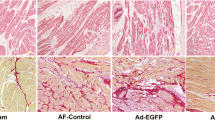
Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia. Concomitant heart failure (HF) poses a particular therapeutic challenge and is associated with prolonged atrial electrical refractoriness compared with non-failing hearts. We hypothesized that downregulation of atrial repolarizing TREK-1 (K2P2.1) K+ channels contributes to electrical remodeling during AF with HF, and that TREK-1 gene transfer would provide rhythm control via normalization of atrial effective refractory periods in this AF subset. In patients with chronic AF and HF, atrial TREK-1 mRNA levels were reduced by 82% (left atrium) and 81% (right atrium) compared with sinus rhythm (SR) subjects. Human findings were recapitulated in a porcine model of atrial tachypacing-induced AF and reduced left ventricular function. TREK-1 mRNA (−66%) and protein (−61%) was suppressed in AF animals at 14-day follow-up compared with SR controls. Downregulation of repolarizing TREK-1 channels was associated with prolongation of atrial effective refractory periods versus baseline conditions, consistent with prior observations in humans with HF. In a preclinical therapeutic approach, pigs were randomized to either atrial Ad-TREK-1 gene therapy or sham treatment. Gene transfer effectively increased TREK-1 protein levels and attenuated atrial effective refractory period prolongation in the porcine AF model. Ad-TREK-1 increased the SR prevalence to 62% during follow-up in AF animals, compared to 35% in the untreated AF group. In conclusion, TREK-1 downregulation and rhythm control by Ad-TREK-1 transfer suggest mechanistic and potential therapeutic significance of TREK-1 channels in a subgroup of AF patients with HF and prolonged atrial effective refractory periods. Functional correction of ionic remodeling through TREK-1 gene therapy represents a novel paradigm to optimize and specify AF management.










Similar content being viewed by others
Abbreviations
- AERP:
-
Atrial effective refractory period
- AF:
-
Atrial fibrillation
- cAF:
-
Chronic atrial fibrillation
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- HF:
-
Heart failure
- K2P :
-
Two-pore-domain K+ channel
- LVEF:
-
Left ventricular ejection fraction
- SR:
-
Sinus rhythm
- TREK:
-
TWIK-related potassium channel
- TWIK:
-
Tandem of P domains in a weak inwardly rectifying K+ channel
References
Aimé-Sempé C, Folliguet T, Rücker-Martin C, Krajewska M, Krajewska S, Heimburger M, Aubier M, Mercadier JJ, Reed JC, Hatem SN (1999) Myocardial cell death in fibrillating and dilated human right atria. J Am Coll Cardiol 34:1577–1586. doi:10.1016/S0735-1097(99)00382-4
Bauer A, McDonald AD, Donahue JK (2004) Pathophysiological findings in a model of persistent atrial fibrillation and severe congestive heart failure. Cardiovasc Res 61:764–770. doi:10.1016/j.cardiores.2003.12.013
Bikou O, Thomas D, Trappe K, Lugenbiel P, Kelemen K, Koch M, Soucek R, Voss F, Becker R, Katus HA, Bauer A (2011) Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc Res 92:218–225. doi:10.1093/cvr/cvr209
Blana A, Kaese S, Fortmüller L, Laakmann S, Damke D, van Bragt K, Eckstein J, Piccini I, Kirchhefer U, Nattel S, Breithardt G, Carmeliet P, Carmeliet E, Schotten U, Verheule S, Kirchhof P, Fabritz L (2010) Knock-in gain-of-function sodium channel mutation prolongs atrial action potentials and alters atrial vulnerability. Heart Rhythm 7:1862–1869. doi:10.1016/j.hrthm.2010.08.016
Burstein B, Nattel S (2008) Atrial structural remodeling as an antiarrhythmic target. J Cardiovasc Pharmacol 52:4–10. doi:10.1097/FJC.0b013e3181668057
Cardin S, Li D, Thorin-Trescases N, Leung TK, Thorin E, Nattel S (2003) Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res 60:315–325. doi:10.1016/j.cardiores.2003.08.014
Cha TJ, Ehrlich JR, Zhang L, Nattel S (2004) Atrial ionic remodeling induced by atrial tachycardia in the presence of congestive heart failure. Circulation 110:1520–1526. doi:10.1161/01.CIR.0000142052.03565.87
Dobrev D, Carlsson L, Nattel S (2012) Novel molecular targets for atrial fibrillation therapy. Nat Rev Drug Discov 11:275–291. doi:10.1038/nrd3682
Enyedi P, Czirjak G (2010) Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90:559–605. doi:10.1152/physrev.00029.2009
Glasscock E, Voigt N, McCauley MD, Sun Q, Li N, Chiang DY, Zhou XB, Molina CE, Thomas D, Schmidt C, Skapura DG, Noebels JL, Dobrev D, Wehrens XH (2015) Expression and function of Kv1.1 potassium channels in human atria from patients with atrial fibrillation. Basic Res Cardiol 110:505. doi:10.1007/s00395-015-0505-6
Goldstein SA, Bockenhauer D, O’Kelly I, Zilberberg N (2001) Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci 2:175–184. doi:10.1038/35058574
Iwasaki YK, Nishida K, Kato T, Nattel S (2011) Atrial fibrillation pathophysiology: implications for management. Circulation 124:2264–2274. doi:10.1161/CIRCULATIONAHA.111.019893
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; Authors/Task Force Members (2016) 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur Heart J. doi:10.1093/eurheartj/ehw210
Kirchhof P, Eckardt L, Franz MR, Mönnig G, Loh P, Wedekind H, Schulze-Bahr E, Breithardt G, Haverkamp W (2003) Prolonged atrial action potential durations and polymorphic atrial tachyarrhythmias in patients with long QT syndrome. J Cardiovasc Electrophysiol 14:1027–1033. doi:10.1046/j.1540-8167.2003.03165.x
Kirchhof P, Eckardt L, Mönnig G, Johna R, Loh P, Schulze-Bahr E, Breithardt G, Borggrefe M, Haverkamp W (2000) A patient with “atrial torsades de pointes”. J Cardiovasc Electrophysiol 11:806–811. doi:10.1111/j.1540-8167.2000.tb00052.x
Kisselbach J, Seyler C, Schweizer PA, Gerstberger R, Becker R, Katus HA, Thomas D (2014) Modulation of K2P2.1 and K2P10.1 K+ channel sensitivity to carvedilol by alternative mRNA translation initiation. Br J Pharmacol 171:5182–5194. doi:10.1111/bph.12596
Lee JM, Lee H, Janardhan AH, Park J, Joung B, Pak HN, Lee MH, Kim SS, Hwang HJ (2016) Prolonged atrial refractoriness predicts the onset of atrial fibrillation: a 12-year follow-up study. Heart Rhythm 13:1575–1580. doi:10.1016/j.hrthm.2016.03.037
Lemoine MD, Duverger JE, Naud P, Chartier D, Qi XY, Comtois P, Fabritz L, Kirchhof P, Nattel S (2011) Arrhythmogenic left atrial cellular electrophysiology in a murine genetic long QT syndrome model. Cardiovasc Res 92:67–74. doi:10.1093/cvr/cvr166
Lugenbiel P, Bauer A, Kelemen K, Schweizer PA, Becker R, Katus HA. Thomas D (2012) Biological heart rate reduction through genetic suppression of Gαs protein in the sinoatrial node. J Am Heart Assoc 1:jah3-e000372. doi:10.1161/JAHA.111.000372
Lugenbiel P, Thomas D, Kelemen K, Trappe K, Bikou O, Schweizer PA, Voss F, Becker R, Katus HA, Bauer A (2012) Genetic suppression of Gαs protein provides rate control in atrial fibrillation. Basic Res Cardiol 107:265. doi:10.1007/s00395-012-0265-5
Lugenbiel P, Wenz F, Govorov K, Schweizer PA, Katus HA, Thomas D (2015) Atrial fibrillation complicated by heart failure induces distinct remodeling of calcium cycling proteins. PLoS One 10:e0116395. doi:10.1371/journal.pone.0116395
Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J (2014) Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. J Am Med Assoc 311:498–506. doi:10.1001/jama.2014.3
Nattel S, Maguy A, Le Bouter S, Yeh YH (2007) Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 87:425–456. doi:10.1152/physrev.00014.2006
Qi XY, Huang H, Ordog B, Luo X, Naud P, Sun Y, Wu CT, Dawson K, Tadevosyan A, Chen Y, Harada M, Dobrev D, Nattel S (2015) Fibroblast inward-rectifier potassium current upregulation in profibrillatory atrial remodeling. Circ Res 116:836–845. doi:10.1161/CIRCRESAHA.116.305326
Sandoz G, Bell SC, Isacoff EY (2011) Optical probing of a dynamic membrane interaction that regulates the TREK1 channel. Proc Natl Acad Sci USA 108:2605–2610. doi:10.1073/pnas.1015788108
Schmidt C, Wiedmann F, Schweizer PA, Katus HA, Thomas D (2014) Inhibition of cardiac two-pore-domain K+ (K2P) channels-an emerging antiarrhythmic concept. Eur J Pharmacol 738:250–255. doi:10.1016/j.ejphar.2014.05.056
Schmidt C, Wiedmann F, Tristram F, Anand P, Wenzel W, Lugenbiel P, Schweizer PA, Katus HA, Thomas D (2014) Cardiac expression and atrial fibrillation-associated remodeling of K2P2.1 (TREK-1) K+ channels in a porcine model. Life Sci 97:107–115. doi:10.1016/j.lfs.2013.12.006
Schmidt C, Wiedmann F, Voigt N, Zhou XB, Heijman J, Lang S, Albert V, Kallenberger S, Ruhparwar A, Szabó G, Kallenbach K, Karck M, Borggrefe M, Biliczki P, Ehrlich JR, Baczkó I, Lugenbiel P, Schweizer PA, Donner BC, Katus HA, Dobrev D, Thomas D (2015) Upregulation of K2P3.1 K+ current causes action potential shortening in patients with chronic atrial fibrillation. Circulation 132:82–92. doi:10.1161/CIRCULATIONAHA.114.012657
Schotten U, Verheule S, Kirchhof P, Goette A (2011) Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 91:265–325. doi:10.1152/physrev.00031.2009
Schweizer PA, Schröter J, Greiner S, Haas J, Yampolsky P, Mereles D, Buss SJ, Seyler C, Bruehl C, Draguhn A, Koenen M, Meder B, Katus HA, Thomas D (2014) The symptom complex of familial sinus node dysfunction and myocardial noncompaction is associated with mutations in the HCN4 channel. J Am Coll Cardiol 64:757–767. doi:10.1016/j.jacc.2014.06.1155
Shinagawa K, Li D, Leung TK, Nattel S (2002) Consequences of atrial tachycardia-induced remodeling depend on the preexisting atrial substrate. Circulation 105:251–257. doi:10.1161/hc0202.102014
Soucek R, Thomas D, Kelemen K, Bikou O, Seyler C, Voss F, Becker R, Koenen M, Katus HA, Bauer A (2012) Genetic suppression of atrial fibrillation using a dominant-negative ether-a-go-go-related gene mutant. Heart Rhythm 9:265–272. doi:10.1016/j.hrthm.2011.09.008
Thomas D, Plant LD, Wilkens CM, McCrossan ZA, Goldstein SA (2008) Alternative translation initiation in rat brain yields K2P2.1 potassium channels permeable to sodium. Neuron 58:859–870. doi:10.1016/j.neuron.2008.04.016
Trappe K, Thomas D, Bikou O, Kelemen K, Lugenbiel P, Voss F, Becker R, Katus HA, Bauer A (2013) Suppression of persistent atrial fibrillation by genetic knockdown of caspase 3: a pre-clinical pilot study. Eur Heart J 34:147–157. doi:10.1093/eurheartj/ehr269
Wakili R, Voigt N, Kääb S, Dobrev D, Nattel S (2011) Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest 121:2955–2968. doi:10.1172/JCI46315
Yeh YH, Wakili R, Qi XY, Chartier D, Boknik P, Kääb S, Ravens U, Coutu P, Dobrev D, Nattel S (2008) Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol 1:93–102. doi:10.1161/CIRCEP.107.754788
Yue L, Xie J, Nattel S (2011) Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res 89:744–753. doi:10.1093/cvr/cvq329
Zellerhoff S, Pistulli R, Mönnig G, Hinterseer M, Beckmann BM, Köbe J, Steinbeck G, Kääb S, Haverkamp W, Fabritz L, Gradaus R, Breithardt G, Schulze-Bahr E, Böcker D, Kirchhof P (2009) Atrial arrhythmias in long-QT syndrome under daily life conditions: a nested case control study. J Cardiovasc Electrophysiol 20:401–407. doi:10.1111/j.1540-8167.2008.01339.x
Acknowledgements
We thank Nadine Weiberg, Jennifer Gütermann, Kai Sona, and Bianca Menrath for excellent technical assistance, and the operating room team at the Department of Cardiac Surgery of Heidelberg University for supporting our work. We are grateful to Jochen Keil for providing technical support. This work was supported in part by research grants from the University of Heidelberg, Faculty of Medicine (Postdoctoral Fellowship to P.L.), from the German Cardiac Society (Fellowship to P.L.), from the DZHK (German Centre for Cardiovascular Research; column B project to D.F. and N.F.), from the German Cardiac Society and the Hengstberger Foundation (Klaus-Georg and Sigrid Hengstberger Scholarship to D.T.), from the German Heart Foundation/German Foundation of Heart Research (Project F/08/14 to D.T.), from the Else Kröner-Fresenius-Stiftung (2014_A242 to D.T.), from the Joachim Siebeneicher Foundation (to D.T.), and from the Ministry of Science, Research and the Arts Baden-Wuerttemberg (Sonderlinie Medizin to D.T.). F.W. was supported by the Cardiology Career Program of the Department of Cardiology, University of Heidelberg, and by the Otto-Hess-Scholarship of the German Cardiac Society. K.G. received support from the German Heart Foundation/German Foundation of Heart Research (Kaltenbach Scholarship). P.A.S was supported by the Heidelberg Research Center for Molecular Medicine (Senior Career Fellowship).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
The study involving human tissue samples was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of the University of Heidelberg (Germany; institutional approval number S-390/2011). Written informed consent was obtained from all patients. Animal experiments have been carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health (NIH publication No. 86-23, revised 1985) and with EU Directive 2010/63/EU, and the current version of the German Law on the Protection of Animals was followed. Experiments involving pigs (institutional approval number G-165/12), mice (institutional approval number T-19/14), and rats (institutional approval number V312-7224-121-4) have been approved by the respective local animal welfare authorities.
Conflict of interest
P.L., P.A.S., H.A.K., and D.T. have filed a patent application for the use of K2P potassium channels for altering cardiac electrophysiology. The remaining authors have reported that they have no relationships relevant to the content of this paper to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lugenbiel, P., Wenz, F., Syren, P. et al. TREK-1 (K2P2.1) K+ channels are suppressed in patients with atrial fibrillation and heart failure and provide therapeutic targets for rhythm control. Basic Res Cardiol 112, 8 (2017). https://doi.org/10.1007/s00395-016-0597-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-016-0597-7