Abstract
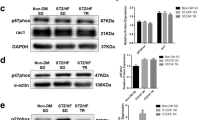
This study aims to determine the effect of exercise on the cardiac function, metabolic profiles and related molecular mechanisms in mice with ischemic-induced heart failure (HF). HF was induced by myocardial infarction (MI) in C57BL6/N mice. Cardiac function and physical endurance were improved in HF mice after exercise. Micro-PET/CT scanning revealed enhanced myocardial glucose uptake in vivo in HF mice after exercise. Exercise reduced mitochondrial structural damage in HF mice. Cardiomyocytes isolated from HF + exercise mice showed increased glycolysis capacity, respiratory function and ATP production. Both mRNA and protein expression of glucose transporter 1 (GLUT1) were upregulated after exercise. Results of ChIP-PCR revealed a novel interaction between transcription factor myocyte enhancer factor 2a (MEF2a) and GLUT1 in hearts of HF + exercise mice. Exercise also activated myocardial AMP-activated protein kinase (AMPK), which in turn phosphorylated histone deacetylase 4 (HDAC4), and thereby modulated the GLUT1 expression through reducing its inhibition on MEF2a in HF mice. Inhibition of HDAC4 also improved cardiac function in HF mice. Moreover, knockdown of GLUT1 impaired the systolic and diastolic function of isolated cardiomyocytes. In conclusion, exercise improves cardiac function and glucose metabolism in HF mice through inhibiting HDAC4 and upregulating GLUT1 expression.








Similar content being viewed by others
Abbreviations
- ACADm:
-
Medium-chain specific acyl-CoA dehydrogenase
- AMPK:
-
AMP-activated protein kinase
- GLUT1:
-
Glucose transporter 1
- IDH2:
-
Isocitrate dehydrogenase 2
- HDAC4:
-
Histone deacetylase 4
- HK2:
-
Hexokinase 2
- H3K9ac:
-
Acetylation of histone 3 lysine 9
- LDHA:
-
Lactate dehydrogenase A
- MEF2a:
-
Myocyte enhancer factor 2a
- OGDH:
-
Oxoglutarate dehydrogenase
- PDH:
-
Pyruvate dehydrogenase
- PFKFB2:
-
Phosphofructo-kinase/fructose-bisphosphatase 2
- SUVmax:
-
Maximum standardized uptake value
References
Ackers-Johnson M, Li PY, Holmes AP, O'Brien SM, Pavlovic D, Foo RS (2016) A simplified, langendorff-free method for concomitant isolation of viable cardiac myocytes and nonmyocytes from the adult mouse heart. Circ Res 119:909–920. https://doi.org/10.1161/CIRCRESAHA.116.309202
Adamopoulos S, Parissis J, Karatzas D, Kroupis C, Georgiadis M, Karavolias G, Paraskevaidis J, Koniavitou K, Coats AJ, Kremastinos DT (2002) Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J Am Coll Cardiol 39:653–663
Ashrafian H, Frenneaux MP, Opie LH (2007) Metabolic mechanisms in heart failure. Circulation 116:434–448. https://doi.org/10.1161/CIRCULATIONAHA.107.702795
Belardinelli R, Georgiou D, Cianci G, Purcaro A (1999) Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 99:1173–1182. https://doi.org/10.1161/01.cir.99.9.1173
Bernardo BC, Ooi JYY, Weeks KL, Patterson NL, McMullen JR (2018) Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: current knowledge and emerging concepts. Physiol Rev 98:419–475. https://doi.org/10.1152/physrev.00043.2016
Bo H, Jiang N, Ma G, Qu J, Zhang G, Cao D, Wen L, Liu S, Ji LL, Zhang Y (2008) Regulation of mitochondrial uncoupling respiration during exercise in rat heart: role of reactive oxygen species (ROS) and uncoupling protein 2. Free Radic Biol Med 44:1373–1381. https://doi.org/10.1016/j.freeradbiomed.2007.12.033
Boss O, Samec S, Desplanches D, Mayet MH, Seydoux J, Muzzin P, Giacobino JP (1998) Effect of endurance training on mRNA expression of uncoupling proteins 1, 2, and 3 in the rat. FASEB J 12:335–339. https://doi.org/10.1096/fasebj.12.3.335
Botker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, Deshwal S, Devaux Y, Di Lisa F, Di Sante M, Efentakis P, Femmino S, Garcia-Dorado D, Giricz Z, Ibanez B, Iliodromitis E, Kaludercic N, Kleinbongard P, Neuhauser M, Ovize M, Pagliaro P, Rahbek-Schmidt M, Ruiz-Meana M, Schluter KD, Schulz R, Skyschally A, Wilder C, Yellon DM, Ferdinandy P, Heusch G (2018) Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol 113:39. https://doi.org/10.1007/s00395-018-0696-8
Bourdier G, Flore P, Sanchez H, Pepin JL, Belaidi E, Arnaud C (2016) High-intensity training reduces intermittent hypoxia-induced ER stress and myocardial infarct size. Am J Physiol Heart Circ Physiol 310:H279–289. https://doi.org/10.1152/ajpheart.00448.2015
Bozi LH, Jannig PR, Rolim N, Voltarelli VA, Dourado PM, Wisloff U, Brum PC (2016) Aerobic exercise training rescues cardiac protein quality control and blunts endoplasmic reticulum stress in heart failure rats. J Cell Mol Med 20:2208–2212. https://doi.org/10.1111/jcmm.12894
Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, Cleland JG, Colucci WS, Butler J, Voors AA, Anker SD, Pitt B, Pieske B, Filippatos G, Greene SJ, Gheorghiade M (2017) Expert consensus document: mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol 14:238–250. https://doi.org/10.1038/nrcardio.2016.203
Burelle Y, Wambolt RB, Grist M, Parsons HL, Chow JC, Antler C, Bonen A, Keller A, Dunaway GA, Popov KM, Hochachka PW, Allard MF (2004) Regular exercise is associated with a protective metabolic phenotype in the rat heart. Am J Physiol Heart Circ Physiol 287:H1055–1063. https://doi.org/10.1152/ajpheart.00925.2003
Campos JC, Queliconi BB, Bozi LHM, Bechara LRG, Dourado PMM, Andres AM, Jannig PR, Gomes KMS, Zambelli VO, Rocha-Resende C, Guatimosim S, Brum PC, Mochly-Rosen D, Gottlieb RA, Kowaltowski AJ, Ferreira JCB (2017) Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure. Autophagy 13:1304–1317. https://doi.org/10.1080/15548627.2017.1325062
Chatterjee A, Seyfferth J, Lucci J, Gilsbach R, Preissl S, Bottinger L, Martensson CU, Panhale A, Stehle T, Kretz O, Sahyoun AH, Avilov S, Eimer S, Hein L, Pfanner N, Becker T, Akhtar A (2016) MOF acetyl transferase regulates transcription and respiration in mitochondria. Cell 167(722–738):e723. https://doi.org/10.1016/j.cell.2016.09.052
Conraads VM, Beckers P, Bosmans J, De Clerck LS, Stevens WJ, Vrints CJ, Brutsaert DL (2002) Combined endurance/resistance training reduces plasma TNF-alpha receptor levels in patients with chronic heart failure and coronary artery disease. Eur Heart J 23:1854–1860. https://doi.org/10.1053/euhj.2002.3239
Davidson SM, Arjun S, Basalay MV, Bell RM, Bromage DI, Botker HE, Carr RD, Cunningham J, Ghosh AK, Heusch G, Ibanez B, Kleinbongard P, Lecour S, Maddock H, Ovize M, Walker M, Wiart M, Yellon DM (2018) The 10th biennial hatter cardiovascular institute workshop: cellular protection-evaluating new directions in the setting of myocardial infarction, ischaemic stroke, and cardio-oncology. Basic Res Cardiol 113:43. https://doi.org/10.1007/s00395-018-0704-z
Doenst T, Nguyen TD, Abel ED (2013) Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113:709–724. https://doi.org/10.1161/CIRCRESAHA.113.300376
Doimo S, Fabris E, Piepoli M, Barbati G, Antonini-Canterin F, Bernardi G, Maras P, Sinagra G (2018) Impact of ambulatory cardiac rehabilitation on cardiovascular outcomes: a long-term follow-up study. Eur Heart J. https://doi.org/10.1093/eurheartj/ehy417
Ferreira JC, Rolim NP, Bartholomeu JB, Gobatto CA, Kokubun E, Brum PC (2007) Maximal lactate steady state in running mice: effect of exercise training. Clin Exp Pharmacol Physiol 34:760–765. https://doi.org/10.1111/j.1440-1681.2007.04635.x
Fillmore N, Mori J, Lopaschuk GD (2014) Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol 171:2080–2090. https://doi.org/10.1111/bph.12475
Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ (2010) A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res 107:1445–1453. https://doi.org/10.1161/CIRCRESAHA.110.223925
Gaudron P, Hu K, Schamberger R, Budin M, Walter B, Ertl G (1994) Effect of endurance training early or late after coronary artery occlusion on left ventricular remodeling, hemodynamics, and survival in rats with chronic transmural myocardial infarction. Circulation 89:402–412. https://doi.org/10.1161/01.cir.89.1.402
Gibb AA, Epstein PN, Uchida S, Zheng Y, McNally LA, Obal D, Katragadda K, Trainor P, Conklin DJ, Brittian KR, Tseng MT, Wang J, Jones SP, Bhatnagar A, Hill BG (2017) Exercise-induced changes in glucose metabolism promote physiological cardiac growth. Circulation 136:2144–2157. https://doi.org/10.1161/CIRCULATIONAHA.117.028274
Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, Schoene N, Schuler G (2000) Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA 283:3095–3101. https://doi.org/10.1001/jama.283.23.3095
Hardie DG (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8:774–785. https://doi.org/10.1038/nrm2249
Hsu CC, Fu TC, Yuan SS, Wang CH, Liu MH, Shyu YC, Cherng WJ, Wang JS (2019) High-intensity interval training is associated with improved long-term survival in heart failure patients. J Clin Med 8 doi: 10.3390/jcm8030409
Jones DA, Rathod KS, Howard JP, Gallagher S, Antoniou S, De Palma R, Guttmann O, Cliffe S, Colley J, Butler J, Ferguson E, Mohiddin S, Kapur A, Knight CJ, Jain AK, Rothman MT, Mathur A, Timmis AD, Smith EJ, Wragg A (2012) Safety and feasibility of hospital discharge 2 days following primary percutaneous intervention for ST-segment elevation myocardial infarction. Heart 98:1722–1727. https://doi.org/10.1136/heartjnl-2012-302414
Khairallah M, Labarthe F, Bouchard B, Danialou G, Petrof BJ, Des Rosiers C (2004) Profiling substrate fluxes in the isolated working mouse heart using 13C-labeled substrates: focusing on the origin and fate of pyruvate and citrate carbons. Am J Physiol Heart Circ Physiol 286:H1461–1470. https://doi.org/10.1152/ajpheart.00942.2003
Kwak HB (2013) Aging, exercise, and extracellular matrix in the heart. J Exerc Rehabil 9:338–347. https://doi.org/10.12965/jer.130049
Lehmann LH, Jebessa ZH, Kreusser MM, Horsch A, He T, Kronlage M, Dewenter M, Sramek V, Oehl U, Krebs-Haupenthal J, von der Lieth AH, Schmidt A, Sun Q, Ritterhoff J, Finke D, Volkers M, Jungmann A, Sauer SW, Thiel C, Nickel A, Kohlhaas M, Schafer M, Sticht C, Maack C, Gretz N, Wagner M, El-Armouche A, Maier LS, Londono JEC, Meder B, Freichel M, Grone HJ, Most P, Muller OJ, Herzig S, Furlong EEM, Katus HA, Backs J (2018) A proteolytic fragment of histone deacetylase 4 protects the heart from failure by regulating the hexosamine biosynthetic pathway. Nat Med 24:62–72. https://doi.org/10.1038/nm.4452
Lindsay RT, Demetriou D, Manetta-Jones D, West JA, Murray AJ, Griffin JL (2019) A model for determining cardiac mitochondrial substrate utilisation using stable (13)C-labelled metabolites. Metabolomics 15:154. https://doi.org/10.1007/s11306-019-1618-y
Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G (2018) Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314:H812–H838. https://doi.org/10.1152/ajpheart.00335.2017
Liu Y, Schneider MF (2013) Opposing HDAC4 nuclear fluxes due to phosphorylation by beta-adrenergic activated protein kinase A or by activity or Epac activated CaMKII in skeletal muscle fibres. J Physiol 591:3605–3623. https://doi.org/10.1113/jphysiol.2013.256263
Lkhagva B, Kao YH, Lee TI, Lee TW, Cheng WL, Chen YJ (2018) Activation of class I histone deacetylases contributes to mitochondrial dysfunction in cardiomyocytes with altered complex activities. Epigenetics 13:376–385. https://doi.org/10.1080/15592294.2018.1460032
Lobera M, Madauss KP, Pohlhaus DT, Wright QG, Trocha M, Schmidt DR, Baloglu E, Trump RP, Head MS, Hofmann GA, Murray-Thompson M, Schwartz B, Chakravorty S, Wu Z, Mander PK, Kruidenier L, Reid RA, Burkhart W, Turunen BJ, Rong JX, Wagner C, Moyer MB, Wells C, Hong X, Moore JT, Williams JD, Soler D, Ghosh S, Nolan MA (2013) Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. Nat Chem Biol 9:319–325. https://doi.org/10.1038/nchembio.1223
Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, Dalal H, Rees K, Singh SJ, Taylor RS (2019) Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev 1:CD003331 doi: 10.1002/14651858.CD003331.pub5
Luo YX, Tang X, An XZ, Xie XM, Chen XF, Zhao X, Hao DL, Chen HZ, Liu DP (2017) SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J 38:1389–1398. https://doi.org/10.1093/eurheartj/ehw138
Marek L, Hamacher A, Hansen FK, Kuna K, Gohlke H, Kassack MU, Kurz T (2013) Histone deacetylase (HDAC) inhibitors with a novel connecting unit linker region reveal a selectivity profile for HDAC4 and HDAC5 with improved activity against chemoresistant cancer cells. J Med Chem 56:427–436. https://doi.org/10.1021/jm301254q
Moore JBT, Tang XL, Zhao J, Fischer AG, Wu WJ, Uchida S, Gumpert AM, Stowers H, Wysoczynski M, Bolli R (2018) Epigenetically modified cardiac mesenchymal stromal cells limit myocardial fibrosis and promote functional recovery in a model of chronic ischemic cardiomyopathy. Basic Res Cardiol 114: 3 10.1007/s00395-018-0710-1
Mu J, Brozinick JT Jr, Valladares O, Bucan M, Birnbaum MJ (2001) A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7:1085–1094. https://doi.org/10.1016/s1097-2765(01)00251-9
Noma T, Nishiyama A, Mizushige K, Murakami K, Tsuji T, Kohno M, Rahman M, Fukui T, Abe Y, Kimura S (2001) Possible role of uncoupling protein in regulation of myocardial energy metabolism in aortic regurgitation model rats. FASEB J 15:1206–1208. https://doi.org/10.1096/fj.000569fje
O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL, Investigators H-A (2009) Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301:1439–1450. https://doi.org/10.1001/jama.2009.454
Ooi JY, Tuano NK, Rafehi H, Gao XM, Ziemann M, Du XJ, El-Osta A (2015) HDAC inhibition attenuates cardiac hypertrophy by acetylation and deacetylation of target genes. Epigenetics 10:418–430. https://doi.org/10.1080/15592294.2015.1024406
Piepoli MF, Conraads V, Corra U, Dickstein K, Francis DP, Jaarsma T, McMurray J, Pieske B, Piotrowicz E, Schmid JP, Anker SD, Solal AC, Filippatos GS, Hoes AW, Gielen S, Giannuzzi P, Ponikowski PP (2011) Exercise training in heart failure: from theory to practice. A consensus document of the heart failure association and the European association for cardiovascular prevention and rehabilitation. Eur J Heart Fail 13:347–357. https://doi.org/10.1093/eurjhf/hfr017
Richter EA, Hargreaves M (2013) Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93:993–1017. https://doi.org/10.1152/physrev.00038.2012
Smith JA, Collins M, Grobler LA, Magee CJ, Ojuka EO (2007) Exercise and CaMK activation both increase the binding of MEF2A to the Glut4 promoter in skeletal muscle in vivo. Am J Physiol Endocrinol Metab 292:E413–420. https://doi.org/10.1152/ajpendo.00142.2006
Smith JA, Kohn TA, Chetty AK, Ojuka EO (2008) CaMK activation during exercise is required for histone hyperacetylation and MEF2A binding at the MEF2 site on the Glut4 gene. Am J Physiol Endocrinol Metab 295:E698–704. https://doi.org/10.1152/ajpendo.00747.2007
Sun X, Zhu H, Dong Z, Liu X, Ma X, Han S, Lu F, Wang P, Qian S, Wang C, Shen C, Zhao X, Zou Y, Ge J, Sun A (2017) Mitochondrial aldehyde dehydrogenase-2 deficiency compromises therapeutic effect of ALDH bright cell on peripheral ischemia. Redox Biol 13:196–206. https://doi.org/10.1016/j.redox.2017.05.018
Sylow L, Kleinert M, Richter EA, Jensen TE (2017) Exercise-stimulated glucose uptake—regulation and implications for glycaemic control. Nat Rev Endocrinol 13:133–148. https://doi.org/10.1038/nrendo.2016.162
Trazzi S, Fuchs C, Viggiano R, De Franceschi M, Valli E, Jedynak P, Hansen FK, Perini G, Rimondini R, Kurz T, Bartesaghi R, Ciani E (2016) HDAC4: a key factor underlying brain developmental alterations in CDKL5 disorder. Hum Mol Genet 25:3887–3907. https://doi.org/10.1093/hmg/ddw231
Vega RB, Konhilas JP, Kelly DP, Leinwand LA (2017) Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab 25:1012–1026. https://doi.org/10.1016/j.cmet.2017.04.025
Ventura-Clapier R, Mettauer B, Bigard X (2007) Beneficial effects of endurance training on cardiac and skeletal muscle energy metabolism in heart failure. Cardiovasc Res 73:10–18. https://doi.org/10.1016/j.cardiores.2006.09.003
Wang Y, Chen P, Wang L, Zhao J, Zhong Z, Wang Y, Xu J (2018) Inhibition of histone deacetylases prevents cardiac remodeling after myocardial infarction by restoring autophagosome processing in cardiac fibroblasts. Cell Physiol Biochem 49:1999–2011. https://doi.org/10.1159/000493672
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C (2017) 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the american college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J Card Fail 23:628–651. https://doi.org/10.1016/j.cardfail.2017.04.014
Zhang L, Wang H, Zhao Y, Wang J, Dubielecka PM, Zhuang S, Qin G, Chin YE, Kao RL, Zhao TC (2018) Myocyte-specific overexpressing HDAC4 promotes myocardial ischemia/reperfusion injury. Mol Med 24:37. https://doi.org/10.1186/s10020-018-0037-2
Funding
This work was supported by funding from the Innovative Research Groups of the National Natural Science Foundation of China (81521001), Major Research Plan of the National Natural Science Foundation of China (91639104), a grant to Aijun Sun from the National Science Fund for Distinguished Young Scholars (81725002), National Natural Science Foundation of China (81800348) and Youth Fund of Zhongshan Hospital, Fudan University 2018ZSQN04.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, H., Jia, D., Zhang, B. et al. Exercise improves cardiac function and glucose metabolism in mice with experimental myocardial infarction through inhibiting HDAC4 and upregulating GLUT1 expression. Basic Res Cardiol 115, 28 (2020). https://doi.org/10.1007/s00395-020-0787-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-020-0787-1