease in VPTT. The thermodynamic reasons for this phenomenon are not yet completely understood. Already in 2010 Hertle et al. [32] employed the non-thermoresponsive N-tert-butylacrylamide (NtBAM) as a comonomer and studied the thermodynamics of the respective copolymer-microgels using the Flory-Rehner theory. However, the classic Flory-Rehner model failed to effectively describe the swelling of PNIPAM-copolymer microgels with elevated NtBAM content. Recently, Leite et al. [33] modeled an interaction parameter using a Hill-type equation for cooperative thermotropic volume phase transitions. They developed a Hill-like model for the interaction parameter \(\chi\) leading to a more adequate description of swelling curves with the Flory-Rehner theory. Friesen et al. further investigated this Hill-like model and found it to outperform the classic model providing reasonably good fit results and physical meaningful parameters for copolymer microgels containing N-isopropylacrylamide (NIPAM), N-n-propylacrylamide (NnPAM) or N-isopropylmethacrylamide (NIPMAM) [34,35,36]. In the present work, we again synthesized systematically PNIPAM-co-NtBAM microgels with different NtBAM and crosslinker (N,N’-methylen-bisacrylamide (BIS)) content extending our previous study [32]. The measured swelling curves acquired by photon correlation spectroscopy (PCS) of these microgels were analyzed by combining the classical Flory-Rehner approach with the Hill-like model to achieve a comprehensive description of the cooperative NIPAM transition with the non-thermoresponsive comonomer NtBAM. The fits, based solely on physically meaningful properties, describe the swelling curves accurately and are superior compared to other methods. The results indicate an exponential decrease in the number of water molecules \(\nu\) cooperatively leaving the microgel segment broadening VPT with increasing NtBAM. An increase in NtBAM content decreases the hydrodynamic radius of the synthesized microgels. Regarding also other studies with non-thermoresponsive comonomers it seems that the hydration and dehydration are influenced by sterically demanding groups.
Theory
Flory-Rehner thermodynamics
The Flory-Rehner model is the most employed model for the description of the thermodynamic properties of polymers or microgels in solutions [32, 37,38,39,40]. The microgels are in thermodynamic equilibrium when the chemical potential \(\mu\) of the solvent S inside the gel equals the chemical potential outside the gel (Eq. 1).
The Flory-Rehner theory for polymer networks in solutions originates from the Flory-Huggins theory, assuming all molecules are represented by a lattice model without volume changes (\(\Delta V=0\)). Consequently, the change in Gibbs energy \(\Delta G\) equates to the change in Helmholtz free energy \(\Delta F\). By considering the molar volume of the solvent \(V_{m,s}\), the alteration in chemical potential (Eq. 2) is given by the osmotic pressure \(\Pi\) and \(\Delta F\).
The osmotic pressure \(\Pi\) of the system comprises elastic interactions \(\Pi _{el}\), the mixing contribution \(\Pi _{mix}\), and the ionic contribution for charged systems \(\Pi _{ion}\), which is not considered in this work [4, 38]. According to Eq. 1 and 2 the resulting osmotic pressure should be zero (Eq. 3) in the equilibrium state.
Within the lattice model, the elastic and mixing contributions, are represented by the Flory-Rehner equation 4 for microgels.
Here, \(N_{\text {Gel}}\) is the average degree of polymerization of a polymer chain or the number of segments between two crosslinking points. \(R_H\) is the hydrodynamic radius, \(\phi\) is the polymer volume ratio and \(\chi\) is the Flory-Huggins interaction parameter. \(R_{H,0}\) and \(\phi _0\) are the values in the reference state, specifically in the collapsed state. The deswelling ratio \(\alpha\) of a microgel is defined by Eq. 5.
The degree of polymerization \(N_{\text {Gel}}\) should be dependent on the amount of crosslinker. Hence, for the bifunctional BIS, it should be \(N_{\text {Gel}} = (N_{\text {NIPAM}} + N_{\text {NtBAM}}) / (2 \cdot N_{\text {BIS}})\). As stated by Wu and Zhou [41], \(N_{\text {Gel}}\) is radially inhomogeneous within the microgels, which leads to the continuous phase transition observed in microgels. Longer subchains undergo the phase transition before shorter subchains, resulting in a continuously distributed range of volume phase transition temperatures (VPTT). In contrast, macroscopic gels generally display discontinuous phase transitions due to their homogeneously distributed chain lengths. In the Flory-Huggins lattice model, the volumes of different components a and b are assumed to be additive. Based on this premise, Godbole et al. [42] proposed an extension of the Flory-Rehner theory, which was subsequently utilized by Friesen et al. [36] for their analysis of copolymer microgels with Eq. 6.
Here, \(\chi _a\) and \(\chi _b\) represent the interaction parameters used for the comonomers with the mole fraction \(x_a\) and \(x_b\).
Interaction parameter \(\chi\)
The original Flory-Huggins interaction parameter \(\chi _{FH}\) represents the interaction energy between polymer segments P and solvent S (Eq. 7).
Here, \(\Delta H_{SP}\) and \(\Delta S_{SP}\) are the interaction enthalpy and entropy, respectively. \(\Theta\) is the spinodal temperature and A is a dimensionless parameter. kB is the Boltzmann constant. The relationship between A, \(\Theta\), \(\Delta H_{SP}\) and \(\Delta S_{SP}\) is given by Eq. 8 [37].
Numerous experimental results couldn’t be adequately described by the original temperature-dependent interaction parameter (Eq. 7). To address this problem, Flory and Erman [43] developed a series expansion (Eq. 9) that incorporates concentration dependency, offering a more accurate description of the experimental observations.
However, this approach lacks a clear physical interpretation for the coefficients \(\chi _n\).
Hill-like model
The Hill-like model, initially proposed by Leite et al. [33] and further investigated by Friesen et al. [34,35,36], represents the interaction parameter \(\chi\) by introducing cooperative effects arising from crosslinking. The state of the monomer unit in a chain or polymer segment P and solvent S before and after the phase transition an be described by Eq. 10, with the solvated state \(\text {PS}_\nu\).
The stoichiometric coefficient \(\nu\), also known as the Hill-parameter, represents the average number of water molecules leaving the hydrophobic solvate layer around the polymer segment during the phase transition. Utilizing the shape of the Hill equation with the relative temperature as a measure for the reaction progress, we arrive at Eq. 11. The Equation used by Friesen et al. [34] has been rewritten in a more compact form and \(t_{0.5}\) was replaced with VPTT,
where, \(\chi _0\) represents the interaction parameter at the initial measured temperature \(T_a\). The parameter a denotes the slope of the baseline and parameter b describes the amplitude of the Hill-transition. In MD simulations [44] and calorimetric studies [45, 46], it has been demonstrated that \(14 \pm 1\) water molecules exit the network polymer chain per segment. This value is in line with the \(\nu\) values obtained by Friesen [34]. Certainly, the precise number of hydration water molecules remains a topic of debate due to the challenges encountered in experimental determination [47]. In a recent \(^2\)H-NMR field cycling relaxometry study of non-crosslinked PNIPAM homo-polymer conducted by Säckel et al. [48] it was observed that only \(\approx 0.4\) water molecules per PNIPAM monomer were directly bounded to the polymer chain.
Materials and Methods
Chemicals
Unless otherwise stated, all chemicals were used without further purification. The microgels containing the N-isopropylacrylamide (NIPAM, Sigma Aldrich, 97%) as a monomer, N-tert-butylacrylamide (NtBAM, TCI, \(>98 \%\)) as comonomer, N,N’-Methylenbisacrylamide (BIS, Sigma Aldrich, 99 %) as crosslinker and potassium peroxodisulfate (KPS, Fluka, \(>99 \%\)) as radical initiator. Water was purified using a Sartorius arium® Pro VF system.
Synthesis of PNIPAM-co-NtBAM microgels
The PNIPAM-co-NtBAM copolymer-microgels were synthesized by surfactant-free radical emulsion polymerisation, analogous to the synthesis protocol of Hertle et al. [32]. Mole fractions (x) were determined by setting the combined molar content of NIPAM and NtBAM to 100 mol%. The total monomer amount was 3.85 mmol in each synthesis. NIPAM (100-x mol%), NtBAM (x mol%), and BIS (5 or 10 mol% with respect to the amount of the other two monomers) were dissolved in water (50 mL, deionized) inside a 100 mL-three neck flask. The mixtures were heated up to 80 °C under magnetical stirring and oxygen was removed by bubbling nitrogen. After 30 min of equilibration, the reaction was initiated by adding KPS (3.5 mol%) using a syringe. The reaction mixture was stirred for 4 h under a nitrogen atmosphere at 80 °C, followed by overnight stirring at room temperature in the presence of air. The yielded opaque dispersion was filtered over glass wool and further purified by three cycles of centrifugation (108800 g = 30000 rpm for 30 min, Avanti-J301, JA\(-\)-30.50 rotor, Beckman Coulter, Fichtenhain, Germany) and decantation of the supernatant. The mass ratio was determined gravimetrically by drying three samples (\(\approx 1\;\text {mL}\)) in a drying oven (80 °C, Memmert, U30, Schwabach, Germany). After purification, PNIPAM-co-NtBAM copolymer-microgels were obtained as a turbid dispersion (\(>\;0.8\;wt\%\)).
Atomic force microscopy (AFM)
The AFM measurements were done using silicon wafers as substrate (1 cm x 1 cm sized, Siegert Wafer GmbH, Aachen, Germany). The wafers were cleaned via a plasmacleaner (Zepto, 0.4mbar O\(_2\), 100%, 60 s, Diener Electronics, Ebhausen, Germany). The freshly cleaned wafers were spin-coated by polyethylenimine (PEI, 100\(\mu\)l, 25wtand excess liquid removed by spin-coating (LabSpin6, SüSS MicroTec, Garching, Germany, 2000 rpm, 240 s). The diluted microgels (100\(\mu\)l, \(\approx\) 0.05 wt%) were coated at 1500 rpm for 4 min. The coated sample were left at room temperature for over 24 h to dry prior to measurement. AFM images were recorded in tapping-mode at room temperature with a Nanosurf Flex AFM (Nanosurf GmbH, Langen, Germany) with TAP300Al-G cantilever (\(k= \text {40 N m}\), \(\nu _e~=~283 \text{kHz}\); Innovative Solution Bulgaria Ltd., Sofia, Bulgaria). Each image was recorded with a resolution of 512 points per line with a scan rate of 2.4 s/line for an area of 10\(\mu\)m x 10\(\mu\)m and 3.0 s/line for an area of 30\(\mu\)m x 30\(\mu\)m.
Temperature dependent photon correlation spectroscopy (PCS, Swelling curves)
Microgel dispersions were prepared inside glas cuvettes by diluting (\(\le \;0.01\;\text {wt}\%\)) microgel dispersion into filtered water (PTFE, hydrophilic, 0.2\(\mu\)m, Fisherbrand, Schwerte, Germany) to exclude dust from the samples. Swelling curves (hydrodynamic radius \(R_H\) against temperature T) were measured using a self-built PCS setup, consisting of a HeNe-LASER (HNL210L, \(\lambda \;=\;\text {632.8 nm}\); Thorlabs Inc.), a multiple-\(\tau\) correlator (ALV-6010, ALV-Laser Vertriebsgesellschaft mbH, Langen, Germany), a thermostatically controlled decaline index-matching bath and a single photon detector (ALV/SO-SIPD; ALV-Laser Vertriebsgesellschaft mbH.). Five measurements at each temperature (280 K to 335 K) were performed at fixed scattering angles \(\theta = 45^\circ\) or \(60^\circ\) and subsequently averaged. In PCS the intensity time autocorrelation function of the scattered light is generated by the hardwired correlator and analyzed by using the program CONTIN [49, 50] or the method of cumulants [51]. This yields the relaxation rate \(\Gamma\). The \(\Gamma\), obtained from the measured intensity time autocorrelation functions is directly proportional to the temperature-dependent diffusion coefficient \(D_T\) and the squared magnitude of the scattering vector \(q^2\).
q depends on the refractive index of the solvent n, the wavelength of the used light source \(\lambda\) and the scattering angle \(\theta\) and is given by Eq. 13.
By assuming perfectly spherical hydrated particles, the Stokes-Einstein equation (Eq. 14) provides the hydrodynamic radius \(R_H\) of the particles given known diffusion rates. Here, \(\eta\) is the dynamic viscosity of the solvent \(\eta\), \(k_B\) the Boltzmann constant and T the temperature.
Fitting data using the Flory-Rehner equation
To fit the swelling curves with the Flory-Rehner equation (Eq. 4 or 6), it is required to solve it to \(R_H (T)\). To accomplish this task, the zero points of the function \(\Pi (R_H)\) are iteratively determined using the Brent method [52] at each measured temperature T for variable radii \(R_H\), employing the Python module Scipy.optimize.root_scalar [53]. The lower boundary for the radius \(R_H\) is set to a volume ratio \(\phi < 1\). The numeric solution to \(R_H(T)\) were used as a model in the python module Lmfit [54] to fit the swelling curves by Levenberg-Marquardt algorithm. The exact code for the transformation to R(H) is provided in the supporting information A1.
Results and discussion
Microgel shape
The structures of the microgel particles adsorbed onto silicon wafers were studied by AFM (Fig. 1). AFM was used as suggested by Suzuki [1] to confirm statistical uniformity of the synthesized microgel particles before starting further investigations. All microgels appear monodisperse with circular crosssections, pointing to spherical structures in solution. The AFM phase images indicate that particles become more rigid with higher NtBAM content.
To analyze the height h and width w of the adsorbed microgels we assumed that the force on the cantilever is constant over the wafer surface and height changes solely result from the microgels. Over 100 height profiles per microgel species were extracted by drawing lines with the software Gwyddion [55] onto the AFM images. The width w is given as distance to the highest positions in the height profiles. The minimum height was set to 0 and the given heights h were averaged for all measured gels and plotted with their standard deviation in Fig. 2.
The small standard deviations in the height profiles suggest that the size distribution is relatively narrow. At 5 mol% BIS, the microgels become narrower as the NtBAM content is increased. The height profiles with 10 mol% BIS are less broad and more homogeneous caused by the stronger crosslinking.
Swelling curves
Besides using AFM the copolymer microgels synthesized in this study were characterized using PCS, employing a fixed scattering angle while systematically varying the temperature. To minimize noise, five measurements were taken at each temperature and the averaged results are plotted in Fig. 3 against the temperature. The swelling curves were smoothed by fitting an arbitrary double sigmoidal function (supporting information S1) to them. The VPTT is determined as inflection point of the swelling curve which corresponds to the minimum of the numerical derivative of the smoothed curves.
Swelling curves for PNIPAM-co-NtBAM at A 5 or B 10 mol% BIS crosslinker content and varying NtBAM copolymer content. An exception to this is the 100 mol% NtBAM microgel with 2.3 mol% BIS, whose swelling curve is plotted in both graphs. A, B display the swelling curves with a representative relative error of 5%, which have been fitted using a double sigmoidal function. While, C, D depict the corresponding derivatives of these curves to determine the VPTT (inflection point of the swelling curves) as a minimum of the numerically calculated derivatives
It is noteworthy from the derivatives that the volume phase transition broadens with increasing NtBAM content. To quantify this broadening, the full width at half minimum (FWHM) of the derivatives is determined. The determined VPTT and FWHM are plotted in Fig. 4.
The VPTT A and full width at half minimum (FWHM) B of the peaks in the derivatives of the swelling curves (Fig. 3) at various NtBAM contents \(x_{\text {NtBAM}}\) are presented. A slope of approximately 0.6 K/mol in VPTT is observed for both crosslinker contents. Dashed lines serve as guide to the eye
The linear decrease in VPTT with increasing comonomer content is in agreement with observations in other copolymer-microgel systems [36, 56]. However, contrary to this finding Keerl et al. [57], reported a non-linear dependence of VPTT for NIPAM and N,N-diethylacrylamide (DEAAM) with a minimum of the VPTT at 60 wt% DEAAM. This minimum is explained by hydrogen bonding between the two components. Due to the chemical similarity between NIPAM and NtBAM the occurring intermolecular NIPAM-NtBAM hydrogen bonds are comparable to the intramolecular NIPAM-NIPAM hydrogen bonds and therefore no minimum in the VPTT is expected with increasing NtBAM content. The phenomenon that the VPTT is reduced by adding the comonomer indicates that the comonomer is successfully incorporated inside the gel. The reported homopropagation rate coefficient for NtBAM with \(k_{p,NtBAM}\, 30~~ ^\circ\text {C} = 13\text{m}^{3} \text{mol}^{-1} s^{-1}\) [58] in an EtOH / H2O is very slow compared to NIPAM \(k_{p,NIPAM}\, 30~~^\circ\text {C} = 30\text{m}^{3} \text{mol}^{-1} s^{-1}\) [59]. This difference in rate coefficients further suggest that NtBAM will not build uneven distributed areas inside the gel. Data for NtBAM in pure water are not available in the literature.
Extrapolating this trend, a microgel synthesized with 100 mol% NtBAM is predicted to exhibit a VPTT of \(-\)29 \({}^{\circ}\text {C}\). The linear increase in FWHM with NtBAM content, further complicates the determination of VPTT compared to a sharp transition like in poly-NIPAM microgels. The broadening of the VPT is a phenomenon observed in other studies involving non-thermoresponsive comonomers, too. In the study by Dirksen et al. [14], the determination of VPTT was impeded and they were unable to extend their analysis beyond 5 mol% of the sterical demanding HMABP comonomer due to the significant broadening observed. An increase in NtBAM content also leads to a reduction in size of the synthesized copolymer microgels in swollen and collapsed state. The corresponding data for swollen microgels at 15 \({}^{\circ }\text {C}\) and collapsed microgels at 50 \({}^{\circ }\text {C}\) are plotted in Fig. 5.
Hydrodynamic radius \(R_H\) at fixed temperatures with varying NtBAM contents. A 15 \({}^{\circ }\text {C}\) in swollen state and B 50 \({}^{\circ }\text {C}\) in collapsed state. Data points were acquired through interpolation of the swelling curves (Fig. 3)
The size decrease in the swollen state could be described as exponential with the NtBAM content, in contrast to a linear trend in NNPAM-co-NIPMAM observed by Wedel et al. [56]. The hydrophobic NtBAM in the swollen state is anticipated to result in reduced water uptake within the polymer network, leading to a decrease in radius. Increasing BIS content from 5 to 10 mol% leads to a decrease in \(R_H\) of about 20–40 nm, see Fig. 3(A, B). This is consistent with findings of Coronado et al. [60], suggesting that enhanced porosity due to increased crosslinking hinders penetration of water molecules into the polymer network and is also in agreement with results by Kratz et al. [61]. Friesen et al. [34] proposed a mechanism suggesting that water remains adsorbed onto the crosslinker during the phase transition due to the absence of a lower critical solution temperature (LCST) in BIS. However, the anticipated increase in hydrodynamic radius in the collapsed state is not observed, as depicted in Fig. 5. The swelling ratio \(\alpha\) (Eq. 5) obtained from the swelling curves in Fig. 6 is almost identical for all swollen and collapsed states of the microgels.
Thermodynamic description by Flory-Rehner
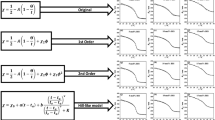
In the study by Hertle et al. [32], the swelling curves of PNIPAM-co-NtBAM microgels were fitted using the Flory-Rehner Eq. 4 and \(\chi _{FE}\). These fits failed to accurately describe the swelling curves for microgels with higher NtBAM contents. Fits of the swelling curves with Eq. 4 and different models for \(\chi\) can be found in the supporting information A3 (Figs. S3 and S4). To achieve better fits of copolymer microgel swelling curves, we employ the hybrid Flory-Rehner model (see Eq. 6) specifically for copolymer microgels. The fitting method is explained in the section ’Fitting data using the Flory-Rehner equation’. Different models for the Flory-Huggins interaction parameter were utilized in the fits: The original temperature dependent \(\chi _{FH}(T)\), with the concentration dependent series expansion from Flory and Erman \(\chi _{FE}(T,\phi )\) expanded up to the second order \(\chi _2\) combined with the cooperative Hill-like model \(\chi _{Hill}(T)\). Friesen et al. [34] considered the parameters \(a_{\text {NIPAM}} = {0.02\,\mathrm{\text {K}^{-1}}}\), \(b_{\text {NIPAM}} = 0.29\), and \(\chi _{0,\text {NIPAM}} = 0.02\) as specific to the substance. Consequently, here these constants were employed as fixed parameters, consistent with their determination in the study conducted by Friesen et al.. The degree of polymerization \(N_{\text {Gel}}\) is expected to be dependent on the quantity of the crosslinker BIS. In SANS measurements conducted by Hertle et al. [32], it is observed that the mesh size does not change significantly with NtBAM and therefore does not affect \(N_{\text {Gel}}\). Therefore, \(N_{\text {Gel}}\) is a shared parameter across the entire dataset used for the global fit. Performing a global fit offers the advantage of reducing the cross-correlation between parameters [62]. This approach allows a comprehensive and simultaneous optimization of parameters, enhancing the overall fitting accuracy. Assuming a statistical distribution of NtBAM within the microgels, this would inherently impact the cooperative interaction between NIPAM and water molecules. To account for this \(\nu\) is set as variable for the fits with \(\chi _{Hill}\). Figure 7 represents an overview of the different parameters, their variability, and their influence on the swelling curves. A more detailed analysis of the parameters influence on the graphs is given in the Supporting Information A2 (Fig. S2).
The cooperative Hill-like model also yields a good description of the curves while employing more physically meaningful parameters.
Swelling curves fitted by the Flory-Rehner equation (4) for hybrid models of the interaction parameter \(\chi _{Hill}\) with A, B \(\chi _{FH}\) or C, D \(\chi _{FE}\). NIPAM is described by the Hill-like modell and NtBAM in A, B with the classical Flory-Huggins \(\chi _{FH}\) parameter and in C, D with the series expansion \(\chi _{FE}\). The parameter \(N_{\text {Gel}}\), \(\Delta H_{SP}\) and \(\Delta S_{SP}\) were shared for fits with same BIS content. The parameters \(a_{NIPAM},b_{NIPAM}\) were taken as constants from the literature [34], while the VPTT parameters were used from the PNIPAM homopolymer
The cooperative behavior of NIPAM is characterized using \(\chi _{Hill}\). Characterizing the NtBAM contribution to swelling using \(\chi _{Hill}\) isn’t feasible due to the absence of a VPTT for PNtBAM. Consequently, we attempted to utilize the \(\chi _{FH}\) and \(\chi _{FE}\) parameters for the NtBAM content. Therefore, the VPTT parameter used for the NIPAM content is acquired from the determination of the PNIPAM homopolymer in Fig. 4 and used as constant over the dataset. The shared parameters for the fits are listed in Table 1.
Both hybrid models demonstrate good agreement with the swelling curves (Fig. 8). The usage of the series expansion \(\chi _{FE}\) model does not significantly outperform the \(\chi _{FH}\) but introduces physically challenging parameters such as \(\chi _2\), complicating their interpretability. In both fits, it is observed that \(N_{\text {Gel}}\) remains nearly constant within the margin of error and decreases linearly with BIS content, as expected. Notably, \(N_{\text {Gel}}\) displays a similar order of magnitude as observed in the non-hybrid models, in the supporting information A3, involving \(\chi _{FE}\) and \(\chi _{Hill}\). The determined \(N_{\text {Gel}}\) values were significantly higher than the estimated \(N_{\text {Gel}} = 10\) or 5, indicating an uneven distribution of BIS within the microgel. By solving Eq. 8\(\Delta H_{SP}\) and \(\Delta S_{SP}\) have been calculated from the acquired \(\Theta\) and A. Within the same model, the values for \(\Delta H_{SP}\) and \(\Delta S_{SP}\) are independent of [BIS] within the margin of error (Table 1). However, they are approximately three times higher for \(\chi _{FH}\) compared to \(\chi _{FE}\). Both \(\Delta H_{SP}\) and \(\Delta S_{SP}\) were negative, resulting in a positive free interaction enthalpy at higher temperatures, as anticipated for thermoresponsive microgels.
The Hill parameter \(\nu\) exhibits a nearly exponential decay when plotted vs. the NtBAM content (Fig. 9). This trend is in contrast to the linear relationships observed in the works of Friesen et al. [34, 36] regarding BIS content or other comonomers such as NNPAM and NIPMAM.
Hence, it is presumed that this exponential reduction in the number of water molecules per segment arises from a different mechanism compared to the linear decrease observed with BIS. The reduction in the parameter \(\nu\) should be attributed to the differences between the isopropyl group of NIPAM and the tert-butyl group of NtBAM. To compare the effects of comonomer chemical structure on \(\nu\) qualitatively it is considered that a broad volume phase transition (VPT) leads to low \(\nu\) values (refer to supporting information A2). Other hydrophobic comonomers like ethyl methacrylate (EMA) and 2-hydroxy-4-(methacryloyloxy)-benzophenone (HMABP) broadened the VPT, but the sterical demanding HMABP leads to a stronger effect in PNIPAM microgels [14, 30]. M. Quesada-Pérez et al. revealed in their simulations, that the abruptness of gel shrinking and with this the sharpness of the phase transition is considerably conditioned by the number of monomeric units per chain [39]. This would lead to longer chains of hydrated water on the polymer chains. An alternative explanation could be based on the cooperative hydration of water molecules within the polymer chains. Okada et al. proposed that water molecules do not randomly adsorb onto the chains, but prefer positions adjacent to existing hydrogen-bound water molecules due to cooperative interactions [63]. The phenomenon is deemed cooperative due to the displacement of methyl groups within NIPAMs isopropyl group by adsorbed water molecules. This displacement creates space for additional water molecules to fit in, leading to a more energetically favorable state compared to random hydration. This aligns with the hypothesis proposed by Ono et al. [47] and molecular dynamics simulations for PNIPAM [44], which suggests that water molecules tend to form clusters that fit well between adjacent isopropylamide groups, thus creating hydrogen bond bridges. A plausible explanation is that the hydrophobic nature of NtBAMs tert-butyl group impedes the cooperative dehydration of water molecules toward neighboring NIPAM monomers within the polymer segment. This could be caused by steric factors, which may impede this cooperative hydration mechanism. When a sterically demanding group is displaced by water, such groups do not provide additional space, thus inhibiting further cooperative hydration of water molecules. Additionally, the size of the tert-butyl group impedes the formation of hydrogen bond bridges between the amide groups of two monomeric units. Hence, a single NtBAM monomer potentially obstructs multiple potential consecutive water molecule chains at once and by this the adsorption on other components as well. This hypothesis is illustrated in Fig. 10.
Illustration of proposed sterical effects from NtBAMs tert-butyl group compared to NIPAMs isopropylgroup on the hydration of polymer chains. The isopropylgroup of NIPAM could get displaced by adsorbing water molecules to create space for further hydration, which is not possible for the more sterical demanding tert-butyl group of NtBAM. Cooperative dehydration between the water molecules is inhibited with NtBAM as a spacer between them, which leads to shorter water chains
Contrary to this, it was observed that an increase in the amount of NtBAM decreases the radii of the obtained microgels (Fig. 5). Certainly, the amount of NtBAM decreases the value of the Hill parameter \(\nu\). The decrease in the amount of NtBAM results in smaller radius \(R_H\), smaller parameter \(\nu\), broader VPT, and in an increase of the deswelling ratio \(\alpha\) (eq. 5) in the temperature range \(285~\,\textrm{K}< T < 320~\,\textrm{K}\) (Fig. 6).
Conclusions
In the present work, PNIPAM-co-NtBAM copolymer microgels are systematically synthesized with varying compositions and their thermoresponsive swelling is analyzed using photon correlation spectroscopy (PCS) and AFM. The applied modified Flory-Rehner theory combining the Hill-like model of the polymer solvent interaction parameter for the NIPAM and a classical Flory-Huggins parameter for the hydrophobic NtBAM leads to a good description of the swelling curves and outperformed other models. The almost identical results in interaction enthalpy and entropy throughout different BIS crosslinker contents suggests that these quantities are characterizing the NtBAM comonomer. However, further investigations, such as calorimetric studies, are needed for confirmation. The hydrodynamic radius in the swollen state and the Hill-parameter \(\nu\) exhibits an exponential decrease with increasing NtBAM content, indicating the influence of one NtBAM monomer on the hydration of neighboring monomers, not solely within their immediate spatial surroundings. The molecular mechanism for the broadening of the VPT, is not quite clear yet. However, considering the results of this work and trends reported in other studies with non-thermoresponsive components suggest that sterically demanding groups have an impact on the polymer chains and their interaction with water molecules.
Data availability
The dataset is available upon request. Contact the corresponding author for access.
References
Suzuki D (2023) Nanogel/microgel science and beyond. Langmuir 39(22):7525–7529. https://doi.org/10.1021/acs.langmuir.3c00560
Plamper FA, Richtering W (2017) Functional microgels and microgel systems. Accounts of chemical research 50(2):131–140. https://doi.org/10.1021/acs.accounts.6b00544
Lyon LA, Serpe MJ (2012) Hydrogel micro and nanoparticles. Wiley-VCH, Weinheim, German. https://doi.org/10.1002/9783527646425
Fernandez-Nieves A (2011) Microgel suspensions: fundamentals and applications. Wiley-VCH, Weinheim
Hoare T, Pelton R (2008) Characterizing charge and crosslinker distributions in polyelectrolyte microgels. Current Opinion in Colloid & Interface Science 13(6):413–42. https://doi.org/10.1016/j.cocis.2008.03.004
Ballauff M, Lu Y (2007) “Smart’’ nanoparticles: preparation, characterization and applications. Polymer 48(7):1815–1823. https://doi.org/10.1016/j.polymer.2007.02.004
Nayak S, Lyon LA (2005) Soft nanotechnology with soft nanoparticles. Angewandte Chemie International Edition 44(47):7686–770. https://doi.org/10.1002/anie.200501321
Thorne JB, Vine GJ, Snowden MJ (2011) Microgel applications and commercial considerations. Colloid and Polymer Science 289(5–6):625–646. https://doi.org/10.1007/s00396-010-2369-5
Sabadasch V, Wiehemeier L, Kottke T et al (2020) Core-shell microgels as thermoresponsive carriers for catalytic palladium nanoparticles. Soft Matter 16(23):5422–543. https://doi.org/10.1039/D0SM00433B
Lu Y, Spyra P, Mei Y et al (2007) Composite hydrogels: robust carriers for catalytic nanoparticles. Macromolecular Chemistry and Physics 208(3):254–260. https://doi.org/10.1002/macp.200600534
Hoare T, Pelton R (2008) Impact of microgel morphology on functionalized microgeldrug interactions. Langmuir 24(3):1005–1012. https://doi.org/10.1021/la7024507
Kittel Y, Kuehne AJC, de Laporte L (2022) Translating therapeutic microgels into clinical applications. Advanced Healthcare Materials 11(6):2101989. https://doi.org/10.1002/adhm.202101989
Barth M, Wiese M, Ogieglo W et al (2018) Monolayer microgel composite membranes with tunable permeability. Journal of Membrane Science 555:473–48. https://doi.org/10.1016/j.memsci.2018.03.037
Dirksen M, Brändel T, Großkopf S et al (2021) UV cross-linked smart microgel membranes as free-standing diffusion barriers and nanoparticle bearing catalytic films. RSC Advances 11(36):22014–22024. https://doi.org/10.1039/d1ra03528b
Uredat S, Gujare A, Runge J et al (2024) A review of stimuli-responsive polymer-based gating membranes. Physical Chemistry Chemical Physics 26(4):2732–274. https://doi.org/10.1039/d3cp05143a
Park TG, Hoffman AS (1990) Immobilization and characterization of beta-galactosidase in thermally reversible hydrogel beads. Journal of Biomedical Materials Research 24(1):21–3. https://doi.org/10.1002/jbm.820240104
Flechner M, Schaller J, Stahl M et al (2022) Adhesion, proliferation, and detachment of various cell types on thermoresponsive microgel coatings. Biotechnology and bioengineering 119(7):1728–173. https://doi.org/10.1002/bit.28095
Serpe MJ, Jones CD, Lyon LA (2003) Layer-by-layer deposition of thermoresponsive microgel thin films. Langmuir 19(21):8759–8764. https://doi.org/10.1021/la034391h
Islam MR, Serpe MJ (2013) Label-free detection of low protein concentration in solution using a novel colorimetric assay. Biosensors and Bioelectronics 49:133–13. https://doi.org/10.1016/j.bios.2013.05.011
Cors M, Wrede O, Genix AC et al (2017) Core-shell microgel-based surface coatings with linear thermoresponse. Langmuir 33(27):6804–6811. https://doi.org/10.1021/acs.langmuir.7b01199
Gao Y, Li X, Serpe MJ (2015) Stimuli-responsive microgel-based etalons for optical sensing. RSC Advances 5(55):44074–4408. https://doi.org/10.1039/C5RA02306H
Mei Y, Lu Y, Polzer F et al (2007) Catalytic activity of palladium nanoparticles encapsulated in spherical polyelectrolyte brushes and core - shell microgels. Chemistry of Materials 19(5):1062–1069. https://doi.org/10.1021/cm062554s
Kureha T, Nagase Y, Suzuki D (2018) High reusability of catalytically active gold nanoparticles immobilized in core-shell hydrogel microspheres. ACS Omega 3(6):6158–616. https://doi.org/10.1021/acsomega.8b00819
Dulle M, Jaber S, Rosenfeldt S et al (2015) Plasmonic gold-poly(N-isopropylacrylamide) core-shell colloids with homogeneous density profiles: a small angle scattering study. Physical chemistry chemical physics: PCCP 17(2):1354–136. https://doi.org/10.1039/c4cp04816d
Welsch N, Ballauff M, Lu Y (2010) Microgels as nanoreactors: applications in catalysis, Springer Berlin Heidelberg, pp 129–16. https://doi.org/10.1007/12_2010_71
Naseem K, Farooqi ZH, Begum R et al (2018) Removal of congo red dye from aqueous medium by its catalytic reduction using sodium borohydride in the presence of various inorganic nano-catalysts: a review. Journal of Cleaner Production 187:296–300. https://doi.org/10.1016/j.jclepro.2018.03.209
Pelton RH, Chibante P (1986) Preparation of aqueous latices with n-isopropylacrylamide. Colloids and Surfaces 20(3):247–256. https://doi.org/10.1016/0166-6622(86)80274-8
Hertle Y, Hellweg T (2013) Thermoresponsive copolymer microgels. Journal of Materials Chemistry B 1(43):5874–588. https://doi.org/10.1039/c3tb21143f
von Nessen K, Karg M, Hellweg T (2013) Thermoresponsive poly-(N-isopropylmethacrylamide) microgels: tailoring particle size by interfacial tension control. Polymer 54(21):5499–5510. https://doi.org/10.1016/j.polymer.2013.08.027
Ma X, Huang X, Zhu L et al (2005) Influence of ethyl methacrylate content on the volume-phase transition of temperature-sensitive poly[(N-isopropylacrylamide)-co-(ethyl methacrylate)] microgels. Polymer International 54(1):83–8. https://doi.org/10.1002/pi.1619
Zhang QS, Zha LS, Ma JH et al (2007) Synthesis and characterization of novel, temperature-sensitive microgels based on n-isopropylacrylamide and tert-butyl acrylate. Journal of Applied Polymer Science 103(5):2962–2967. https://doi.org/10.1002/app.25423
Hertle Y, Zeiser M, Hasenöhrl C et al (2010) Responsive P(NIPAM-co-NtBAM) microgels: Flory-Rehner description of the swelling behaviour. Colloid and Polymer Science 288(10–11):1047–105. https://doi.org/10.1007/s00396-010-2232-8
Leite DC, Kakorin S, Hertle Y et al (2018) Smart starch-poly( N-isopropylacrylamide) hybrid microgels: synthesis, structure, and swelling behavior. Langmuir 34(37):10943–1095. https://doi.org/10.1021/acs.langmuir.8b00706
Friesen S, Hannappel Y, Kakorin S et al (2021) Accounting for cooperativity in the thermotropic volume phase transition of smart microgels. Gels 7(2):4. https://doi.org/10.3390/gels7020042
Friesen S, Hannappel Y, Kakorin S et al (2022) Comparison of different approaches to describe the thermotropic volume phase transition of smart microgels. Colloid and Polymer Science 300(300):1235–1245. https://doi.org/10.1007/s00396-022-04950-w
Friesen S, Kakorin S, Hellweg T (2022) Modified Flory-Rehner theory describes thermotropic swelling transition of smart copolymer microgels. Polymers 14(10):199. https://doi.org/10.3390/polym14101999
Flory PJ (1953) Principles of polymer chemistry. Cornell University Press, Ithaca, NY
Quesada-Pérez M, Maroto-Centeno JA, Forcada J et al (2011) Gel swelling theories: the classical formalism and recent approaches. Soft Matter 7(22):1053. https://doi.org/10.1039/c1sm06031g
Quesada-Pérez M, Ramos J, Forcada J et al (2012) Computer simulations of thermo-sensitive microgels: quantitative comparison with experimental swelling data. The Journal of chemical physics 136. https://doi.org/10.1063/1.4729946
Crassous JJ, Wittemann A, Siebenbürger M et al (2008) Direct imaging of temperature-sensitive core-shell latexes by cryogenic transmission electron microscopy. Colloid and Polymer Science 286(6–7):805–812. https://doi.org/10.1007/s00396-008-1873-3
Wu C, Zhou S (1997) Volume phase transition of swollen gels: discontinuous or continuous? Macromolecules 30(3):574–579. https://doi.org/10.1021/ma960499a
Godbole RV, Khabaz F, Khare R et al (2017) Swelling of random copolymer networks in pure and mixed solvents: multi-component Flory-Rehner theory. The Journal of Physical Chemistry B 121(33):7963–7977. https://doi.org/10.1021/acs.jpcb.7b02194
Erman B, Flory PJ (1986) Critical phenomena and transitions in swollen polymer networks and in linear macromolecules. Macromolecules 19(9):2342–2353. https://doi.org/10.1021/ma00163a003
Tavagnacco L, Zaccarelli E, Chiessi E (2018) On the molecular origin of the cooperative coil-to-globule transition of poly(N-isopropylacrylamide) in water. Phy Chem Chem Phys 20(15):9997–1001. https://doi.org/10.1039/c8cp00537k
Shibayama M, Morimoto M, Nomura S (1994) Phase separation induced mechanical transition of poly(N-isopropylacrylamide)/water isochore gels. Macromolecules 27(18):5060–506. https://doi.org/10.1021/ma00096a031
Shibayama M, Sy Mizutani, Nomura S (1996) Thermal properties of copolymer gels containing N -isopropylacrylamide. Macromolecules 29(6):2019–2024. https://doi.org/10.1021/ma951390q
Ono Y, Shikata T (2007) Contrary hydration behavior of n-isopropylacrylamide to its polymer, p(nipam), with a lower critical solution temperature. The Journal of Physical Chemistry B 111(7):1511–151. https://doi.org/10.1021/jp068954k
Säckel C, von Klitzing R, Siegel R et al (2024) Water dynamics in solutions of linear poly (n-isopropyl acrylamide) studied by 2h nmr field-cycling relaxometry. Frontiers in Soft Matter. https://doi.org/10.3389/frsfm.2024.1379816
Berne BJ, Pecora R (1976) Dynamic light scattering: with applications to chemistry, biology and physics. A Wiley-Interscience publication, Wiley, New York NY
Provencher SW (1982) A constrained regularization method for inverting data represented by linear algebraic or integral equations. Computer Physics Communications 27(3):213–22. https://doi.org/10.1016/0010-4655(82)90173-4
Frisken BJ (2001) Revisiting the method of cumulants for the analysis of dynamic light-scattering data. Applied Optics 40(24):4087–4091. https://doi.org/10.1364/AO.40.004087
Brent RP (2002) Algorithms for minimization without derivatives, Unabridged republication of the work publ. by Prentice-Hall (1973) edn. Dover books on mathematics, Dover Publications, Mineola, NY
Virtanen P, Gommers R, Oliphant TE et al (2020) SciPy 1.0: fundamental algorithms for scientific computing in Python. Nature Methods 17(3):261–27. https://doi.org/10.1038/s41592-019-0686-2
Newville M, Stensitzki T, Allen DB, et al (2014) LMFIT: non-linear least-square minimization and curve-fitting for Python. https://doi.org/10.5281/ZENODO.11813
Nečas D, Klapetek P (2012) Gwyddion: an open-source software for spm data analysis. Open Physics 10(1). https://doi.org/10.2478/s11534-011-0096-2
Wedel B, Zeiser M, Hellweg T (2012) Non NIPAM based smart microgels: systematic variation of the volume phase transition temperature by copolymerization. Zeitschrift für Physikalische Chemie 226(7–8):737–74. https://doi.org/10.1524/zpch.2012.0267
Keerl M, Smirnovas V, Winter R et al (2008) Copolymer microgels from mono- and disubstituted acrylamides: phase behavior and hydrogen bonds. Macromolecules 41(18):6830–6839. https://doi.org/10.1021/ma800785w
Refai I, Agboluaje M, Hutchinson RA (2022) Radical copolymerization kinetics of n-tert-butyl acrylamide and methyl acrylate in polar media. Polymer Chemistry 13(14):2036–204. https://doi.org/10.1039/d2py00087c
Ganachaud F, Balic R, Monteiro MJ et al (2000) Propagation rate coefficient of poly(n-isopropylacrylamide) in water below its lower critical solution temperature. Macromolecules 33(23):8589–859. https://doi.org/10.1021/ma000619l
Coronado R, Pekerar S, Lorenzo AT et al (2011) Characterization of thermo-sensitive hydrogels based on poly(N-isopropylacrylamide)/hyaluronic acid. Polymer Bulletin 67(1):101–12. https://doi.org/10.1007/s00289-010-0407-6
Kratz K, Hellweg T, Eimer W (2001) Structural changes in pnipam microgel particles as seen by sans, dls, and em techniques. Polymer 42(15):6631–6639. https://doi.org/10.1016/S0032-3861(01)00099-4
Herman P, Lee JC (2012) The advantage of global fitting of data involving complex linked reactions. Methods in Molecular Biology (Clifton, NJ) 796:399–42. https://doi.org/10.1007/978-1-61779-334-9_22
Okada Y, Tanaka F (2005) Cooperative hydration, chain collapse, and flat LCST behavior in aqueous poly(N-isopropylacrylamide) solutions. Macromolecules 38(10):4465–4470. https://doi.org/10.1021/ma0502497
Acknowledgements
We acknowledge René Haverkamp and Simon Friesen for fruitful discussions related to this project and we would like to thank Marco Annegarn for providing essential tools for PCS data analysis, which have been very helpful in our work.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krüger, J., Kakorin, S. & Hellweg, T. Volume phase transition of NIPAM based copolymer microgels with non-thermoresponsive comonomers. Colloid Polym Sci (2024). https://doi.org/10.1007/s00396-024-05288-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00396-024-05288-1