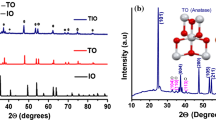
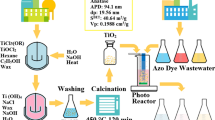
Abstract
Adsorption and photocatalytic decolorization methods were used to remove Reactive Orange 16 dye from textile wastewater by using ethyleneglycoldimethacrylate and 1-vinyl-1,2,4-triazole, m-poly(EGDMA-VTA)-TiO2 polymer composite particles with magnetic synthesized by suspension polymerization. The characterization of the synthesized m-poly(EGDMA-VTA)-TiO2 particules were carried out by using XRD, FTIR, SEM–EDS-elemental mapping, ESR, and BET analyses. Both adsorption and photocatalytic decolorization processes of RO16 dye were applied to the polymer particles. The effects of solution pH, amount of adsorbent, initial dye concentration, temperature, and time on the adsorption capacity were investigated. The removal of R016 dye reached a maximum at pH 3. Dye substance removal decreased due to increasing temperature and adsorbent amount. As a result of experimental studies, the adsorption of RO16 dye was explained by the Langmuir isotherm, while its kinetics was stated by a pseudo-second-order mechanism. Additionally, thermodynamic functions (ΔHo, ΔGo, and ΔSo) have been determined. At the end of adsorption, the decolorization kinetics were elucidated by examining the adsorbent amount, time, and dye concentration parameters for the photocatalytic decolorization of non-adsorbed dyes. It was determined that the photocatalytic activity was highest at low dye concentration and high photocatalyst content. Additionally, it was determined that decolorization kinetics studies were compatible with the Langmuir–Hinshelwood model.

















Similar content being viewed by others
Availability of data and materials
This declaration is “not applicable.”
Abbreviations
- C e :
-
Concentration of RO16 at equilibrium (mg L−1)
- C 0 :
-
Initial concentration of RO16 in solution of RO16 (mg L−1)
- E a :
-
Activation energy of adsorption (kJ mol−1)
- E fe :
-
Free energy of adsorption (kJ mol−1)
- ∆G :
-
Gibbs free energy of adsorption (J mol−1)
- ∆H :
-
Isosteric enthalpy of adsorption (J mol−1)
- ∆S :
-
Entropy change of the adsorption process (J mol−1 K−1)
- R:
-
The gas constant (J mol−1 K−1)
- q e :
-
The amount of RO16 adsorbed on the adsorbent at equilibrium (mg g−1)
- q t :
-
The amount of RO16 adsorbed on the adsorbent at any time (mg g−1)
- q m :
-
The maximum amount of RO16 adsorbed per unit mass adsorbent (mg g−1)
- Q L :
-
The maximum amount of RO16 ions adsorbed per unit mass adsorbent (mg g−1)
- K L :
-
The Langmuir constant related to the affinity of binding sites (mL mg−1)
- n :
-
The heterogenity factor
- K F :
-
The Freundlich constant (mg g−1) (L mg−1)1/n
- QD-R :
-
The maximum amount of RO16 adsorbed per unit mass adsorbent (mg g−1)
- K D-R :
-
The Dubinin-Radushkevich constant (mol2 J−2)
- ε :
-
The polanyi potential (J mol−1)
- R L :
-
The dimensionless separation factor
- k 1 :
-
The rate constant of pseudo-first-order adsorption (min−1)
- k 2 :
-
The rate constant of pseudo-second-order adsorption ((g mg−1) min−1)
- k0 :
-
Independent temperature factor ((g mg−1) min−1)
- k R :
-
The rate constant for the modified Ritchie second-order model (min−1)
- k dif :
-
The intraparticle diffusion rate constant (mg (g min0.5)−1)
- R 2 :
-
Linear regression coefficient
- t :
-
Time (min)
- T :
-
Temperature (K)
- kapp :
-
The apparent first-order rate constant (min−1)
- Kr :
-
The reaction rate (mg (L min)−1)
- Ks :
-
The adsorption constant (L mg−1)
References
Al-Tohamy R, Ali SS, Li F, Okasha KM, Mahmoud YAG, Elsamahy T, Jiao H, Fu Y, Sun J (2022) Ecotoxicol Environ Saf. https://doi.org/10.1016/J.ECOENV.2021.113160
Chandanshive V, Kadam S, Rane N, Jeon BH, Jadhav J, Govindwar S (2020) Chemosphere. https://doi.org/10.1016/J.CHEMOSPHERE.2020.126513
Al-Mamun MR, Kader S, Islam MS, Khan MZH (2019) J Environ Chem Eng. https://doi.org/10.1016/j.jece.2019.103248
Sudha M, Saranya A, Selvakumar G, Sivakumar N (2014) Int J Curr Microbiol App Sci. 3(2):670
Artifon W, Cesca K, de Andrade CJ, Ulson de Souza AA, de Oliveira D (2021) Process Biochem. https://doi.org/10.1016/J.PROCBIO.2021.10.030
Benkhaya S, Harfi SE, Harfi AE (2017) App J Environ Eng Sci. https://doi.org/10.48422/IMIST.PRSM/ajees-v3i3.9681
Asad S, Amoozegar MA, Pourbabaee AA, Sarbolouki MN, Dastgheib SMM (2007) Biores Technol. https://doi.org/10.1016/j.biortech.2006.08.020
Haroun AA, Abdelghaffar F, Hakeim OA (2021) Biointerf Res Appl Chem. https://doi.org/10.33263/BRIAC114.1165311665
Alghamdi AA, Al-Odayni A-B, Saeed WS, Almutairi MS, Alharthi FA, Aouak T, Al-Kahtani A (2019) Molecules. https://doi.org/10.3390/molecules24203685
Oliveira GAR, Ferraz ERA, Chequer FMD, Grando MD, Angeli JPF, Tsuboy MS, Marcarini JC, Mantovani MS, Osugi ME, Lizier TM, Zanoni MVB, Oliveira DP (2010) Mutation Research/Genetic Toxicology and Environmental Mutagenesis. https://doi.org/10.1016/J.MRGENTOX.2010.09.001
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Adv Coll Interface Sci. https://doi.org/10.1016/J.CIS.2014.04.002
Wijannarong S, Aroonsrimorakot S, Thavipoke P, Kumsopa C, Sangjan S (2013) APCBEE Procedia. https://doi.org/10.1016/j.apcbee.2013.05.048
Saitoh T, Shibata K, Hiraide M (2014) J Environ Chem Engin. https://doi.org/10.1016/J.JECE.2014.08.005
Georgiou D, Melidis P, Aivasidis A, Gimouhopoulos K (2002) Dyes Pigments. https://doi.org/10.1016/S0143-7208(01)00078-X
Gupta VK (2009) Suhas. J Environ Manage. https://doi.org/10.1016/j.jenvman.2008.11.017
Ram C, Pareek RK, Singh V (2012) Inter J Theor App Sci 4(2):82
Ledakowicz S, Paździor K (2021) Molecules. https://doi.org/10.3390/molecules26040870
Cebeci MS, Selçuk SF (2020) Academic Platform Journal of Engineering and Science 8(3):533
Danish MSS, Estrella LL, Alemaida IMA, Lisin A, Moiseev N, Ahmadi M, Nazari M, Wali M, Zaheb H, Senjyu T (2021) Metals. https://doi.org/10.3390/met11010080
Zhang P, Wang T, Chang X, Gong J (2016) Acc Chem Res. https://doi.org/10.1021/acs.accounts.6b00036
Elgohary EA, Mahmoud Mohamed YA, HA el Nazer HA, Baaloudj O, Alyami MSS, el Jery A, Amine Assadi A, Amrane A, Comparelli R (2021) Catalysts. https://doi.org/10.3390/catal11121498
Li W, Wang J, He G, Yu L, Noor N, Sun Y, Zhou X, Hu J, Parkin IP (2017) J Mater Chem. https://doi.org/10.1039/c6ta09116d
Elhadj M, Samira A, Mohamed T, Djawad F, Asma A, Djamel N (2020) Sep Sci Technol. https://doi.org/10.1080/01496395.2019.1577896
Li M, Zhao H, Lu ZY (2020) Microporous Mesoporous Mater. https://doi.org/10.1016/j.micromeso.2019.109774
Sawut A, Wu T, Simayi R, Wu T, Gong X, Wang Z (2023) Colloids Surf, A. https://doi.org/10.1016/j.colsurfa.2023.132531
Uzun L, Kara A, Tüzmen N, Karabakan A, Beşirli N, Denizli A (2006) J App Poly Sci. https://doi.org/10.1002/app.24830
Kara A (2004) J Hazar Mater. https://doi.org/10.1016/j.jhazmat.2003.08.016
Yalçın Ş, Kara A, Demirel S (2022) Univ J Natural Appl Sci. https://doi.org/10.19113/sdufenbed.982112
Malega F, Indrayana PT, Suharyadi E (2018) Journal Ilmiah Pendidikan Fisika Al-BiRuNi. https://doi.org/10.24042/jipfalbiruni.v7i2.2913
Thamaphat K, Limsuwan P, Ngotawornchai B (2008) Agriculture and Natural Resources 42(5):357
Özel Ş (2019) Master's thesis, Bursa Uludağ Üniversitesi
Denizli A, Arpa Ç, Bektas S, Genç Ö (2002) Adsorpt Sci Technol. https://doi.org/10.1260/026361702760120953
Kara A, Demirbel E, Tekin N, Osman B, Beşirli N (2015) J Hazar Mater. https://doi.org/10.1016/J.JHAZMAT.2014.12.011
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Pure Appl Chem. https://doi.org/10.1515/pac-2014-1117
Kara A, Demirbel E (2011) Water Air Soil Pollut. https://doi.org/10.1007/s11270-011-1032-1
Rouquerol J, Baron G, Denoyel R, Giesche H, Groen J, Klobes P, Levitz P, Neimark AV, Rigby S, Skudas R, Sing K, Thommes M (2012) K. Unger. Pure Appl Chem. https://doi.org/10.1351/PAC-REP-10-11-19
Benhouria A, Islam MA, Zaghouane-Boudiaf H, Boutahala M, Hameed BH (2015) Chem Engineer J. https://doi.org/10.1016/j.cej.2015.02.030
Panda SK, Aggarwal I, Kumar H, Prasad L, Kumar A, Sharma A, Vo DVN, Thuan DV, Mishra V (2021) Environ Chem Lett. https://doi.org/10.1007/s10311-020-01173-9
Kheradmand A, Negarestani M, Kazemi S, Shayesteh H, Javanshir S, Ghiasinejad H (2022) Sci Rep. https://doi.org/10.1038/s41598-022-19056-0
Rápó E, Tonk S (2021) Molecules. https://doi.org/10.3390/molecules26175419
Kannusamy P, Sivalingam T (2013) Colloids and Surfaces B: Biointerfaces. https://doi.org/10.1016/j.colsurfb.2013.03.015
Albroomi H, Elsayed M, Baraka A, Abdelmaged M (2015) J Turk Chem Soc Section A Chem. 2(1):17
Kara A (2009) J App Poly Sci. https://doi.org/10.1002/app.29169
Errais E, Duplay J, Darragi F, M’Rabet I, Aubert A, Huber F, Morvan G (2011) Desalination. https://doi.org/10.1016/J.DESAL.2011.02.031
Lagergren S (1898) Handlingar 25(4):1
Ho YS, McKay G (1999) Process Biochem. https://doi.org/10.1016/S0032-9592(98)00112-5
Wu F-C, Tseng R-L, Juang R-S (2009) Chem Engineer J. https://doi.org/10.1016/j.cej.2009.04.042
Can N, Ömür BC, Altındal A (2016) Anadolu Univ J Sci Technol A- Appl Sci Eng. https://doi.org/10.18038/aubtda.266443
Abualnaja KM, Alprol AE, Abu-Saied MA, Ashour M, Mansour AT (2021) Sustainability. https://doi.org/10.3390/su13137077
Osman B, Özer ET, Kara A, Güçer Ş, Beşirli N (2012) J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2012.03.069
Langmuir I (1918) J Am Chem Soc. https://doi.org/10.1021/JA02242A004/ASSET/JA02242A004.FP.PNG_V03
Freundlich HMF (1906) J Phys Chem 57:385
Sarı A, Tuzen M (2009) J Hazar Mater. https://doi.org/10.1016/j.jhazmat.2008.09.047
Tassist A, Lounici H, Abdi N, Mameri N (2010) J Hazar Mater. https://doi.org/10.1016/j.jhazmat.2010.06.078
Namasivayam C, Kavitha D (2002) Dyes Pigm. https://doi.org/10.1016/s0143-7208(02)00025-6
Ebelegi A, Ayawei N, Wankasi D (2020) Open J Phys Chem. https://doi.org/10.4236/ojpc.2020.103010
Özcan A, Ömeroǧlu Ç, Erdoǧan Y, Özcan AS (2007) J Hazar Mater. https://doi.org/10.1016/J.JHAZMAT.2006.06.138
Wu CH (2007) J Hazar Mater. https://doi.org/10.1016/J.JHAZMAT.2006.09.083
Kansal SK, Hassan Ali A, Kapoor S (2010) Desalination. https://doi.org/10.1016/J.DESAL.2010.04.017
Chekir N, Tassalit D, Benhabiles O, Kasbadji Merzouk N, Ghenna M, Abdessemed A, Issaadi R (2017) Int J Hydr Energy https://doi.org/10.1016/j.ijhydene.2016.11.057
Lahmar H, Benamira M, Akika FZ, Trari M (2017) J Phys Chem Solids. https://doi.org/10.1016/J.JPCS.2017.06.021
Khezrianjoo S, Revanasiddappa HD (2012) Chem Sci J. CSJ-85
Asenjo NG, Santamaría R, Blanco C, Granda M, Álvarez P, Menéndez R (2013) Carbon. https://doi.org/10.1016/j.carbon.2012.12.010
Ohtani B (2011) Advances in Inorganic Chem. https://doi.org/10.1016/b978-0-12-385904-4.00001-9
Doğdu G (2022) Nanocatalyst Düzce University. J Sci Technol 10:1998
Acknowledgements
The authors thank Tübitak for their support. A patent application has been filed for this study (TurkPatent 2022/018576, PCT/TR2023/050372).
Funding
This research is supported by Bursa Uludağ University General Research Project numbered FGA-2021–656. It was carried out in cooperation with Bursa Uludağ University and BUTEKOM Bursa Technology Coordination and R&D Center within the scope of Tübitak 2244 PhD Project (Project number: 119C122) in the Fields of Sustainability in Textiles and Composite Materials.
Author information
Authors and Affiliations
Contributions
Author Contributions: G.K.M: Literature review, experimental studies, preparation of graphics, writing A.K: Subject determination, analysis, data analysis, writing and general coordination, N.T: experimental design, manuscript editing, S.D writing editing, preparation of references and the article in a format suitable for the journal. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This declaration is “not applicable.”
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mutlu, G.K., Kara, A., Tekin, N. et al. Synthesis and characterization of 1-vinyl-1,2,4-triazole, m-poly(EGDMA-VTA)-TiO2 polymer composite particles and the using of Reactive Orange 16 dye in adsorption and photocatalytic decolorization. Colloid Polym Sci 302, 623–642 (2024). https://doi.org/10.1007/s00396-023-05213-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-023-05213-y