Abstract
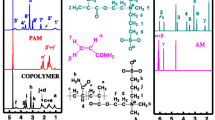
Sulfobetaine 3-[N,N-dimethyl-N-(2-methacryloxylethyl)ammonio]-propane sulfonate (DMAPS) was used to copolymerize with acrylamide (AM) to prepare salt-sensitive copolymers. The optimal reaction conditions, such as initiator concentration, temperature, and reaction time, to achieve the largest weight averaged molecular weight (M w) and the lowest polydispersity index (PDI) were studied. The reactivity ratios of AM and DMAPS (r 1, r 2) were calculated to be (0.45, 0.84) by the Fineman-Ross method, and (0.47, 0.91) by the Kelen-Tüdös method. The Q-e values of DMAPS were calculated to be (0.30, −0.38). DMAPS was found to be more easily to add into the copolymer than AM to form random copolymers with a slight alternating tendency. Solution rheology and molecular size of the copolymers as a function of M w were studied in 1 M NaCl solution, a near θ-solution, by rotational rheometer and light scattering methods, respectively. Undisturbed dimension of the copolymers was evaluated according to Stockmayer-Fixman relation.










Similar content being viewed by others
References
Myagchenkov VA, Kurenkov VF (1991) Applications of acrylamide polymers and copolymers—a review. Polym Plast Technol 30(2–3):109–135. doi:10.1080/03602559108020132
York AW, Kirkland SE, McCormick CL (2008) Advances in the synthesis of amphiphilic block copolymers via RAFT polymerization: stimuli-responsive drug and gene delivery. Adv Drug Deliv Rev 60(9):1018–1036. doi:10.1016/j.addr.2008.02.006
Kuang W, Zhao X (2013) Synthesis, characterization, and properties of novel hydrophobically associating fluorinated copolymers for DNA delivery. React Funct Polym 73(5):703–709. doi:10.1016/j.reactfunctpolym.2013.02.010
Xue W, Huglin MB, Liao B (2006) Observations on the swelling characteristics of the zwitterionic hydrogel of poly(1-3-sulfopropyl)-2-vinyl-pyridinium-betaine hydrogel. Eur Polym J 42(11):3015–3023. doi:10.1016/j.eurpolymj.2006.07.015
Lee WF, Wu RJ (1996) Superabsorbent polymeric materials.1. Swelling behaviors of crosslinked poly(sodium acrylate-co-hydroxyethyl methacrylate) in aqueous salt solution. J Appl Polym Sci 62(7):1099–1114. doi:10.1002/(sici)1097-4628(19961114)62:7<1099::aid-app16>3.0.co;2-1
Lee WF, Wu RJ (1997) Superabsorbent polymeric materials.2. Swelling behavior of crosslinked poly sodium acrylate-co-3-dimethyl(methacryloyloxyethyl) ammonium propane sulfonate in aqueous salt solution. J Appl Polym Sci 64(9):1701–1712. doi:10.1002/(sici)1097-4628(19970531)64:9<1701::aid-app5>3.0.co;2-j
Li L, Marchant RE, Dubnisheva A, Roy S, Fissell WH (2011) Anti-biofouling sulfobetaine polymer thin films on silicon and silicon nanopore membranes. J Biomater Sci Polym Ed 22(1–3):91–106. doi:10.1163/092050609x12578498982998
Wu L, Jasinski J, Krishnan S (2012) Carboxybetaine, sulfobetaine, and cationic block copolymer coatings: a comparison of the surface properties and antibiofouling behavior. J Appl Polym Sci 124(3):2154–2170. doi:10.1002/app.35233
Gunkel G, Huck WTS (2013) Cooperative adsorption of lipoprotein phospholipids, triglycerides, and cholesteryl esters are a key factor in nonspecific adsorption from blood plasma to antifouling polymer surfaces. J Am Chem Soc 135(18):7047–7052. doi:10.1021/ja402126t
Hoshi Y, Xu Y, Ober CK (2013) Photo-cleavable anti-fouling polymer brushes: a simple and versatile platform for multicomponent protein patterning. Polymer 54(7):1762–1767. doi:10.1016/j.polymer.2013.02.027
McCormick CL, Salazar LC (1992) Water-soluble copolymers.46. Hydrophilic sulfobetaine copolymers of acrylamide and 3-(2-acrylamido-2-methylpropanedimethyl-ammonio)-1-propanesulphonate. Polymer 33(21):4617–4624. doi:10.1016/0032-3861(92)90422-s
Donovan MS, Lowe AB, Sanford TA, McCormick CL (2003) Sulfobetaine-containing diblock and triblock copolymers via reversible addition-fragmentation chain transfer polymerization in aqueous media. J Polym Sci A Polym Chem 41(9):1262–1281. doi:10.1002/pola.10658
Woodfield PA, Zhu Y, Pei Y, Roth PJ (2014) Hydrophobically modified sulfobetaine copolymers with tunable aqueous UCST through postpolymerization modification of poly(pentafluorophenyl acrylate). Macromolecules 47(2):750–762. doi:10.1021/ma402391a
Soto VMM, Galin JC (1984) Poly(sulphopropylbetaines).1. Synthesis and characterization. Polymer 25(1):121–128
Soto VMM, Galin JC (1984) Poly(sulphopropylbetaines).2. Dilute-solution properties. Polymer 25(2):254–262
Wang H, Hirano T, Seno M, Sato T (2003) Radical polymerization behavior of 3-(N-2-methacryloyloxyethyl-N, N-dimethyl)ammonatopropanesulfonate in water. Eur Polym J 39(11):2107–2114. doi:10.1016/s0014-3057(03)00156-3
Gauthier M, Carrozzella T, Penlidis A (2002) Sulfobetaine zwitterionomers based on n-butyl acrylate and 2-ethoxyethyl acrylate: monomer synthesis and copolymerization behavior. J Polym Sci A Polym Chem 40(4):511–523
Liaw DJ, Lee WF (1985) Thermal-degradation of poly 3-dimethyl(methylmethacryloylethyl) ammonium propanesulfonate. J Appl Polym Sci 30(12):4697–4706. doi:10.1002/app.1985.070301216
Huglin MB, Radwan MA (1991) Properties of poly N-2-(methyacryloyloxy)ethyl-N, N-dimethyl-N-3-sulfopropylammonium betaine in dilute-solution. Makromol Chem Macromol Chem Phys 192(10):2433–2445
Liaw DJ, Huang CC (2002) Effect of salt and surfactant on aqueous solution properties of pyrene-labeled poly(3-dimethyl (methylmethacryloyl ethyl) ammonium propane sulfonate). Macromol Symp 179(1):209–222. doi:10.1002/1521-3900(200203)179:1<209::aid-masy209>3.0.co;2-o
Huglin MB, Rego JM (1991) Observations on the copolymerization of charged monomers. Polym Commun 32(5):130–133
Rego JM, Huglin MB (1991) Influence of composition on properties of hydrogels of 2-hydroxyethyl methacrylate with a sulfobetaine comonomer. Polym J 23(12):1425–1434. doi:10.1295/polymj.23.1425
Kostova B, Kamenska E, Ivanov I, Kamenova I, Rachev D, Georgiev G (2008) Copolymer zwitteriones as new sustained drug delivery systems. God Sofii Univ Sv Kliment Okhridski Khim Fak 100(1):91–105
Chang Y, Yandi W, Chen W-Y, Shih Y-J, Yang C-C, Chang Y, Ling Q-D, Higuchi A (2010) Tunable bioadhesive copolymer hydrogels of thermoresponsive poly(N-isopropyl acrylamide) containing zwitterionic polysulfobetaine. Biomacromolecules 11(4):1101–1110. doi:10.1021/bm100093g
Han D-S, Gong M-S (2010) New efficient polyelectrolyte containing zwitterionic sulfobetaine salt for the high sensitive resistive humidity sensor. Macromol Res 18(3):260–265. doi:10.1007/s13233-010-0307-5
Kathmann EE, White LA, McCormick CL (1997) Water soluble polymers.69. pH and electrolyte responsive copolymers of acrylamide and the zwitterionic monomer 4-(2-acrylamido-2-methyl propyldimethyl-ammonio) butanoate: synthesis and solution behaviour. Polymer 38(4):871–878. doi:10.1016/s0032-3861(96)00586-1
Armentrout RS, McCormick CL (2000) Water soluble polymers. 76. Electrolyte responsive cyclocopolymers with sulfobetaine units exhibiting polyelectrolyte or polyampholyte behavior in aqueous media. Macromolecules 33(2):419–424. doi:10.1021/ma991133b
Liaw DJ, Huang CC, Wu PL (2002) Effect of surfactant and various-salts on aqueous solution properties of naphthalene-labeled poly(hydrochloride-quaternized 2-norbornene-5-methylamine) made by ring-opening metathesis polymerization (ROMP). Macromol Chem Phys 203(15):2177–2187. doi:10.1002/1521-3935(200211)203:15<2177::aid-macp2177>3.0.co;2-s
Virtanen J, Arotcarena M, Heise B, Ishaya S, Laschewsky A, Tenhu H (2002) Dissolution and aggregation of a poly(NIPA-block-sulfobetaine) copolymer in water and saline aqueous solutions. Langmuir 18(14):5360–5365. doi:10.1021/la0118208
Varghese S, Chang C-W, Hwang Y (2013) Synthetic heparin mimetic and acrylamide polymer hydrogel matrices for self-renewal and expansion of stem cells. US20130177980A1
Che Y-J, Tan Y, Cao J, Xu G-Y (2010) Aggregation behavior of copolymer containing sulfobetaine structure in aqueous solution. J Macromol Sci B Phys 49(4):695–710. doi:10.1080/00222341003598281
Georgiev G, Dyankova K, Vassileva E, Friedrich K (2006) High synthesis and some mechanical properties of polysulfobetaine - polyacrylamide double networks. E-Polymers. doi:054
Rubinstein M, Colby RH (2003) Polymer physics. Oxford University Press, New York
Huglin MB (1972) Light scattering from polymer solutions. Academic, London
Berry GC (1966) Thermodynamic and conformational properties of polystyrene. I. Light-scattering studies on dilute solutions of linear polystyrenes. J Chem Phys 44(12):4550–4564. doi:10.1063/1.1726673
Pan Z (2003) Polymer chemistry, 3rd edn. Chemical Industry Press, Peking
Fineman M, Ross SD (1950) Linear method for determining monomer reactivity ratios in copolymerization. J Polym Sci 5(2):259–262. doi:10.1002/pol.1950.120050210
Kelen T, Tüdös F (1975) Analysis of the linear methods for determining copolymerization reactivity ratios. I. A new improved linear graphic method. J Macromol Sci A Chem 9(1):1–27. doi:10.1080/00222337508068644
Alfrey T, Goldfinger G (1944) The mechanism of copolymerization. J Chem Phys 12(6):205–209. doi:10.1063/1.1723934
McCormick CL, Hutchinson BH, Morgan SE (1987) Water-soluble copolymers: 16. Studies of the behavior of acrylamide-N-(1,1-dimethyl-3-oxobutyl)acrylamide copolymers in aqueous salt-solutions. Makromol Chem Macromol Chem Phys 188(2):357–370
McCormick CL, Chen GS (1984) Water-soluble copolymers: 9. Copolymers of acrylamide with N-(1,1-dimethyl-3-oxybutyl)acrylamide and N, N-dimethylacrylamide - synthesis and characterization. J Polym Sci A Polym Chem 22(12):3633–3647. doi:10.1002/pol.1984.170221202
McCormick CL, Blackmon KP (1986) Water-soluble copolymers: 23. Copolymers of acrylamide with N-(1,1-dimethyl-3-oxobutyl)-N-(N-propyl)acrylamide—synthesis and characterization. Angew Makromol Chem 144:73–86. doi:10.1002/apmc.1986.051440106
Mumick PS, McCormick CL (1994) Water-soluble copolymers: 54. N-isopropylacrylamide-co-acrylamide copolymers in drag reduction - synthesis, characterization, and dilute-solution behavior. Polym Eng Sci 34(18):1419–1428. doi:10.1002/pen.760341809
McCormick CL, Blackmon KP (1986) Water-soluble copolymers: 12. Copolymers of acrylamide with sodium-3-acrylamido-3-methylbutanoate—synthesis and characterization. J Polym Sci A Polym Chem 24(10):2635–2645. doi:10.1002/pola.1986.080241020
McCormick CL, Blackmon KP, Elliott DL (1986) Water-soluble copolymers: 20. Copolymers of acrylamide with sodium 3-(N-propyl)acrylamido-3-methylbutanoate—solution properties. J Macromol Sci Chem A23(12):1469–1486. doi:10.1080/00222338608081137
McCormick CL, Blackmon KP (1986) Water-soluble copolymers: 21. Copolymers of acrylamide with 2-acrylamido-2-methylpropanedimethylammonium chloride—synthesis and characterization. Polymer 27(12):1971–1975. doi:10.1016/0032-3861(86)90192-8
McCormick CL, Chen GS (1982) Water-soluble copolymers: 4. Random copolymers of acrylamide with sulfonated co-monomers. J Polym Sci A Polym Chem 20(3):817–838. doi:10.1002/pol.1982.170200319
McCormick CL, Salazar LC (1993) Water-soluble copolymers: 42. Cationic polyelectrolytes of acrylamide and 2-acrylamido-2-methylpropanetrimethylammonium chloride. J Polym Sci A Polym Chem 31(5):1099–1104. doi:10.1002/pola.1993.080310501
Kathmann EE, White LA, McCormick CL (1996) Water-soluble copolymers: 67. Polyelectrolytes of N-vinylformamide with sodium 3-acrylamido-3-methylbutanoate, sodium 2-acrylamido-2-methylpropanesulfonate, and sodium acrylate: synthesis and characterization. Macromolecules 29(16):5268–5272. doi:10.1021/ma9518520
Bune YV, Barabanova AI, Bogachev YS, Gromov VF (1997) Copolymerization of acrylamide with various water-soluble monomers. Eur Polym J 33(8):1313–1323. doi:10.1016/s0014-3057(96)00258-3
Tanaka H (1986) Copolymerization of cationic monomers with acrylamide in an aqueous-solution. J Polym Sci A Polym Chem 24(1):29–36. doi:10.1002/pol.1986.170240103
Smets G, Hesbain AM (1959) Hydrolysis of polyacrylamide and acrylic acid-acrylamide copolymers. J Polym Sci 40(136):217–226. doi:10.1002/pol.1959.1204013616
Riahinezhad M, Kazemi N, McManus N, Penlidis A (2013) Optimal estimation of reactivity ratios for acrylamide/acrylic acid copolymerization. J Polym Sci A Polym Chem 51(22):4819–4827. doi:10.1002/pola.26906
Baade W, Hunkeler D, Hamielec AE (1989) Copolymerization of acrylamide with cationic monomers in solution and inverse-microsuspension. J Appl Polym Sci 38(1):185–201. doi:10.1002/app.1989.070380117
Igarashi S (1963) Representation of composition and blockiness of the copolymer by a triangular coordinate system. J Polym Sci B Polym Lett 1(7):359–363. doi:10.1002/pol.1963.110010706
Pyun CW (1970) Comonomer and stereosequence distributions in high polymers. J Polym Sci A2 Polym Phys 8(7):1111–1126. doi:10.1002/pol.1970.160080707
Georgiev GS (1978) General method for evaluation of alternating tendency in copolymerization. J Macromol Sci A Chem 12(8):1175–1195. doi:10.1080/00222337808063182
Alfrey T, Price CC (1947) Relative reactivities in vinyl copolymerization. J Polym Sci 2(1):101–106. doi:10.1002/pol.1947.120020112
Brandrup J, Immergut EH, Grulke EA (1999) Polymer handbook, 4th edn. Wiley, New York
Shukla A, Srivastava AK (2003) Free radical copolymerization of acrylamide and linalool with functional group as a pendant. High Perform Polym 15(3):243–257. doi:10.1177/0954008303015003002
Ye T, Song Y, Zheng Q (2014) Solubility and solution rheology of acrylamide-sulfobetaine copolymers. Colloid Polym Sci 292(9):2185–2195. doi:10.1007/s00396-014-3246-4
Macosko CW (1994) Rheology: principles, measurements, and applications. Wiley, New York
Sakai T (1968) Huggins constant k’ for flexible chain polymers. J Polym Sci A2 Polym Phys 6(8):1535–1549. doi:10.1002/pol.1968.160060810
Halabalova W, Simek L, Dostal J, Bohdanecky M (2004) Note on the relation betweeen the parameters of the Mark-Houwink-Kuhn-Sakurada equation. Int J Polym Anal Charact 9(1–3):65–75. doi:10.1080/10236660490890484
Bohdanecky M, Petrus V, Horsky J (1995) Hydrodynamic properties of dilute aqueous-solutions of poly(N-ethylmethacrylamide). Macromolecules 28(24):8344–8349. doi:10.1021/ma00128a051
Bohdanecky M (1996) Mark-Houwink-Kuhn-Sakurada exponent at the Theta condition. Its invariancy with respect to the cross-sectional dimensions of polymer chains. Macromolecules 29(6):2265–2268. doi:10.1021/ma9507507
McCarthy KJ, Burkhardt CW, Parazak DP (1987) Mark-Houwink-Sakurada constants and dilute-solution behavior of heterodisperse poly(acrylamide-co-sodium acrylate) in 0.5m and 1m NaCl. J Appl Polym Sci 33(5):1699–1714. doi:10.1002/app.1987.070330523
Bai LB, Zheng RR, Li WL, Wu YG, Ba XW, Wang HJ (2013) A synthetic approach for water soluble hyperbranched poly(N, N-ethylidenebis(N-2-chloroacetyl acrylamide)) with high degree of branching via atom transfer radical polymerization/self-condensing vinyl polymerization. Chin J Polym Sci 31(7):1038–1045. doi:10.1007/s10118-013-1257-0
Bohdanecky M, Netopilik M (1995) The Mark-Houwink-Kuhn-Sakurada exponent of polymers with long side-groups—is a(0)=1/2 a reliable criterion of the theta-state. Polymer 36(17):3377–3384. doi:10.1016/0032-3861(95)99439-2
Stockmayer WH, Fixman M (1963) On the estimation of unperturbed dimensions from intrinsic viscositiesxcin. J Polym Sci C Polym Symp 1(1):137–141. doi:10.1002/polc.5070010109
Kurata M, Stockmayer W (1963) Intrinsic viscosities and unperturbed dimensions of long chain molecules. In: Fortschritte Der Hochpolymeren-Forschung, vol 3/2. Advances in Polymer Science. Springer Berlin Heidelberg, pp 196–312. doi: 10.1007/BFb0050490
Buhler E, Boué F (2004) Chain persistence length and structure in hyaluronan solutions: ionic strength dependence for a model semirigid polyelectrolyte. Macromolecules 37(4):1600–1610. doi:10.1021/ma0215520
Hester RD, Mitchell PH (1980) A new universal GPC calibration method. J Polym Sci Polym Chem Ed 18(6):1727–1738. doi:10.1002/pol.1980.170180608
Fevola MJ, Bridges JK, Kellum MG, Hester RD, McCormick CL (2004) pH-responsive polyzwitterions: a comparative study of acrylamide-based polyampholyte terpolymers and polybetaine copolymers. J Appl Polym Sci 94(1):24–39. doi:10.1002/app.20700
Ezell RG, Gorman I, Lokitz B, Ayres N, McCormick CL (2006) Stimuli-responsive ampholytic terpolymers of N-acryloyl-valine, acrylamide, and (3-acrylamidopropyl)trimethylammonium chloride: synthesis, characterization, and solution properties. J Polym Sci A Polym Chem 44(9):3125–3139. doi:10.1002/pola.21408
Fevola MJ, Bridges JK, Kellum MG, Hester RD, McCormick CL (2004) pH-responsive ampholytic terpolymers of acrylamide, sodium 3-acrylamido-3-methylbutanoate, and (3-acrylamidopropyl)trimethylammonium chloride. I. Synthesis and characterization. J Polym Sci A Polym Chem 42(13):3236–3251. doi:10.1002/pola.20173
Feng XS, Taton D, Chaikof EL, Gnanou Y (2005) Toward an easy access to dendrimer-like poly(ethylene oxide)s. J Am Chem Soc 127(31):10956–10966. doi:10.1021/ja0509432
Trollsas M, Atthof B, Wursch A, Hedrick JL, Pople JA, Gast AP (2000) Constitutional isomers of dendrimer-like star polymers: design, synthesis, and conformational and structural properties. Macromolecules 33(17):6423–6438. doi:10.1021/ma000321v
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ye, T., Song, Y. & Zheng, Q. Synthesis and solution property of acrylamide-sulfobetaine copolymers. Colloid Polym Sci 293, 797–807 (2015). https://doi.org/10.1007/s00396-014-3467-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-014-3467-6