Abstract
Heart-specific CD4+ T cells have been implicated in development and progression of myocarditis in mice and in humans. Here, using mouse models of experimental autoimmune myocarditis (EAM) we investigated the role of heart non-specific CD4+ T cells in the progression of the disease. Heart non-specific CD4+ T cells were obtained from DO11.10 mice expressing transgenic T cell receptor recognizing chicken ovalbumin. We found that heart infiltrating CD4+ T cells expressed exclusively effector (Teff) phenotype in the EAM model and in hearts of patients with lymphocytic myocarditis. Adoptive transfer experiments showed that while heart-specific Teff infiltrated the heart shortly after injection, heart non-specific Teff effectively accumulated during myocarditis and became the major heart-infiltrating CD4+ T cell subset at later stage. Restimulation of co-cultured heart-specific and heart non-specific CD4+ T cells with alpha-myosin heavy chain antigen showed mainly Th1/Th17 response for heart-specific Teff and up-regulation of a distinct set of extracellular signalling molecules in heart non-specific Teff. Adoptive transfer of heart non-specific Teff in mice with myocarditis did not affect inflammation severity at the peak of disease, but protected the heart from adverse post-inflammatory fibrotic remodelling and cardiac dysfunction at later stages of disease. Furthermore, mouse and human Teff stimulated in vitro with common gamma cytokines suppressed expression of profibrotic genes, reduced amount of α-smooth muscle actin filaments and decreased contraction of cardiac fibroblasts. In this study, we provided a proof-of-concept that heart non-specific Teff cells could effectively contribute to myocarditis and protect the heart from the dilated cardiomyopathy outcome.
Similar content being viewed by others
Introduction
Myocarditis most commonly results from cardiotropic infections or tissue damage, followed by the activation of heart-specific autoimmunity [3, 34]. Lymphocytic myocarditis, characterized by extensive infiltration of lymphocytes and monocytes with signs of cardiomyocyte necrosis, represents the most common type of the disease [9]. Clinical presentation of the disease ranges from acute or chronic to fulminant myocarditis [35]. In about one-third of biopsy-proven cases, myocarditis progresses to dilated cardiomyopathy (DCM), characterized by extensive fibrosis, ventricular dilation and heart failure [9, 14]. The mechanisms underlying variable clinical outcomes of myocarditis remain, however, poorly understood.
Mouse models of experimental autoimmune myocarditis (EAM) reflect key aspects of the human disease [34]. In the classical EAM model, immunization with alpha-myosin heavy chain (α-MyHC) peptide and complete Freund’s adjuvant (CFA) induces myocarditis in susceptible mice. On follow-up, inflammation in the myocardium resolves and some mice develop progressive cardiac fibrosis, ventricular dilation and heart failure [4, 5, 21]. In the α-MyHC/CFA model, autoreactive CD4+ T cells play a central role in disease induction. Mechanistically, circulating α-MyHC-reactive CD4+ T cells, which escape the negative selection in the thymus [27] set the biological basis for EAM development. Following α-MyHC/CFA immunization, antigen-presenting cells (APCs) activate α-MyHC-reactive CD4+ T cells. During the initial phase, naïve CD4+ T (Tn) cells become activated with antigen in the lymphatic system, where they undergo phenotypic changes. Tn cells convert into the effector (Teff) phenotype, expand and acquire new migratory properties [16]. In the effector phase, α-MyHC-reactive Teff migrate into cardiac tissue, where they re-encounter α-MyHC antigen. This antigen-dependent response in the heart triggers production of proinflammatory cytokines, which induce myocarditis. Accordingly, adoptive transfer of CD4+ T cells isolated from α-MyHC/CFA immunized mice can convey disease from the host to recipient [30, 36]
The adverse post-inflammatory remodeling, including an excessive fibrotic response, characterizes progression of myocarditis to DCM and end-stage heart failure [6, 19]. In fact, accumulation of cardiac fibroblasts and their transformation into myofibroblasts is the hallmark of cardiac fibrosis [24]. CD4+ T cells and particularly the Th17 subset have been implicated in transition from myocarditis to DCM phenotype [2, 29]. Insight from other models also points to autoreactive CD4+ T cells as key mediators of cardiac fibrosis [15, 26].
In contrast to well-defined antigen-dependent responses, our understanding of antigen-independent mechanisms of CD4+ T cells is limited. It has been demonstrated that such antigen-independent stimulation of Teff, called also a bystander activation, could effectively contribute to CD4+ T cell expansion and function in a specific inflammatory condition [10]. Innate stimuli [18, 32] and cytokines of the common gamma family (γc-cytokines IL-2, IL-7, IL-15 and IL-21) [33] have been recognized as mediators of the bystander activation of CD4+ T cells. So far, however, the role of antigen-independent mechanisms and heart non-specific CD4+ T cells in myocarditis remained elusive.
Methods
EAM induction and adoptive T cell transfer
EAM was induced in 6–8 weeks old BALB/c or CD45.1-tg mice using an established protocol (see Supplemental Methods). In respective experiments, EAM mice were injected intravenously with either DO11.10+ Teff, DO11.10+ Tn or TCR-M Teff (3–5 × 106 per mouse) on days 17 and 20. For adoptive transfer, 6–8 weeks old BALB/c mice were sub-lethally irradiated (5.5 Gy) using a Gammatron (Co-60) and intravenously injected with 3–5 × 106 CD45.1+ Teff, TCR-M Teff and/or DO11.10+ Teff per mouse. Sublethal irradiation depletes host’s T cells and thereby enables efficient engraftment and propagation of adoptively transferred T cells. Mice were euthanised by exposure to > 70% carbon dioxide or anesthetic overdose. All animal experiments were approved by local authorities and performed in accordance with Swiss federal and Polish law and the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (NIH Publication, 8th Edition, 2011).
Generation of T eff
Erythrocyte-lysed splenocytes/iLN cells were isolated from α-MyHC/CFA immunized CD45.1-tg mice at day 17 (CD45.1+ Teff), untreated TCR-M-tg (TCR-M Teff) or untreated DO11.10-tg (DO11.10+ Teff) mice. Teff were generated by ex vivo CD4+ T cell activation with the respective antigen in the presence of APCs for 72 h (see Supplemental Methods). Activated CD4+ T cells purified by MACSorting using mouse anti-CD4 magnetic beads (Miltenyi) were used as Teff for adoptive transfer experiments. For in vitro use, Teff isolated from spleens/LN and hearts were additionally purified by FACSorting for CD4+CD44hiCD62Llow cells. Tn were isolated from spleens of DO11.10-tg mice and purified using CD4 positive MACSorting and FACSorting for CD4+CD44lowCD62Lhi cells using FACS Aria III.
Human CD4+ T cell subsets were sorted from peripheral blood mononuclear cells (PBMCs) isolated from peripheral blood buffy coats of healthy donors (Blutspende Zurich, Switzerland), obtained with informed consent in accordance with the Declaration of Helsinki. PBMCs were isolated using Lympholyte (Cederlane) density gradient separation according to manufacturer’s instructions. CD4+ T cells were enriched by MACSorting using human anti-CD4 magnetic beads (Miltenyi). CD4+ T cell subpopulations were further sorted using FACS Aria III (BD Bioscience) based on the respective surface marker phenotypes: CD45RA+CD45RO−CCR7+CD27+ (Tn) and CD45RA−CD45RO+CCR7−CD27− (Teff).
CD4+ T cell proliferation
CD4+ T cells were isolated from mouse erythrocyte-lysed splenocytes/LNs or human PBMCs by MACS or sorted from hearts digested with Liberase (Roche). CD4+ T cell subpopulations were isolated using FACS Aria III. Cells were labeled with 2.5 μM CFSE or 5 μM CellTrace Violet Cell Proliferation Kit (both Life Technologies) and either injected into recipient mice or cultured in vitro in the respective conditions (see Supplemental Methods). Cell proliferation was analyzed with LSRII Fortessa analyzer (BD Bioscience) and FlowJo software (Tree Star) using the Proliferation platform.
CD4+ T cell and cardiac fibroblast co-culture
CD4+ T cell subpopulations were isolated from mouse or human CD4+ T cell fractions with FACS Aria III cell sorter and cultured on confluent mouse or human cardiac fibroblasts, respectively (see Supplemental methods).
Flow cytometry and cell sorting
Single cell suspensions were prepared either from digested mouse hearts, erythrocyte-lysed splenocytes/LNs, or mouse and human PBMCs (see Supplemental methods). Freshly isolated or cultured cells were subjected to CD4 positive MACSorting and/or stained with the appropriate combination of fluorochrome-conjugated mouse/human antibodies (see Supplemental methods). Cells were analyzed with LSRII Fortessa analyzer. In the respective experiments, T cell fractions were sorted with FACSAria III (purity > 98%). For intracellular protein staining, cardiac fibroblasts were stained with anti-mouse/human αSMA (Sigma) and anti-human collagen I-biotin (Acris) antibodies (see Supplemental methods) and analyzed with LSRII Fortessa analyzer.
Histopathology, immunohistochemistry and immunocytochemistry
Conventional hematoxylin/eosin and Masson’s trichrome staining were used to assess cardiac inflammation and fibrosis, respectively (see Supplemental methods). Immunopositive cells (for CD3, CD45, CD45.1, CD90.1) and fibrotic markers (Masson’s Trichrome; αSMA; periostin, vimentin, both Abcam) were quantified using Olympus BX51 microscope and cellSens (Olympus) or Fiji software. Formalin fixed, paraffin embedded human heart tissue from 10 lymphocytic myocarditis patients were obtained from the Biobank of the Institute for Pathology and Neuropathology, University Hospital Tubingen in accordance with local ethical regulations and stained using rabbit anti-human CD4 (EPR6855, Abcam), mouse anti-human CD45RO (UCHL-1, eBioscience) and mouse anti-human CD45RA (HI100, eBioscience); see Supplemental Methods. Image acquisition was performed with a Zeiss AxioObserver Z1 widefield microscope and processed by ImageJ.
Human and mouse cardiac fibroblasts were stained with anti-mouse/human αSMA antibody and phalloidin (Sigma); see Supplemental methods. Image acquisition was performed with a Leica DM IRE2 microscope equipped with the Nomarski Interference Contrast and the Operetta high-content screening platform (Perkin–Elmer).
Echocardiography
Transthoracic echocardiography was performed using a Vevo 2100 system equipped with 30-MHz transducer (VisualSonics). Anesthesia was induced by 5% isoflurane and confirmed by the absence of the withdrawal reflex of one of the hind paws. During echocardiogram acquisition isofluorane was reduced to 1.5–2%. The heart was imaged in the bidimensional (2-D) mode, in the parasternal long-axis, short-axis and apical 4-chamber views. Detailed description is available in the Supplemental methods.
Statistics
Where relevant, data were analyzed by unpaired, two-tailed Student’s t test, one-way ANOVA followed by the Dunnett’s post hoc test (for normally distributed data), or by Mann–Whitney test (for nonparametric data). Differences were considered statistically significant for p < 0.05. All analyses were performed with GraphPad Prism 6 software and values are expressed as mean with SEM or SD.
For the extensive materials and methods we refer to the Supplemental methods.
Results
CD44hiCD62Llow cells represent the effector subset of CD4+ T cells in EAM
To characterize the phenotype of inflammatory CD4+ T cells in human myocarditis, we analyzed cardiac biopsies of 10 patients with acute lymphocytic, virus-negative myocarditis (Supp. Table 1). Biopsies were evaluated using histology and immunofluorescence staining for detection of CD4, CD45RA (expressed on resting/naïve T cells) and CD45RO (expressed on activated and memory T cells). As shown by the representative staining (Supp. Fig. 1), nearly all CD4+ cells stained positive for CD45RO and negative for CD45RA, indicating that heart infiltrating CD4+ T cells in patients with acute lymphocytic myocarditis almost exclusively express a phenotype of activated Teff. Next, we characterized heart-specific CD4+ T cells in the mouse model of EAM. CD4+ T cells were isolated from blood and lymphoid organs of mice immunized with α-MyHC and CFA (Fig. 1a) 5–7 days after immunization (before myocarditis onset) and were further stimulated ex vivo for 3–5 days with α-MyHC antigen in the presence of APCs. Cells isolated from inguinal lymph nodes (LNs) located at the site of α-MyHC antigen injection showed the most pronounced α-MyHC-specific proliferation, suggesting local activation of heart-reactive CD4+ T cells (Supp. Fig. 2). To identify α-MyHC-responsive subsets, we sorted four populations of CD4+ T cells, based on their surface expression of CD44 and CD62L markers (Fig. 1b). A robust proliferative response to α-MyHC was observed only for the CD44hiCD62Llow Teff population (Fig. 1c, Supp. Fig. 3). During the early phase of myocarditis (EAM day 17), α-MyHC-reactive Teff were detectable not only in inguinal LNs, but also in mediastinal LNs, mesenteric LNs, blood, spleen and the heart (Fig. 1d). As expected, nearly all CD4+ T cells infiltrating cardiac tissue displayed the CD44hiCD62Llow Teff phenotype. When isolated and exposed to α-MyHC antigen presented by APCs, these heart-infiltrating Teff extensively proliferated and produced inflammatory cytokines (Fig. 1d, e).
Teff phenotype of heart-reactive CD4+ T cells in α-MyHC/CFA-induced EAM. BALB/c mice received subcutaneous injection of α-MyHC/CFA into the right inguinal region as shown in panel a. CD4+ T cells obtained from the right iLN 7 days after immunization were sorted into four fractions, labelled with CFSE and cultured for 3 days on APCs with or without α-MyHC. Panel b shows the gating strategy for CD4+ T cell subpopulations in viable CD4+ CD45+ population isolated from iLN: Teff effector, Tcm central memory, Tn naïve and Tun undefined T cells. Percentage of proliferating CFSE-labelled CD4+ T cell subpopulations is quantified in panel c. Data are representative of three independent experiments, *p < 0.05 (unpaired Student’s t test). Panel d shows representative phenotype analyses of viable CD4+CD45+ cells and frequencies of CD4+CD44hiCD62Llow cells (Teff) in indicated organs at day 17 (active myocarditis). Teff from respective organs were sorted, labelled with CFSE and stimulated with α-MyHC peptide in presence of APCs for 5 days. Proliferation is shown as dilution of CFSE dye in viable CD3+CD4+ population. Supernatant levels of indicated cytokines secreted by Teff isolated from the heart are presented in panel e. Data are representative of two independent experiments. iLN inguinal lymph node, mesLN mesenteric lymph node, medLN mediastinal lymph node, n.d. not detected
Heart non-specific T eff effectively accumulate in the inflamed myocardium
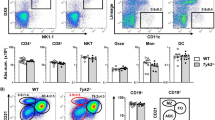
Adoptive transfer of α-MyHC-re-stimulated CD4+ T cells isolated from inguinal LNs and spleens of α-MyHC/CFA immunized wild-type donor mice expressing CD45.1+ alloantigen, effectively induced myocarditis in sub-lethally irradiated CD45.2+ wild-type recipients (Fig. 2a). More than 80% of donor CD45.1+CD4+ cells displayed Teff phenotype and these cells effectively accumulated in the hearts of the recipients (Fig. 2b), confirming the potential of heart-reactive Teff to trigger myocarditis.
Cells with Teff signature effectively infiltrate cardiac tissue. Indicated Teff (3–5 × 106) were adoptively transferred into sub-lethally irradiated BALB/c recipients. Top panel a shows representative heart histology of the respective recipient 10 days after adoptive transfer. Bottom panel a shows immunohistochemistry for CD45.1 and CD90.1 in hearts of indicated recipients. Scale bars = 100 μm. Panel b shows analysis of donor CD4+CD45.1+ cells in blood (top) and heart (bottom) 10 days after adoptive transfer of CD45.1+ Teff. Analysis of donor CD45.1+ and DO11.10+ Teff in indicated organs 10 days after adoptive transfer is shown in panel c and quantification of total heart-inflammatory CD3+CD4+ DO11.10+ T cells is presented in panel d. Arrows show gating strategies and numbers indicate percentage of cells in the adjacent gate. Data are representative of six mice, p value calculated with unpaired Student’s t-test. CD45.1+ Teff—CD4+ cells from α-MyHC-stimulated (72 h) splenocytes/iLN cells of α-MyHC/CFA immunized CD45.1-tg mice (EAM d17); TCR-M Teff—CD4+ cells from α-MyHC-stimulated (72 h) splenocytes of TCR-M-tg (CD90.1+) mice; DO11.10+ Teff—CD4+ cells from OVA-stimulated (72 h) splenocytes of DO11.10-tg mice
We adopted this T cell transfer model to study mechanisms of T cell trafficking to the heart. To address the differential role of heart-specific and heart non-specific T cells, we used CD4+ T cells from TCR-M and DO11.10 transgenic mouse strains, which expressed transgenic TCRs on their CD4+ T cells. In TCR-M mice, T cells react exclusively to α-MyHC antigen and these mice develop spontaneous myocarditis [31]. On the other hand, chicken ovalbumin (OVA)-reactive DO11.10+ CD4+ T cells from DO11.10 transgenic mice were used as a source of heart non-specific CD4+ T cells. To obtain Teff for the adoptive transfer experiments, splenic and iLN cells from the respective donors were activated with their respective cognate antigen for 72 h and CD4+ T cells were sorted for experiments. Adoptive transfer of heart non-specific DO11.10+ Teff failed to trigger inflammation (Fig. 2a), but DO11.10+ CD4+ T cells entered to a limited extent non-inflamed cardiac tissue (Fig. 2c). In contrast, heart-reactive TCR-M Teff (which express CD90.1 alloantigen [31]) efficiently accumulated in the recipient hearts after adoptive transfer, although they induced myocarditis less efficiently than CD45.1+CD4+ T cells obtained from α-MyHC/CFA immunized mice (Fig. 2a).
Next, we co-transferred heart non-specific DO11.10+ Teff together with α-MyHC-re-stimulated CD45.1+ Teff from α-MyHC/CFA immunized mice. In inflamed hearts, we found not only myocarditis-inducing α-MyHC-reactive CD45.1+ Teff, but also a massive accumulation of heart non-specific DO11.10+ Teff (Fig. 2c). The total amount of DO11.10+ Teff was substantially higher in the inflamed hearts compared to the non-inflamed organs 10 days after adoptive transfer (Fig. 2d), demonstrating that the inflammatory response effectively attracted heart non-specific Teff into the heart.
To compare antigen-dependent and antigen-independent expansion of Teff in the inflamed myocardium, we co-delivered α-MyHC-reactive CD45.1+ Teff from α-MyHC/CFA immunized mice together with heart-specific TCR-M Teff and heart non-specific DO11.10+ Teff. Four days after adoptive transfer, we detected all three injected Teff in the recipient heart and TCR-M cells showed the most efficient heart-specific accumulation (Fig. 3a, b). However, at the peak of inflammation at day 10, DO11.10+ CD4+ T cells represented more than 50% of Teff in inflamed hearts, becoming the dominant heart-infiltrating CD4+ T cell population (Fig. 3b).
Differential kinetic of antigen-dependent and antigen-independent expansion of Teff during myocarditis. CD45.1+ Teff (4 × 106), TCR-M Teff (4.5 × 106) and DO11.10+ Teff (4.5 × 106) cells were co-delivered into sub-lethally irradiated BALB/c recipients. Panel a shows analysis of donor CD4+ T cell frequency in indicated organs 4 days after adoptive transfer. Arrows show gating strategies and numbers indicate percentage of cells in the adjacent gate. Data are representative of four mice per time point. Panel b presents frequencies of indicated donor Teff among heart-infiltrating CD4+ T cells at days 4 and 10 after adoptive transfer. Donor CD45.1+, TCR-M and DO11.10+ Teff were generated as described in Fig. 2
To better characterize antigen-dependent and antigen-independent CD4+ T cell responses, splenocytes of mice adoptively co-transferred with CD45.1+ Teff (obtained from α-MyHC/CFA immunized mice) and DO11.10+ Teff were isolated and cultured with or without α-MyHC peptide for 24 h. Next, CD45.1+ Teff and DO11.10+ Teff were sorted and analyzed for the whole genome transcriptomics (Fig. 4a). The analysis showed differential response of CD45.1+ Teff and DO11.10+ Teff to α-MyHC stimulation (Fig. 4b). Antigen-dependent stimulation of CD45.1+ Teff resulted in response with Th cytokines (Il17a, Il17f, Ifng, Il5, Il22), chemokines (Ccl20, Cxcl10) and hematopoietic growth factors (Csf1, Csf2, Fig. 4c). In contrast, antigen-independent response of DO11.10+ Teff resulted in the up-regulation of inflammatory cytokines of TNF superfamily (Tnf, Lta), IL-6 family (Osm, Lif) and others (Mif, Il4, Flt3l, Fig. 4c).
Transcriptomic analysis of heart-specific and heart non-specific Teff cells. CD45.1+ Teff (3 × 106) and DO11.10+ Teff (3 × 106) cells were co-delivered into sub-lethally irradiated BALB/c recipients. 10 days later, the whole splenocytes were isolated, stimulated with α-MyHC (or kept unstimulated for controls) for 24 h and CD45.1+ and DO11.10+ CD4+ cells were sorted for the whole transcriptome sequencing (RNAseq). Panel a illustrates the experimental setup. The number and the overlap of all differentially expressed genes (p < 0.05, log2ratio > 0.5 or < −0.5) in response to α-MyHC in CD45.1+ Teff and in DO11.10+ Teff is shown in panel b. Panel c shows heatmap of genes of the cytokine activity GO:0005125 category up-regulated (p < 0.05, log2ratio > 0.75) in CD45.1+ Teff and/or DO11.10+ Teff in response to α-MyHC treatment. Panel d shows heatmap of genes of the extracellular space GO:0005615 category differentially expressed (p < 0.05, log2ratio > 0.75 or < − 0.75) between CD45.1+ Teff and DO11.10+ Teff under stimulatory (+α-MyHC) condition. Cells obtained from 3 independent mice were used for RNAseq. Donor CD45.1+ and DO11.10+ Teff were generated as described in Fig. 2
Heart non-specific T eff do not affect myocarditis severity, but protect from post-inflammatory fibrosis and cardiac dysfunction
To address functional consequences of differential CD4+ T cell responses, we analyzed heart sections of mice receiving α-MyHC-reactive CD45.1+ Teff (obtained from α-MyHC/CFA immunized mice) with or without heart non-specific DO11.10+ Teff. Accumulation of heart non-specific DO11.10+ Teff neither affected myocarditis severity scores, nor the total number of CD3+ and CD45+ infiltrates in cardiac sections (Fig. 5). Next, we addressed the impact of heart non-specific Teff on cardiac fibrosis in α-MyHC/CFA-immunized BALB/c mice. Immunized mice received two doses of either antigen-activated DO11.10+ or TCR-M Teff, or non-activated DO11.10+ Tn shortly before the onset of myocardial fibrosis at d17 and d20. Both DO11.10+ and TCR-M Teff infiltrated the inflamed myocardium (Supp. Fig. 4). Injection of DO11.10+ Teff protected the heart from post-inflammatory fibrosis at later stage (day 40), as indicated by Masons Trichrome staining (Fig. 6a, b), reduced hydroxyproline content (Fig. 6c), expression of profibrotic genes (Suppl. Fig. 5) and the amount of αSMA-positive and periostin-postive myofibroblasts (Fig. 6a, b). Instead, injection of TCR-M Teff or DO11.10+ Tn did not affect post-inflammatory fibrosis (Suppl. Fig. 6). Next, we addressed whether DO11.10+ Teff cells can affect cardiac function. As expected, echocardiography analysis showed significantly compromised cardiac function in α-MyHC/CFA-immunized mice at day 40 with ejection fraction reduced from 58% before immunisation to 40% at d40 and fractional shortening from 29% before immunisation to 18% at d40. Treatment with DO11.10+ Teff cells prevented development of cardiac dysfunction in most animals (Fig. 6d and Suppl. Fig. 7). This better cardiac functions in mice receiving DO11.10+ Teff cells were paralleled by a lower heart weights comparing with α-MyHC/CFA-immunized mice treated with PBS (Fig. 6e). All these data suggest that heart non-specific Teff cells can prevent development of DCM phenotype in the EAM model.
Heart non-specific Teff do not affect myocarditis severity. Myocarditis-inducing CD45.1+ Teff (4–6 × 106) were injected with or without DO11.10+ Teff (4–6 × 106) into sub-lethally irradiated BALB/c recipients. Representative histology (top) and immunohistochemistry for CD45 (middle) and CD3 (bottom) in hearts of indicated recipients 10 days after adoptive transfer are shown in panel a. Scale bar = 100 μm. Myocarditis severity scores and quantification of heart-infiltrating CD3+ and CD45+ cells are presented in panel b. p values calculated with unpaired Student’s t test. Donor CD45.1+ and DO11.10+ Teff were generated as described in Fig. 2
Heart non-specific Teff prevents postinflammatory fibrosis and cardiac dysfunction. α-MyHC/CFA immunized BALB/c recipients received control solution (PBS) or DO11.10+ Teff (4–5 × 106) at days 17 and 20 of EAM and were analyzed for fibrotic and functional changes at day 40. Panel a shows representative Masson’s Trichrome staining and immunohistochemistry stainings for periostin, vimentin and αSMA in heart sections. Scale bar = 200 μm. Quantifications of the fibrotic and immunopositive areas are shown in panel b. p values calculated with the Mann–Whitney test. Panel c shows hydroxyproline content at day 40. Echocardiography measurements were performed in mice before immunization (d0) and at d40 of EAM. Panel d shows difference for d40 and d0 (Δ = d40–d0) for selected parameters. EF ejection fraction, FS fractional shortening, CO cardiac output. p values calculated with the Student’s t test. All echocardiographic parameters at d0 and d40 are available in the Suppl. Table 2. Analysis of heart weights at d40 is shown in panel e. HW/TL heart weight to tibial length. p values calculated with the Student’s t test. Donor DO11.10+ Teff were generated as described in Fig. 2
Bystander activation of T eff suppress myofibroblast phenotype of mouse and human cardiac fibroblasts
The transcriptomic analysis showed that in contrast to heart-specific CD45.1+ CD4+ T cells, unstimulated DO11.10+ Teff expressed low levels of extracellular molecules. In stimulatory condition (+α-MyHC), however, a set of 56 genes encoding extracellular factors became specifically upregulated in heart non-specific DO11.10+ Teff, but not in α-MyHC-reactive CD45.1+ Teff (Fig. 4d). This suggested that antigen-independent stimulation of Teff might trigger production of certain antifibrotic factors. To address this option, we co-cultured DO11.10+ Teff with mouse cardiac fibroblasts in the presence or absence of IL-2, IL-7, IL-15 and IL-21 (further called γc-cytokines). Analysis of profibrotic markers in cardiac fibroblasts showed that DO11.10+ Teff stimulated with γc-cytokines effectively suppressed expression of profibrotic genes Acta2, Col1a1 and Fn1 and reduced formation of αSMA fibers (Supp. Fig. 8).
Next, we addressed, whether or not, human Teff can affect the myofibroblast phenotype of human cardiac fibroblasts. A panel of phenotypic markers (CD45RO, CD45RA, CCR7, CD27) allows identification of CD4+ T cell subsets in humans (Supp. Fig. 9A). Using these markers, we sorted Tn (CD45RO−CD45RA+CCR7+CD27+) and Teff (CD45RO+CD45RA−CCR7−CD27−) from human peripheral blood and analyzed their proliferation in vitro. In contrast to Tn cells, human Teff showed some proliferation in response to γc-cytokine stimulation (Supp. Fig. 9B). In co-cultures with human cardiac fibroblasts, only Teff stimulated with γc-cytokines down-regulated expression of profibrotic genes ACTA2, COL1A1, and FN1 in fibroblasts (Fig. 7a). Furthermore, in co-cultures with Teff, cardiac fibroblasts showed less αSMA-positive fibers (Fig. 7b). The reduced αSMA and collagen-I protein content was confirmed by intracellular flow cytometry analysis (Fig. 7c). Since αSMA is implicated in the contractile function of fibroblasts, we observed impaired contraction of cardiac fibroblasts upon pre-treatment with γc-cytokine-stimulated Teff (Fig. 7d). Suppression of myofibroblast signature in cardiac fibroblasts by Teff was, however, not associated with changes in proliferation or apoptosis (Supp. Fig. 10). Our data suggested that the antifibrotic effect of Teff required stimulation with γc-cytokines.
Human Teff stimulated with γc-cytokines suppress myofibroblast signature of human cardiac fibroblasts. Human cardiac fibroblasts were co-cultured with sorted human Tn and Teff in the presence of IL-2, IL-7, IL-5 and IL-21 (γc-cytokines) for 7 days (human Tn and Teff were isolated as shown in Supp. Fig. 7). Panel a shows gene expression levels (normalized to GAPDH) in cardiac fibroblasts at day 7 at indicated conditions. Data are representative of three independent experiments, p values calculated with one-way ANOVA, * p < 0.05 the Dunnett post hoc test compared to fibroblasts with γc-cytokines. Panel b shows representative immunofluorescence for αSMA (green) and F-actin (phalloidin staining, red) and quantification of αSMA-positive cardiac fibroblasts cultured for 7 days in presence of γc-cytokines with or without human Teff. Data are representative of three independent experiments, p value calculated with unpaired Student’s t-test. Scale bar = 50 μm. Panel c shows relative intracellular levels of αSMA and collagen I in cardiac fibroblasts in presence of γc-cytokines and Teff measured with flow cytometry. Data are shown as fold change from three independent experiments, p values calculated with unpaired Student’s t-test. Panel d shows contraction of cardiac fibroblasts in 3D collagen matrix after 5 days. Cardiac fibroblasts were pretreated with γc-cytokine-stimulated Tn or Teff for 7 days. Data are representative of two independent experiments, p values calculated with one-way ANOVA, * p < 0.05 the Dunnett post hoc test compared to fibroblasts with γc-cytokines. ACTA2 alpha smooth muscle actin, COL1A1 collagen I, FN1 fibronectin, VIM vimentin
Discussion
Observations from animal models have pointed to a critical role of CD4+ T cells and heart-specific autoimmunity in the development of myocarditis and cardiac fibrosis, however, surprisingly little experimental data addressed migration and expansion of autoreactive CD4+ T cells in the EAM model. In α-MyHC/CFA immunized mice, essentially only α-MyHC-reactive T cells could be activated with their antigen and turn into Teff phenotype. Our mouse data confirmed that α-MyHC-reactive Teff cells efficiently expand and accumulate in the inflamed cardiac tissue. Furthermore, we demonstrated Teff phenotype of heart infiltrating CD4+ T cells also in human myocarditis. Animals housed under high hygiene standards lack, however, other antigen-activated Teff cells, which are commonly present in humans due to exposure to various infectious agents. To address migratory properties of heart non-specific Teff, we used a T cell adoptive transfer model of DO11.10+ CD4+ T cells activated in vitro with OVA antigen and found that Teff cardiotropism was not restricted to heart-specific CD4+ T cells. It seems that expression of specific homing molecules, rather than antigen-specificity primary controls migration of CD4+ T cells into the cardiac tissue. In fact, such cardiotropic signature has been recently suggested [23]. In our model, we observed more efficient accumulation of heart-specific CD4+ T cells during the early phase of inflammation, while heart non-specific CD4+ T cells were more prevalent at later phases. This differential kinetics could be the result of differential cell expansion/survival in the periphery and/or differential expression of homing molecules by these two types of CD4+ T cells.
Antigen-specific responses of α-MyHC-reactive CD4+ T cells are crucial for myocarditis induction. Our results pointed to Th1/Th17 skewed antigen-dependent response of heart-specific Teff. On the one hand, Th1 cytokines are considered to be involved in the myocarditis induction at a very early stage [37]. Th17 polarized responses, on the other hand, have been associated with worsened myocarditis outcome due to increased cardiac fibrosis and heart failure in both, EAM and human patients [2, 29, 31]. These data might suggest that myocardial fibrosis and DCM phenotype after α-MyHC/CFA immunization is a direct consequence of the Th1/Th17 polarisation of α-MyHC-reactive CD4+ T cells. Furthermore, adoptive transfer experiments demonstrated that heart-specific CD4+ T cell mediated myocarditis induction paralleled a strong bystander activation of heart non-specific CD4+ T cells. Such antigen-independent expansion of activated Teff has been described in the context of viral infections [13], in breast cancer [17] and after vaccination [10]. Insights from other disease models point to various innate stimuli [18, 32] and γc-cytokines [12, 33] as potential mediators. We assume that in myocarditis, mainly γc-cytokines produced by heart-specific CD4+ T cells promote the antigen-independent response of heart non-specific CD4+ T cells.
In our model heart non-specific Teff effectively competed with heart-specific Teff and eventually partially replaced them in the inflamed myocardium at later stages of disease. Our data showed that heart non-specific Teff produced a distinct set of cytokines compared to heart-specific Teff. This is not surprising, considering that heart-specific Teff responded to antigen (α-MyHC), while heart non-specific Teff showed antigen-independent response only. Interestingly, heart non-specific Teff expressed high levels of pro-inflammatory cytokines, such as Tnf, Lta or Mif. This result could explain why heart non-specific Teff did not affect the severity of the acute cardiac inflammatory response.
In contrast, adoptive transfer of heart non-specific Teff successfully prevented development of post-inflammatory fibrosis in the EAM model. We found that bystander activation of the heart non-specific Teff induced production of surprisingly high number of extracellular molecules. Some of these factors might be antifibrotic and thus actively inhibit fibrotic processes. Sfrp2 represents an example of such antifibrotic agent, which was specifically expressed by activated heart non-specific Teff. We have recently demonstrated that systemic administration of sFRP2 during acute phase of myocarditis prevented development of fibrotic changes in the EAM model by blocking profibrotic Wnt signalling [5]. The in vitro data have also pointed to the secretion of antifibrotic factors by mouse and human Teff activated in antigen-independent manner. Furthermore, heart non-specific Teff by replacing heart-specific Teff could reduce cardiac levels of profibrotic cytokines, such as IL-17A, in the inflamed heart. We consider these two mechanisms being responsible for antifibrotic effect of the heart non-specific Teff (Supp. Fig. 11). Noteworthy, the analysis of cytokine and extracellular factors production by heart-specific and heart non-specific Teff was performed on restimulated splenic cells only. Lack of data obtained from the heart-infiltrating Teff is a major limitation of this study. We did not perform such analysis due to technical difficulties.
Cardiac fibrosis is a key driver of DCM [6]. Accordingly, in the EAM model, development of cardiac fibrosis was associated with impaired cardiac function and increased heart size [4]. Our data confirmed that function of fibrotic heart are significantly compromised in the α-MyHC/CFA EAM model (ejection fraction reduced from 58% before immunisation to 40% at d40). It is, therefore, not surprising that antifibrotic effect of heart non-specific Teff protected cardiac function and development of DCM phenotype. It should be noted that interplay between cardiac inflammation and fibrosis is of high relevance, not only in myocarditis, but also in other heart diseases [1]. These results further stress the importance of antifibrotic therapies for the positive outcome of myocarditis. A number of antifibrotic agents successfully prevented development of DCM phenotype in animal models [4, 5, 20, 25].
In contrast to the EAM model, heart-specific T cells are usually not the primary inducers of myocarditis in humans [7, 22]. Instead, viruses and parasites represent the most common triggers of lymphocytic infiltration in the myocardium and heart-specific autoimmunity is predominantly associated with the disease progression [3, 8]. By shedding light on the importance of antigen-independent responses, our data provide a link between insights from animal models and observations in humans. Accordingly, our findings can potentially explain the difficulties in identifying heart-specific CD4+ T cells in human myocarditis. We suggest that inflammation-driven, antigen-independent migration and proliferation of Teff could account for extensive cardiac accumulation of heart non-specific CD4+ T cells. So far, there are no concrete data on the numbers of heart-reactive CD4+ T cells in human myocarditis, but considering that heart-specific CD4+ T cells are rather infrequent in humans, we hypothesize that a significant proportion of heart-infiltrating CD4+ T cells in myocarditis patients do not react against cardiac antigens. From this point of view, and given the antifibrotic and cardioprotective properties of heart non-specific Teff, significant infiltrations with CD4+ T lymphocytes could even indicate favourable outcome in myocarditis. Indeed, most patients with lymphocytic myocarditis recover, while only some develop DCM phenotype [11, 28]. The observation that chronic inflammation and long-term antigen activation alters Teff responses [16] supports our idea that a bystander activation of CD4+ T cell protects myocarditis patients from progression to heart failure.
So far, investigations addressing the role of CD4+ T cells in myocarditis focused on heart-specific autoimmunity. Our data underline potential importance of antigen-independent CD4+ T cell responses in the progression from myocarditis to DCM and attribute them a cardioprotective role. Individual differences in the capability of the immune system to express such functions could explain variable clinical outcomes of lymphocytic myocarditis. The relevance of our findings has to be validated in human patients, since EAM model only partially mimics the complexity of naturally occurring myocarditis. At the same time, further research is needed to elucidate molecular mechanisms and to enable translation of these findings into appropriate therapeutic approaches.
Abbreviations
- EAM:
-
Experimental autoimmune myocarditis
- DCM:
-
Dilated cardiomyopathy
- α-MyHC:
-
Alpha-myosin heavy chain
- CFA:
-
Complete Freud’s adjuvant
- T n/T eff :
-
Naïve/effector CD4+ T cell
- TCR:
-
T cell receptor
- APC:
-
Antigen presenting cell
- γc-cytokines:
-
Common gamma cytokines
- IL:
-
Interleukin
- αSMA:
-
α-Smooth-muscle actin
- LN:
-
Lymph node
- OVA:
-
Ovalbumin
- Th:
-
CD4+ T helper cell
References
Bacmeister L, Schwarzl M, Warnke S, Stoffers B, Blankenberg S, Westermann D, Lindner D (2019) Inflammation and fibrosis in murine models of heart failure. Basic Res Cardiol 114(3):19. https://doi.org/10.1007/s00395-019-0722-5
Baldeviano GC, Barin JG, Talor MV, Srinivasan S, Bedja D, Zheng D, Gabrielson K, Iwakura Y, Rose NR, Cihakova D (2010) Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res 106:1646–1655. https://doi.org/10.1161/circresaha.109.213157
Błyszczuk P (2019) Myocarditis in humans and in experimental animal models. Front Cardiovasc Med 6:64. https://doi.org/10.3389/fcvm.2019.00064
Blyszczuk P, Berthonneche C, Behnke S, Glönkler M, Moch H, Pedrazzini T, Lüscher TF, Eriksson U, Kania G (2013) Nitric oxide synthase 2 is required for conversion of pro-fibrogenic inflammatory CD133(+) progenitors into F4/80(+) macrophages in experimental autoimmune myocarditis. Cardiovasc Res 97:219–229. https://doi.org/10.1093/cvr/cvs317
Blyszczuk P, Müller-Edenborn B, Valenta T, Osto E, Stellato M, Behnke S, Glatz K, Basler K, Lüscher TF, Distler O, Eriksson U, Kania G (2017) Transforming growth factor-β-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. Eur Heart J 38:1413–1425. https://doi.org/10.1093/eurheartj/ehw116
Blyszczuk P, Valaperti A, Eriksson U (2008) Future therapeutic strategies in inflammatory cardiomyopathy: insights from the experimental autoimmune myocarditis model. Cardiovasc Hematol Disord Drug Targets 8:313–321. https://doi.org/10.5167/uzh-11964
Caforio AL, Angelini A, Blank M, Shani A, Kivity S, Goddard G, Doria A, Schiavo A, Testolina M, Bottaro S, Marcolongo R, Thiene G, Iliceto S, Shoenfeld Y (2015) Passive transfer of affinity-purified anti-heart autoantibodies (AHA) from sera of patients with myocarditis induces experimental myocarditis in mice. Int J Cardiol 179:166–177. https://doi.org/10.1016/j.ijcard.2014.10.165
Caforio AL, Marcolongo R, Jahns R, Fu M, Felix SB, Iliceto S (2013) Immune-mediated and autoimmune myocarditis: clinical presentation, diagnosis and management. Heart Fail Rev 18:715–732. https://doi.org/10.1007/s10741-012-9364-5
Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM, Diseases ESoCWGoMaP (2013) Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 34(2636–2648):2648a–2648d. https://doi.org/10.1093/eurheartj/eht210
Di Genova G, Savelyeva N, Suchacki A, Thirdborough SM, Stevenson FK (2010) Bystander stimulation of activated CD4+ T cells of unrelated specificity following a booster vaccination with tetanus toxoid. Eur J Immunol 40:976–985. https://doi.org/10.1002/eji.200940017
Felker GM, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Baughman KL, Hare JM (2000) Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol 36:227–232. https://doi.org/10.1016/s0735-1097(00)00690-2
Guo L, Junttila IS, Paul WE (2012) Cytokine-induced cytokine production by conventional and innate lymphoid cells. Trends Immunol 33:598–606. https://doi.org/10.1016/j.it.2012.07.006
Haas A, Zimmermann K, Oxenius A (2011) Antigen-dependent and -independent mechanisms of T and B cell hyperactivation during chronic HIV-1 infection. J Virol 85:12102–12113. https://doi.org/10.1128/jvi.05607-11
Heymans S, Eriksson U, Lehtonen J, Cooper LT (2016) The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol 68:2348–2364. https://doi.org/10.1016/j.jacc.2016.09.937
Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S (2012) Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125:1652–1663. https://doi.org/10.1161/circulationaha.111.044164
Jelley-Gibbs DM, Lepak NM, Yen M, Swain SL (2000) Two distinct stages in the transition from naive CD4 T cells to effectors, early antigen-dependent and late cytokine-driven expansion and differentiation. J Immunol 165:5017–5026. https://doi.org/10.4049/jimmunol.165.9.5017
Joncker NT, Marloie MA, Chernysheva A, Lonchay C, Cuff S, Klijanienko J, Sigal-Zafrani B, Vincent-Salomon A, Sastre X, Lantz O (2006) Antigen-independent accumulation of activated effector/memory T lymphocytes into human and murine tumors. Int J Cancer 118:1205–1214. https://doi.org/10.1002/ijc.21472
Kamath AT, Sheasby CE, Tough DF (2005) Dendritic cells and NK cells stimulate bystander T cell activation in response to TLR agonists through secretion of IFN-alpha beta and IFN-gamma. J Immunol 174:767–776. https://doi.org/10.4049/jimmunol.174.2.767
Kania G, Blyszczuk P, Eriksson U (2009) Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc Med 19:247–252. https://doi.org/10.1016/j.tcm.2010.02.005
Kania G, Blyszczuk P, Stein S, Valaperti A, Germano D, Dirnhofer S, Hunziker L, Matter CM, Eriksson U (2009) Heart-infiltrating prominin-1+/CD133+ progenitor cells represent the cellular source of transforming growth factor beta-mediated cardiac fibrosis in experimental autoimmune myocarditis. Circ Res 105:462–470. https://doi.org/10.1161/circresaha.109.196287
Kania G, Blyszczuk P, Valaperti A, Dieterle T, Leimenstoll B, Dirnhofer S, Zulewski H, Eriksson U (2008) Prominin-1+/CD133+ bone marrow-derived heart-resident cells suppress experimental autoimmune myocarditis. Cardiovasc Res 80:236–245. https://doi.org/10.1093/cvr/cvn190
Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper LT, Bohm M (2012) Update on myocarditis. J Am Coll Cardiol 59:779–792. https://doi.org/10.1016/j.jacc.2011.09.074
Komarowska I, Coe D, Wang G, Haas R, Mauro C, Kishore M, Cooper D, Nadkarni S, Fu H, Steinbruchel DA, Pitzalis C, Anderson G, Bucy P, Lombardi G, Breckenridge R, Marelli-Berg FM (2015) Hepatocyte growth factor receptor c-Met instructs T cell cardiotropism and promotes T cell migration to the heart via autocrine chemokine release. Immunity 42:1087–1099. https://doi.org/10.1016/j.immuni.2015.05.014
Kong P, Christia P, Frangogiannis NG (2014) The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 71:549–574. https://doi.org/10.1007/s00018-013-1349-6
Kraft L, Erdenesukh T, Sauter M, Tschöpe C, Klingel K (2019) Blocking the IL-1β signalling pathway prevents chronic viral myocarditis and cardiac remodeling. Basic Res Cardiol 114:11. https://doi.org/10.1007/s00395-019-0719-0
Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, Delage C, Calise D, Dutaur M, Parini A, Pizzinat N (2014) CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation 129:2111–2124. https://doi.org/10.1161/circulationaha.113.007101
Lv H, Havari E, Pinto S, Gottumukkala RV, Cornivelli L, Raddassi K, Matsui T, Rosenzweig A, Bronson RT, Smith R, Fletcher AL, Turley SJ, Wucherpfennig K, Kyewski B, Lipes MA (2011) Impaired thymic tolerance to α-myosin directs autoimmunity to the heart in mice and humans. J Clin Invest 121:1561–1573. https://doi.org/10.1172/jci44583
McCarthy RE, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM, Baughman KL (2000) Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med 342:690–695. https://doi.org/10.1056/nejm200003093421003
Myers JM, Cooper LT, Kem DC, Stavrakis S, Kosanke SD, Shevach EM, Fairweather D, Stoner JA, Cox CJ, Cunningham MW (2016) Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight. https://doi.org/10.1172/jci.insight.85851
Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW (1987) Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol 139:3630–3636
Nindl V, Maier R, Ratering D, De Giuli R, Zust R, Thiel V, Scandella E, Di Padova F, Kopf M, Rudin M, Rulicke T, Ludewig B (2012) Cooperation of Th1 and Th17 cells determines transition from autoimmune myocarditis to dilated cardiomyopathy. Eur J Immunol 42:2311–2321. https://doi.org/10.1002/eji.201142209
O’Donnell H, Pham OH, Li LX, Atif SM, Lee SJ, Ravesloot MM, Stolfi JL, Nuccio SP, Broz P, Monack DM, Baumler AJ, McSorley SJ (2014) Toll-like receptor and inflammasome signals converge to amplify the innate bactericidal capacity of T helper 1 cells. Immunity 40:213–224. https://doi.org/10.1016/j.immuni.2013.12.013
Rochman Y, Spolski R, Leonard WJ (2009) New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol 9:480–490. https://doi.org/10.1038/nri2580
Rose NR (2009) Myocarditis: infection versus autoimmunity. J Clin Immunol 29:730–737
Sagar S, Liu PP, Cooper LT Jr (2012) Myocarditis. Lancet 379:738–747. https://doi.org/10.1016/s0140-6736(11)60648-x
Smith SC, Allen PM (1991) Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol 147:2141–2147
Thelemann C, Haller S, Blyszczuk P, Kania G, Rosa M, Eriksson U, Rotman S, Reith W, Acha-Orbea H (2016) Absence of nonhematopoietic MHC class II expression protects mice from experimental autoimmune myocarditis. Eur J Immunol 46:656–664. https://doi.org/10.1002/eji.201545945
Valaperti A, Marty RR, Kania G, Germano D, Mauermann N, Dirnhofer S, Leimenstoll B, Blyszczuk P, Dong C, Mueller C, Hunziker L, Eriksson U (2008) CD11b+ monocytes abrogate Th17 CD4+ T cell-mediated experimental autoimmune myocarditis. J Immunol 180:2686–2695. https://doi.org/10.4049/jimmunol.180.4.2686
Acknowledgements
Martina Zarak-Crnkovic acknowledges the support from the Integrative Molecular Medicine PhD program. The authors acknowledge the assistance and support of the Center for Microscopy and Image Analysis and the Flow Cytometry Facility of the University of Zurich, as well as the Functional Genomics Center Zurich.
Funding
Research grants from the GZO Regional Health Center, Swiss National Foundation 310030_149785, Hartmann-Mueller Foundation, Swiss Life Jubiläumsstiftung, Olga Mayenfisch Foundation, Swiss Heart Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zarak-Crnkovic, M., Kania, G., Jaźwa-Kusior, A. et al. Heart non-specific effector CD4+ T cells protect from postinflammatory fibrosis and cardiac dysfunction in experimental autoimmune myocarditis. Basic Res Cardiol 115, 6 (2020). https://doi.org/10.1007/s00395-019-0766-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-019-0766-6