Abstract
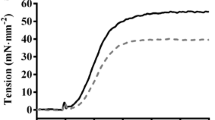
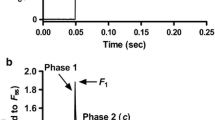
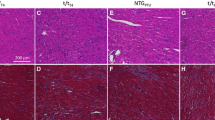
Interplay between the protein kinase C (PKC)-mediated phosphorylation of troponin T (TnT)- and myosin heavy chain (MHC)-mediated effects on thin filaments takes on a new significance because: (1) there is significant interaction between the TnT- and MHC-mediated effects on cardiac thin filaments; (2) although the phosphorylation of TnT by PKC isoforms is common to both human and rodent hearts, human hearts predominantly express β-MHC while rodent hearts predominantly express α-MHC. Therefore, we tested how α- and β-MHC isoforms differently affected the functional effects of phosphorylated TnT. Contractile measurements were made on cardiac muscle fibers from normal rats (α-MHC) and propylthiouracil-treated rats (β-MHC), reconstituted with the recombinant phosphomimetic-TnT (T204E; threonine 204 replaced by glutamate). Ca2+-activated maximal tension decreased differently in α-MHC + T204E (~68 %) and β-MHC + T204E (~35 %). However, myofilament Ca2+ sensitivity decreased similarly in α-MHC + T204E and β-MHC + T204E, demonstrating that a decrease in Ca2+ sensitivity alone cannot explain the greater attenuation of tension in α-MHC + T204E. Interestingly, dynamic contractile parameters (rates of tension redevelopment, crossbridge (XB) recruitment dynamics, XB distortion dynamics, and XB detachment kinetics) decreased only in α-MHC + T204E. Thus, the transition of thin filaments from the blocked- to closed-state was attenuated in α-MHC + T204E and β-MHC + T204E, but the closed- to open-state transition was attenuated only in α-MHC + T204E. Our study demonstrates that the effects of phosphorylated TnT and MHC isoforms interact to bring about different functional states of cardiac thin filaments.









Similar content being viewed by others
References
Belin RJ, Sumandea MP, Sievert GA, Harvey LA, Geenen DL, Solaro RJ, de Tombe PP (2011) Interventricular differences in myofilament function in experimental congestive heart failure. Pflugers Arch 462:795–809. doi:10.1007/s00424-011-1024-4
Bell MG, Lankford EB, Gonye GE, Ellis-Davies GC, Martyn DA, Regnier M, Barsotti RJ (2006) Kinetics of cardiac thin-filament activation probed by fluorescence polarization of rhodamine-labeled troponin C in skinned guinea pig trabeculae. Biophys J 90:531–543. doi:10.1529/biophysj.105.072769
Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ (1999) Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation 99:384–391. doi:10.1161/01.CIR.99.3.384
Brenner B (1988) Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci USA 85:3265–3269. doi:10.1073/pnas.85.9.3265
Brenner B, Eisenberg E (1986) Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci USA 83:3542–3546. doi:10.1073/pnas.83.10.3542
Campbell K (1997) Rate constant of muscle force redevelopment reflects cooperative activation as well as cross-bridge kinetics. Biophys J 72:254–262. doi:10.1016/S0006-3495(97)78664-8
Campbell KB, Chandra M, Kirkpatrick RD, Slinker BK, Hunter WC (2004) Interpreting cardiac muscle force-length dynamics using a novel functional model. Am J Physiol Heart Circ Physiol 286:H1535–H1545. doi:10.1152/ajpheart.01029.2003
Canton M, Menazza S, Sheeran FL, Polverino de Laureto P, Di Lisa F, Pepe S (2011) Oxidation of myofibrillar proteins in human heart failure. J Am Coll Cardiol 57:300–309. doi:10.1016/j.jacc.2010.06.058
Canton M, Skyschally A, Menabo R, Boengler K, Gres P, Schulz R, Haude M, Erbel R, Di Lisa F, Heusch G (2006) Oxidative modification of tropomyosin and myocardial dysfunction following coronary microembolization. Eur Heart J 27:875–881. doi:10.1093/eurheartj/ehi751
Chandra M, Tschirgi ML, Ford SJ, Slinker BK, Campbell KB (2007) Interaction between myosin heavy chain and troponin isoforms modulate cardiac myofiber contractile dynamics. Am J Physiol Regul Integr Comp Physiol 293:R1595–R1607. doi:10.1152/ajpregu.00157.2007
Chandra M, Tschirgi ML, Rajapakse I, Campbell KB (2006) Troponin T modulates sarcomere length-dependent recruitment of cross-bridges in cardiac muscle. Biophys J 90:2867–2876. doi:10.1529/biophysj.105.076950
Chandra M, Tschirgi ML, Tardiff JC (2005) Increase in tension-dependent ATP consumption induced by cardiac troponin T mutation. Am J Physiol Heart Circ Physiol 289:H2112–H2119. doi:10.1152/ajpheart.00571.2005
Chizzonite RA, Zak R (1984) Regulation of myosin isoenzyme composition in fetal and neonatal rat ventricle by endogenous thyroid hormones. J Biol Chem 259:12628–12632
Czuriga D, Toth A, Pasztor ET, Balogh A, Bodnar A, Nizsaloczki E, Lionetti V, Recchia FA, Czuriga I, Edes I, Papp Z (2012) Cell-to-cell variability in troponin I phosphorylation in a porcine model of pacing-induced heart failure. Basic Res Cardiol 107:244. doi:10.1007/s00395-012-0244-x
de Tombe PP, Stienen GJ (1995) Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ Res 76:734–741
Fabiato A, Fabiato F (1979) Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 75:463–505
Ford SJ, Chandra M, Mamidi R, Dong W, Campbell KB (2010) Model representation of the nonlinear step response in cardiac muscle. J Gen Physiol 136:159–177. doi:10.1085/jgp.201010467
Goldspink PH, Montgomery DE, Walker LA, Urboniene D, McKinney RD, Geenen DL, Solaro RJ, Buttrick PM (2004) Protein kinase Cepsilon overexpression alters myofilament properties and composition during the progression of heart failure. Circ Res 95:424–432. doi:10.1161/01.RES.0000138299.85648.92
Guo X, Wattanapermpool J, Palmiter KA, Murphy AM, Solaro RJ (1994) Mutagenesis of cardiac troponin I. Role of the unique NH2-terminal peptide in myofilament activation. J Biol Chem 269:15210–15216
Hasenfuss G, Mulieri LA, Blanchard EM, Holubarsch C, Leavitt BJ, Ittleman F, Alpert NR (1991) Energetics of isometric force development in control and volume-overload human myocardium. Comparison with animal species. Circ Res 68:836–846. doi:10.1161/01.RES.68.3.836
Hershberger RE, Pinto JR, Parks SB, Kushner JD, Li D, Ludwigsen S, Cowan J, Morales A, Parvatiyar MS, Potter JD (2009) Clinical and functional characterization of TNNT2 mutations identified in patients with dilated cardiomyopathy. Circ Cardiovasc Genet 2:306–313. doi:10.1161/CIRCGENETICS.108.846733
Heusch G, Schulz R (2011) A radical view on the contractile machinery in human heart failure. J Am Coll Cardiol 57:310–312. doi:10.1016/j.jacc.2010.06.057
Hill AV (1938) The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond Ser B Biol Sci 126:136–195. doi:10.1098/rspb.1938.0050
Ho CY, Lever HM, DeSanctis R, Farver CF, Seidman JG, Seidman CE (2000) Homozygous mutation in cardiac troponin T: implications for hypertrophic cardiomyopathy. Circulation 102:1950–1955. doi:10.1161/01.CIR.102.16.1950
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318
Inoue T, Kobirumaki-Shimozawa F, Kagemoto T, Fujii T, Terui T, Kusakari Y, Hongo K, Morimoto S, Ohtsuki I, Hashimoto K, Fukuda N (2013) Depressed Frank-Starling mechanism in the left ventricular muscle of the knock-in mouse model of dilated cardiomyopathy with troponin T deletion mutation DeltaK210. J Mol Cell Cardiol 63:69–78. doi:10.1016/j.yjmcc.2013.07.001
Jideama NM, Noland TA Jr, Raynor RL, Blobe GC, Fabbro D, Kazanietz MG, Blumberg PM, Hannun YA, Kuo JF (1996) Phosphorylation specificities of protein kinase C isozymes for bovine cardiac troponin I and troponin T and sites within these proteins and regulation of myofilament properties. J Biol Chem 271:23277–23283. doi:10.1074/jbc.271.38.23277
Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B, Smoot L, Mullen MP, Woolf PK, Wigle ED, Seidman JG, Seidman CE (2000) Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med 343:1688–1696. doi:10.1056/NEJM200012073432304
Kimura C, Maeda K, Maeda Y, Miki M (2002) Ca(2+)- and S1-induced movement of troponin T on reconstituted skeletal muscle thin filaments observed by fluorescence energy transfer spectroscopy. J Biochem 132:93–102. doi:10.1093/oxfordjournals.jbchem.a003204
Kobayashi M, Debold EP, Turner MA, Kobayashi T (2013) Cardiac muscle activation blunted by a mutation to the regulatory component, troponin T. J Biol Chem 288:26335–26349. doi:10.1074/jbc.M113.494096
Kooij V, Boontje N, Zaremba R, Jaquet K, dos Remedios C, Stienen GJ, van der Velden J (2010) Protein kinase C alpha and epsilon phosphorylation of troponin and myosin binding protein C reduce Ca2+ sensitivity in human myocardium. Basic Res Cardiol 105:289–300. doi:10.1007/s00395-009-0053-z
Mamidi R, Chandra M (2013) Divergent effects of alpha- and beta-myosin heavy chain isoforms on the N terminus of rat cardiac troponin T. J Gen Physiol 142:413–423. doi:10.1085/jgp.201310971jgp
Mamidi R, Mallampalli SL, Wieczorek DF, Chandra M (2013) Identification of two new regions in the N-Terminal region of cardiac troponin T that have divergent effects on cardiac contractile function. J Physiol. doi:10.1113/jphysiol.2012.243394
Mamidi R, Michael JJ, Muthuchamy M, Chandra M (2013) Interplay between the overlapping ends of tropomyosin and the N terminus of cardiac troponin T affects tropomyosin states on actin. FASEB J. doi:10.1096/fj.13-232363
Manning EP, Tardiff JC, Schwartz SD (2011) A model of calcium activation of the cardiac thin filament. Biochemistry 50:7405–7413. doi:10.1021/bi200506k
Marsiglia JDC, Credidio FL, de Oliveira TGM, Reis RF, Antunes MdO, de Araujo AQ, Pedrosa RP, Barbosa-Ferreira JMB, Mady C, Krieger JE, Arteaga-Fernandez E, Pereira ADC (2013) Screening of MYH7, MYBPC3, and TNNT2 genes in Brazilian patients with hypertrophic cardiomyopathy. Am Heart J 166:775–782. doi:10.1016/j.ahj.2013.07.029
McKillop DF, Geeves MA (1993) Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J 65:693–701. doi:10.1016/S0006-3495(93)81110-X
Michael JJ, Gollapudi SK, Ford SJ, Kazmierczak K, Szczesna-Cordary D, Chandra M (2012) Deletion of 1–43 amino acids in cardiac myosin essential light chain blunts length dependency of Ca2+ sensitivity and crossbridge detachment kinetics. Am J Physiol Heart Circ Physiol. doi:10.1152/ajpheart.00572.2012
Mogensen J, Murphy RT, Shaw T, Bahl A, Redwood C, Watkins H, Burke M, Elliott PM, McKenna WJ (2004) Severe disease expression of cardiac troponin C and T mutations in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 44:2033–2040. doi:10.1016/j.jacc.2004.08.027
Pan BS, Johnson RG Jr (1996) Interaction of cardiotonic thiadiazinone derivatives with cardiac troponin C. J Biol Chem 271:817–823. doi:10.1074/jbc.271.2.817
Piazzesi G, Linari M, Reconditi M, Vanzi F, Lombardi V (1997) Cross-bridge detachment and attachment following a step stretch imposed on active single frog muscle fibres. J Physiol 498(Pt 1):3–15
Reiser PJ, Kline WO (1998) Electrophoretic separation and quantitation of cardiac myosin heavy chain isoforms in eight mammalian species. Am J Physiol 274:H1048–H1053
Shitaka Y, Kimura C, Miki M (2005) The rates of switching movement of troponin T between three states of skeletal muscle thin filaments determined by fluorescence resonance energy transfer. J Biol Chem 280:2613–2619. doi:10.1074/jbc.M408553200
Solzin J, Iorga B, Sierakowski E, Gomez Alcazar DP, Ruess DF, Kubacki T, Zittrich S, Blaudeck N, Pfitzer G, Stehle R (2007) Kinetic mechanism of the Ca2+-dependent switch-on and switch-off of cardiac troponin in myofibrils. Biophys J 93:3917–3931. doi:10.1529/biophysj.107.111146
Stienen GJ, Zaremba R, Elzinga G (1995) ATP utilization for calcium uptake and force production in skinned muscle fibres of Xenopus laevis. J Physiol 482(Pt 1):109–122
Sumandea MP, Pyle WG, Kobayashi T, de Tombe PP, Solaro RJ (2003) Identification of a functionally critical protein kinase C phosphorylation residue of cardiac troponin T. J Biol Chem 278:35135–35144. doi:10.1074/jbc.M306325200
Takeda S, Yamashita A, Maeda K, Maeda Y (2003) Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature 424:35–41. doi:10.1038/nature01780
Tardiff JC, Factor SM, Tompkins BD, Hewett TE, Palmer BM, Moore RL, Schwartz S, Robbins J, Leinwand LA (1998) A truncated cardiac troponin T molecule in transgenic mice suggests multiple cellular mechanisms for familial hypertrophic cardiomyopathy. J Clin Invest 101:2800–2811. doi:10.1172/JCI2389
Tschirgi ML, Rajapakse I, Chandra M (2006) Functional consequence of mutation in rat cardiac troponin T is affected differently by myosin heavy chain isoforms. J Physiol 574:263–273. doi:10.1113/jphysiol.2006.107417
Van Driest SL, Vasile VC, Ommen SR, Will ML, Tajik AJ, Gersh BJ, Ackerman MJ (2004) Myosin binding protein C mutations and compound heterozygosity in hypertrophic cardiomyopathy. J Am Coll Cardiol 44:1903–1910. doi:10.1016/j.jacc.2004.07.045
Wang J, Liu X, Sentex E, Takeda N, Dhalla NS (2003) Increased expression of protein kinase C isoforms in heart failure due to myocardial infarction. Am J Physiol Heart Circ Physiol 284:H2277–H2287. doi:10.1152/ajpheart.00142.2002
Acknowledgments
This work was funded by National Heart, Lung, and Blood Institute Grant R01-HL-075643 to Dr. Murali Chandra and a Poncin Fellowship to John Jeshurun Michael.
Conflict of interest
The authors declare that they have no conflict of interest. Our manuscript does not contain any clinical studies or patient data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michael, J.J., Gollapudi, S.K. & Chandra, M. Effects of pseudo-phosphorylated rat cardiac troponin T are differently modulated by α- and β-myosin heavy chain isoforms. Basic Res Cardiol 109, 442 (2014). https://doi.org/10.1007/s00395-014-0442-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-014-0442-9