Abstract
Purpose
There is an ongoing debate whether vegetarian (VG) and especially vegan (VN) diets are nutritionally adequate in early childhood. Hence, the Vegetarian and Vegan Children Study (VeChi Diet Study) aimed to assess the food and nutrient intake of VG and VN infants.
Methods
The study examined the diets of 1–3-year-old VG, VN, and omnivorous (OM) children (n = 430). Dietary intake was assessed via a 3-day weighed dietary record and compared between groups using ANCOVA. Lifestyle data were collected using a questionnaire. Here, the results of micronutrient and fatty acid intakes are presented.
Results
Most nutrient intakes (with and without supplements) differed significantly between VN children and the two other groups, with a more favourable overall micronutrient intake in VN, followed by VG children, [e.g., the highest intake of vitamin E (8.3 mg/d vs. VG 7.4 mg/d and OM 5.1 mg/d), vitamin B1 (569 µg/d vs. VG 513 µg/d and OM 481 µg/d), folate (143 µg/d vs. VG 116 µg/d and OM 108 µg/d), magnesium (241 mg/d vs. VG 188 mg/d and OM 164 mg/d), and iron (8.9 mg/d vs. VG 7.3 mg/d and OM 6.0 mg/d)] as well as fat quality [highest intake of polyunsaturated fatty acids (8.7 E% vs. VG 6.9 E% and OM 4.5 E%) and lowest intake of saturated fatty acids (9.1 E% vs. VG 11.9 E% and OM 14.0 E%)]. In contrast, OM children had the highest intake of vitamin B2 (639 µg/d vs. VG 461 µg/d and VN 429 µg/d), calcium (445 mg/d vs. VG 399 mg/d and VN 320 mg/d), iodine (47 µg/d vs. VG 33 µg/d and VN 31 µg/d), and DHA (35.4 mg/d vs. VG 16.6 mg/d and VN 18.4 mg/d). Without supplementation, OM children had the highest average vitamin B12 intake (1.5 µg/d vs. VG 0.6 µg/d and VN 0.2 µg/d), whereas VN children had the highest average vitamin B12 intake with supplementation (73.8 µg/d vs. VG 1.3 µg/d and OM 1.7 µg/d). Without supplementation, none of the groups’ median intakes met the harmonised Average Requirement (h-AR) for vitamin D and iodine. Moreover, VG and VN children did not achieve h-ARs for vitamin B2, vitamin B12, and iron—if a low absorption of iron is anticipated; VN children also did not do so for calcium.
Conclusion
In early childhood, VN and VG diets can provide most micronutrients in desirable amounts and a preferable fat quality compared to an OM diet. Special focus should be paid to (potentially) critical nutrients, particularly vitamin D, iodine, and DHA for all children regardless of diet, as well as vitamin B2, vitamin B12, calcium, and iron for VG and VN children.
Trail registration
This study was registered with the German Clinical Trials Register (DRKS00010982) on (September 2, 2016).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vegetarian (VG, excluding meat and fish) and vegan (VN, excluding all animal foods) diets have become increasingly popular recently [1]. Nevertheless, only a few studies have investigated the food intake, nutritional status, and health of VG and VN children. These studies were heterogeneous, mostly cross-sectional, and of small sample sizes. Furthermore, they were mainly from the 1970s to 1990s [2, 3]. Since then, the food market has changed, for example, offering more dairy or meat alternatives and dietary supplements. Hence, new studies are needed to estimate the food and nutrient intake of children on a modern VG or VN diet.
Only four studies have examined the micronutrient intake of VG, VN, and OM children during the last 10 years [4,5,6,7]. Potentially critical micronutrients identified in these studies are calcium, sometimes iron, and vitamin D for VG children. However, they mostly had a higher intake of vitamin E, vitamin B1, vitamin C, and magnesium, lower intake of vitamin B2, vitamin B12 (without supplements), and zinc, and sometimes higher intake of (pro)vitamin A, and folate than OM subjects [4, 6, 8,9,10,11,12,13,14,15,16]. The intake of saturated fatty acids (SFA) [4, 5] and dietary cholesterol [5] of VG children was lower, and polyunsaturated fatty acid intake (PUFA) [4] was higher than in OM subjects.
VN children generally met the recommended intakes of essential nutrients in older studies [17, 18]. In one study, they did not reach the recommendations for vitamin B12 [17], in two studies those for vitamin D [18, 19], and in all three studies for calcium [17,18,19]. In the study by Sanders and Purves (1981), as well as in the follow-up in 1988 [19], a few VN children had a low intake of vitamin B2 and vitamin B12 [18]. VN children had a higher intake of linoleic acid (LA), a high ratio of LA: α-linolenic acid (ALA), and PUFA, but a lower intake of SFA than OM children [17]. None of these rather outdated studies examined the nutritional status of VN children with biomarkers, and the contribution of supplements to the nutrient intake was also not evaluated in detail. In more recent studies with VN children, they had higher intakes of vitamin E [4], β-carotene equivalents [6], vitamin B1 [4], vitamin C [4, 6], folate [4,5,6], magnesium [4, 6], zinc [4, 5], iron [4,5,6], MUFA [4, 5], and PUFA [4,5,6], but lower intakes of vitamin B2 [4], vitamin B12 (without supplements) [4, 6], vitamin D [6], calcium [4, 6], SFA [4,5,6], and dietary cholesterol [5, 6] than VG and/or OM children.
The nutritional status of VG children in most studies was generally normal [5, 9, 10, 14, 20, 21]. In some studies, the concentrations of vitamin B12 (without supplements) [6], vitamin D [11, 13], iron [9, 15, 21], or zinc [5] were lower than the cutoffs and/or concentrations in OM children. Regarding blood lipids, VG had lower average total cholesterol (C) [6, 22], LDL-C [22], HDL-C [6], but higher VLDL-C [6], and triglycerides [6, 22] than OM children. In other studies, the blood lipids did not differ in comparison to OM participants [4, 5] or were within the reference ranges [10].
Regarding nutritional status, VN children had higher folate concentrations [4, 5], but poorer status markers for iron [4, 6], vitamin A [5], vitamin D [5, 6], and vitamin B12 (without supplements) in some studies [6]. Regarding blood lipids, VN children had lower DHA [5], triglyceride [4], HDL-C [5, 6], non-HDL-C [4], LDL-C [4,5,6], and total C [5, 6] concentrations than VG and/or OM children. However, in the VeChi Youth Study, there were no significant differences in blood concentrations of haemoglobin between VN, VG, and OM participants. Nevertheless, VN subjects had lower ferritin concentrations than OM children, but mostly in the normal range. Furthermore, no significant differences in blood concentrations of vitamin B2 and 25-OH vitamin D were found between the groups. Additionally, VG but not VN participants on average had a poorer vitamin B12 status than OM subjects [4].
Dietary habits appear to vary by age group and country. Hence, the overall aim of the Vegetarian and Vegan Children Study (VeChi Diet Study) was to examine the food and nutrient intake of 430 VG, VN, and OM children aged 1–3 years in Germany. Results on anthropometric data and macronutrient intakes were published recently [23]. This paper describes micronutrient and fat intake and assesses the nutrient adequacy of the three diet groups.
Subjects and methods
Study population
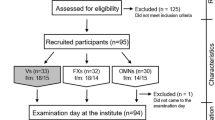
The VeChi Diet Study is a cross-sectional study collecting data on diet, lifestyle, body weight (BW), and body height (BH) from VG, VN, and OM children. The study was conducted from October 2016 to April 2018 throughout Germany. Study design, sample size calculation, recruitment, and initial results are described elsewhere in detail [23]. In short, the study population consisted of 127 VG, 139 VN, and 164 OM children aged 1–3 years (Fig. S1, Online Resource 1). Families were recruited mainly via the study website (www.vechi-studie.de), VN/VG/child nutrition Facebook groups, a mailing list of Giessen University, magazines and journals, VN/VG websites, daycare centres, and VN/VG conventions. Furthermore, participating families were asked to recruit friends of their children. The subjects were without diagnosed diseases that could affect the studied variables. Moreover, diets with predominantly uncooked (raw) food (≥ 70%) were excluded.
The observational and non-invasive VeChi Diet Study was conducted according to the guidelines of the Declaration of Helsinki and its later amendments. The Ethics Committee of the University of Bonn approved it (046/17). All examinations were performed with parental written consent.
Diet group classification
Parents were asked in an online questionnaire: “How is your child raised?”.
-
Vegetarian (no meat, sausage, or fish, but with dairy products and/or eggs).
-
Vegan (no meat, sausage, fish, dairy products, or eggs).
-
Omnivorous (with meat and/or sausage and/or fish).
Parents of VG or VN children were asked whether there are exceptions in food intake. Accordingly, VG and VN children who usually eat meat or fish ≥ 1 time/week were reclassified as OM (2.1%: 8 VG, 1 VN). VN who usually eat dairy products and/or eggs ≥ 1 time/week were categorized as VG (5.6%: 24 VN).
Dietary assessment
Dietary intake was assessed using weighed dietary records (paper-and-pencil) during 3 consecutive days (weekdays and weekends). The parents weighed and recorded all foods, beverages, and supplements that the participating children consumed, as well as plate waste, to the nearest gram using their own electronic kitchen scales. When exact weighing was not possible—e.g., when eating out—household measures (e.g., spoons, cups) and a photo booklet with foods in toddlers’ portion sizes [24], supplemented with special VG and VN foods, allowed semi-quantitative recording. Besides written information on dietary recording, a video tutorial was provided on the study website. The protocols were returned via mail or email. The study staff assessed missing data, requesting the information from the parents via email. When children ate at a daycare, preschool teachers recorded the foods the children ate, supplemented by recipes from the caterer if available. When recipes were not available, they were simulated. As measurement of individual breast milk intakes was not possible, amounts were estimated by multiplying the numbers of breast meals with age-specific amounts of breast milk meal volumes (for details see [23]). Energy and nutrient intakes were calculated using the nutrient food composition database LEBTAB. In this database, the composition of staple foods is based on the standard German food composition tables BLS 3.02. The energy and nutrient contents of commercial food products, i.e., processed foods and ready-to-eat meals or snack foods, were estimated via recipe simulation using labelled ingredients and nutrient contents [25]. LEBTAB is continuously updated by adding products or supplements recorded by study participants. Supplements were included in the calculation of individual nutrient intake with the exact composition and dosage as supplementation, in particular of vitamin B12, as recommended for vegans [26,27,28,29].
Assessment of covariables
Data on sociodemographic variables, lifestyle, and further variables, e.g., birth weight, were collected via an online questionnaire. Reported BW and BH were either proxy assessed by the parents or by a paediatrician during the last paediatric checkup. Sex-specific weight-for-height, height-for-age, and weight-for-age z-scores were calculated using WHO Anthro version 3.2.2 for SPSS [30]. Socioeconomic status (SES) was calculated according to [31]. Physical activity was categorized into active or very active (i.e., ‘playing outside’ and/or ‘attendance in play/sport groups’ ≥ 4–7 times per week) or less active (< 4 times/week).
Data analysis and statistics
Statistical analysis was performed with SPSS Version 20 (IBM SPSS Statistics, Chicago, IL, USA). Energy and nutrient intake (with and without dietary supplements) were calculated as individual means of the 3 recorded days (kcal, mg, or µg/d). Fortified foods, e.g., multivitamin juices, fortified ready-to-eat cereals, and plant oils fortified with DHA, were included in the food intake. Fatty acids, carbohydrates, and protein were additionally calculated as percentages of energy intake (E%). We used the harmonised Average Requirement (h-AR), a combination of the AR of the European Food Safety Authority (EFSA) and the Estimated AR (EAR) of the Institute of Medicine (IOM) of the US National Academies [32] to estimate the percentage of subjects at risk of nutrient deficiency. To reflect the different bioavailability, EFSA published two different AR values for iron for 1–3-year-old children: one for moderate absorption (5 mg/d) and one for low absorption (10 mg/d). The comparison of the medians with both AR values is presented. Nevertheless, we assume moderate absorption for an OM diet and low absorption for a VG and VN diet. Since no ARs for potassium, vitamin K, and fatty acids are available, only group comparisons were carried out.
Participants’ characteristics are presented as mean ± standard deviation (SD) for the variables with a normal distribution. For non-normally distributed variables, median and interquartile ranges (IQRs) are presented. Differences in categorical characteristics between diet groups were tested using Chi2 test or Fisher’s exact test. For continuous characteristics, ANOVA for parametric or the Kruskal–Wallis test for non-parametric data was applied. In the case of significant differences, pairwise Bonferroni post hoc tests (parametric) or Mann–Whitney U tests (non-parametric data) were applied.
Continuous variables were included in the analysis of covariance (ANCOVA) as covariates [children’s age (years), breast milk intake (g/day), paternal BMI (kg/m2), socioeconomic status [Winkler index], weight-for-height z-score]. Categorical variables were included as fixed factors (sex, physical activity, season). In case of unequal categorical variables (e.g., urbanicity), these variables were dummy coded. Total energy intake (TEI) was only considered as a confounder for variables that were not calculated as E%. In the basic model, sex and age were included. Then, each covariate was checked separately for interaction effects with sex and age. Those covariates or interactions with a p ≤ 0.1 and/or a partial eta squared (η2) ≥ 0.06 were added to the model. The backward method was used to build the fully adjusted model with the same criteria (p ≤ 0.1 and/or partial η2 ≥ 0.06). If heterogeneity of variances as an assumption of ANCOVA was violated for the fully adjusted model, simple bootstrap ANCOVA with 1,000 samples and bias-corrected accelerated (BCa) 95% confidence intervals (CI) were calculated.
Data transformations \(\left( {\log \left[ x \right],\log \left[ {x + 1} \right],{\text{ square root}}\left[ x \right]} \right)\) were applied if assumptions of ANCOVA were violated. Due to a high number of tests, the chance of type I errors had to be reduced. Consequently, p ≤ 0.001 is considered statistically significant. An η2 ≥ 0.01 is interpreted as small, η2 ≥ 0.06 as medium, and η2 ≥ 0.14 as large effect sizes [33].
Sensitivity analyses without outliers (modulus of standardized residuals > 3) were carried out. Results are presented if there was remarkable variance from the main analysis.
Due to the skewness of the data and the resulting violation of assumptions, ANCOVA of vitamin B12 and vitamin D intake could not be conducted. Instead, the vitamin B12 and D intakes were adjusted for 4.184 MJ (= 1,000 kcal/day) and tested for differences with a Kruskal–Wallis and a Dunn–Bonferroni post hoc test. Effect size r is calculated with \(r = \left| {\frac{z}{\sqrt n }} \right|\).
Results
Sample characteristics
Sample characteristics are presented in Table 1. The age of the study participants ranged from 0.9–4.3 years and 51.9% were boys (not shown). The majority of families lived in metropolitan or medium-sized urban cities, and the median SES was high. The proportion of children using any dietary supplement or vitamin B12 supplements was highest among VN children. The diet groups did not differ concerning most characteristics.
Micronutrient intake
Intakes (without supplements) of all vitamins except vitamin A (retinol eq) and vitamin D differed significantly between groups. VN children had the highest intake of vitamins E, K, B1, B6, folate, and C. In contrast, OM children had the highest intake of vitamins B2 and B12. Between VG and OM children, there were only significant differences in the intake of vitamins E, B2 and B12 (Table 2).
For minerals (Table 2), intakes (without supplements) of all minerals except zinc differed significantly between groups. VN children showed the highest intake of potassium, magnesium, and iron, whereas OM children had the highest intake of calcium and iodine. Between VG and OM children, only magnesium, iron, and iodine intake differed significantly.
When supplements were taken into account, vitamins B12 and D intake differed significantly between VN and VG, as well as VN and OM children (Table 3). The differences between the groups’ average nutrient intake were the same if supplements were included; only the effect sizes were usually greater without supplements. Table S1 (Online Resource 1) additionally shows the mean intake of vitamins, minerals, and long-chain n-3 fatty acids obtained from food and dietary supplements.
Fig. S2 (Online Resource 1) shows the intake of vitamins and minerals (without supplements) as percentages of the h-ARs [32] (%h-AR) by diet group. In all diet groups, median %h-AR was below 100% for vitamin D and iodine, as well as for iron if a low absorption is anticipated. Median %h-AR reached or was above 100% for vitamins A, E, B1, B6, C, folate, magnesium, zinc, and iron if a moderate absorption is anticipated. For vitamin B12, VN children clearly fell below the h-AR when supplements were not considered. Less than half of VN children reached the h-AR for calcium and vitamin B2, whereas the median intake of OM children reached the h-AR of both nutrients, while the median intake of VG children was between the other two groups (below the h-AR for vitamins B2 and B12, above the h-AR for calcium).
Fatty acid and cholesterol intake
The intake of SFA, PUFA, LA, ALA, DHA, and cholesterol differed significantly between all groups in the fully adjusted model with large effect sizes (Table 4). DHA and EPA intake with supplements differed significantly between VN and OM, as well as VG and OM children, respectively (Table 3). Without supplements, the difference in DHA intake was also significant between VG and VN children (Table 4). OM children had the highest intake of SFA, arachidonic acid (AA), EPA, DHA, and cholesterol. In contrast, VN children had the highest intake of PUFA, LA, and ALA.
Discussion
Micronutrient intake
In our study, VN children had the highest intake of several vitamins and minerals. In contrast, OM children had the highest intake of vitamin B2, calcium, and iodine. Intake of vitamin D and iodine was below the h-AR in all groups. Only the median intake of vitamins B2 and B12 as well as iron by VG and VN, and calcium by VN children, was below the h-AR.
Our results confirm that, in VN diets, vitamin B12 supplementation is essential to ensure sufficient intake as bioactive vitamin B12 only occurs in animal foods [34, 35]. The proportion of VN children who received vitamin B12 supplements was very high. However, the remaining VN children are at risk of a deficiency. Apart from supplements, other sources of vitamin B12 are fortified products. Indeed, a recent regional survey of food retailers in Germany revealed that a variety of vegan foods are fortified with vitamin B12 [36]. Nevertheless, regarding the usual amounts of these food products consumed, vitamin B12 levels are generally too low to meet h-AR. It is important to note that the median vitamin B12 intake of VG children was also below the h-AR [32]. In a recent review, as well as in the VeChi Youth Study, a higher risk of vitamin B12 deficiency not only for VN, but also for VG was described [4, 35]. Hence, both VN and VG children should be encouraged to use vitamin B12 supplements on a regular and reliable basis and should be screened for vitamin B12 deficiency. In contrast, the median vitamin B12 intake of OM children seemed to be adequate.
Dairy products are the main source of vitamin B2 and calcium in Germany [37]. Not only VN but also VG children consumed less of this food group than OM children (data not shown and [38]). In our study, the intake of vitamin B2 and calcium was substantially lower among VN and VG than among OM children. However, only the median intake of VN children did not reach the h-AR of calcium, whereas the median intake of VG children just reached the h-AR. Therefore, vitamin B2 supplementation or consumption of vitamin B2-fortified foods might be recommended for all VG and VN children. In contrast, the VeChi Youth Study showed a high prevalence (> 35%) of vitamin B2 blood concentrations below the reference value regardless of the diet group [4].
The average intake of vitamin D (without supplementation) of all groups was also inadequate in our study. Calcium and vitamin D are key nutrients to build and maintain a normal bone mass [39]. In a recent systematic review and meta-analysis of 20 cohort and cross-sectional studies (n = 37,134), VG and VN adults had lower bone mineral densities and VN a higher fracture rate than OM adults [39]. However, this meta-analysis has been criticized due to the lack of statistical adjustment in particular of the BMI [40, 41]. Our results are mostly in agreement with two cross-sectional studies from Poland (n = 50, 2–10 years and n = 187, 5–10 years) where VG and VN children had calcium and vitamin D intakes below the reference values (only without vitamin D supplementation in [6]). More relevantly, 25-OH vitamin D blood levels were lower and the concentration of serum bone metabolism markers was poorer than those of OM children [6, 13]. In the study by Desmond et al. [6], VG and VN subjects had less bone mineral content than OM subjects, which was more pronounced for VN children. Supplementation of vitamin D resolved low 25-OH vitamin D concentrations. Likewise, the VeChi Youth Study showed a high prevalence (> 30%) of 25-OH vitamin D in all diet groups being below the reference value [4]. However, Hovinen et al. [5] showed only borderline sufficient vitamin D concentrations in all VN participants. In another recent study from Poland with 53 VG and 53 OM children, there were no significant differences in calcium or vitamin D intake, as well as 25-OH vitamin D blood levels (80% of both groups included vitamin D supplements). Furthermore, the groups did not differ regarding the absolute values of bone mineral density, but VG children had on average lower total and lumbar spine BMD z-scores [7]. This is in accordance with the VeChi Youth Study [4]. Therefore, a higher intake of calcium should be encouraged for VN children and perhaps also for VG children since the median intake of the latter only just reached the h-AR. Whether vitamin D supplementation is necessary beyond infancy is under discussion in Germany [42, 43].
Iodine intake was inadequate in our study in all diet groups. Major dietary sources of iodine in the diet of children in Germany are iodised salt and dairy [44]. Salt was not recorded quantitatively in our dietary records; hence, intakes presented here might be underestimated. However, this bias should not differ between the groups. In our study, OM children had the highest average iodine intake, due to the highest consumption of dairy and fish (data not shown), but also of iodised salt and foods, in particular bread, produced with iodised salt (Table 1). This is in line with the results of the VeChi Youth Study where VN children and adolescents had significantly lower urinary iodine excretion than OM and VG subjects. However, the excretion of all groups was on average below the reference value [45]. As iodine intake during childhood is associated with cognitive development, there is an urgent need for increased intake, for example via broader use of iodised salt for food production, in particular bread [46]. Alternatively, on an individual basis, iodine can be supplemented as recommended for breastfed infants in Germany without iodine-fortified weaning food [47, 48].
VN and VG diets are regarded as risk factors for iron deficiency [26, 49]. Nevertheless, available studies on iron deficiencies among VG and VN children and adolescents yielded heterogeneous results [50]. For example, Desmond et al. [6] found a higher prevalence of iron deficiency anaemia in VN children, whereas Hovinen et al. [5] found no differences between VN and OM children in serum ferritin. The ferritin concentrations in the VeChi Youth Study were significantly higher in OM participants than in VG and VN ones. Nevertheless, the prevalence of haemoglobin and ferritin concentrations below the cutoff was low [4]. As in other studies [4,5,6], VN and VG children in our study had higher iron intakes than OM children. The observed high iron intake reflects the high iron content of some plant foods, e.g., whole grain or legumes. Nevertheless, the bioavailability of this non-heme iron is lower than that of heme iron from meat. Besides, the absorption of non-heme iron is dependent on promoting factors (e.g., organic acids such as ascorbic acid) and impairing factors (e.g., phytate, polyphenols) [34, 49]. Food preparation, i.e., leavening, fermentation, and soaking of legumes and grains, reduces the content of phytate and improves iron absorption [49]. Moreover, some ‘partial physiologic adaptive responses in the absorption of non-heme iron are discussed for VG and VN. Besides, this cannot be verified for our participants with the current study design, which did not allow measurement of the bioavailability of nutrients. However, if we assume moderate absorption for an OM diet and low absorption for a VG and VN diet, VG and VN children are below the h-AR for low absorption and OM children are above the h-AR for moderate absorption. On this basis, higher iron intake should be encouraged only in VG and VN children.
Plant foods are the main sources of folate in the diet [51]. As a result, the median folate intake of VN was significantly higher than that of VG and OM children. The higher folate intake of VN children is in line with other recent studies [4,5,6]. However, all groups met the h-AR on average.
Fatty acids and cholesterol intake
Altogether, the fat quality of the diet of VN children was the most favourable, followed by that of VG children; the VN children’s intake of MUFA and PUFA was highest and intake of SFA was lowest. Only OM children exceeded on average the recommended maximum daily intake of dietary cholesterol [80 mg/1000 kcal (4.184 MJ)] for children [43].
In contrast, the EPA and DHA intake of VN and VG were substantially lower than those of OM children. In OM diets, fatty fish is the major source for preformed DHA. The long-chain n-3 fatty acid is especially important in early childhood. Besides others, it plays a central role in eye and brain development in childhood [52]. For VG or VN diets, plant supplements from microalgae (e.g., Schizochytrium sp.) with DHA (+ EPA) are available, e.g., as fortified plant oils. The endogenous formation of DHA is low because the conversion rate of ALA into DHA is poor. However, it might be increased by replacing LA with ALA in the diet because both fatty acids compete for the same enzymes [52]. There was a trend to a higher LA:ALA ratio from the OM to the VG to the VN diet group. To improve the LA:ALA ratio, the consumption of good ALA sources (i.e., linseed oil, rapeseed oil) are recommended, especially in VG and VN children where sources of preformed EPA and DHA are limited.
Strengths and limitations
Our study has some limitations. The cross-sectional design of the VeChi Diet Study allows no conclusion on the long-term food intake of the children. Nevertheless, follow-up investigations are planned. Furthermore, our data are based on proxy reporting by the parents (or the family paediatrician in case of BW and BH). Hence, the anthropometric data are especially susceptible to bias (discussed in [23]) [53, 54]. However, we used weighed dietary records because they provide the best estimate for children aged 0.5–4 years [55]. Parents were instructed to maintain the usual diet, and every protocol was checked for completeness and plausibility. Missing information was immediately collected from the parents. As seen in other investigations, underreporting is unlikely (only 1%) in 1–5-year-old children [56]. Other studies such as the DONALD study [57] have been using this method successfully for many years. Nevertheless, the data have not been confirmed with objective biomarkers. Nutrient intake was compared to the h-AR. Unfortunately, there are no h-ARs for vitamin K, potassium, and fatty acids. Furthermore, in general, the data basis to derive DRVs for children is sparse and ARs do not take the special requirements of a VN diet into account, except for iron and zinc.
The online questionnaire was not validated, but the questions were partly taken from a questionnaire of a representative, validated health survey in Germany (German Health Interview and Examination Survey for Children and Adolescents) [58], complemented by VG and VN-specific questions. Another limitation is the estimation of breast milk intake, which is based on reliable data of the DONALD study but is not as exact as weighing the children before and after each breastfeeding. Additionally, the mothers’ supplementation was not gathered. Moreover, the composition of breast milk of VN and VG women might vary from that of OM women. Additionally, three days of dietary record might not be enough to observe foods that are not consumed daily such as fish. Therefore, the DHA and iodine intake of OM children could be higher than reported here. Since 3 days of dietary records might also not be sufficient to represent regular supplement intake, this question was also asked in the online questionnaire. The number of participants who reported taking a supplement in the online questionnaire but not in the dietary record was highest among the OM children. Because the number of VN and VG children in Germany is not known, it was not possible to select a representative sample. Additionally, the OM control group does not represent the average German population of that age. As in all nutrition studies, participants tend to be more health conscious than the average population. The majority of the families lived in medium-sized urban or metropolitan cities and had a high median SES. As a consequence, the differences between the diet groups might have been even higher when compared to the average population than to the health-conscious control group in our study.
However, a major strength of the VeChi Diet Study is the detailed dietary record that is well suited for young children and the assessment of special food groups of VN and VG diets (e.g., meat and dairy alternatives). The nutrient database LEBTAB ensures high accuracy in nutrient intake due to brand-specific estimations of ingredients and nutrient contents by recipe simulation including fortified foods and supplements. Therefore, the LEBTAB database is more accurate when assessing the food intake of VG and VN than the national database BLS. Therefore, the VeChi Diet Study comprises a unique data pool of the understudied population of VG and VN children at the age of 1–3 years. For the future, repeated examinations of this cohort, preferably complemented with biomarkers of the nutritional status and measurements of child development, are in preparation.
Conclusion
In the VeChi Diet Study, VN and VG children had a more favourable intake of several micronutrients and fatty acids than that of OM children, regardless of the intake of dietary supplements. Critical nutrients for all three diet groups were vitamin D (without supplements), iodine, and DHA, with OM children having the highest intakes. For VG and VN children, vitamins B2 and B12, and iron are considered critical, as well as calcium for VN children. Therefore, for VG and VN children foods rich in vitamin B2 (e.g., yeast, nuts, legumes), iron (in combination with foods that increase the bioavailability of iron), vitamin B2-fortified plant-based dairy alternatives, and iodised salt are recommended. Furthermore, DHA supplementation should be encouraged, in addition to reliable supplementation of vitamin B12 and possibly vitamin B2. For VN children, calcium-fortified plant-based dairy alternatives and mineral water with high calcium content can improve calcium intake. OM children should consume more PUFA, fatty fish or more ecological alternatives, DHA supplements (from microalgae), and less cholesterol and SFA containing animal foods. Supplementation of vitamin D should be considered in all diet groups, especially in autumn and winter. To evaluate the health and nutritional status of VN and VG children, our data should be complemented with biomarkers and longitudinal studies of VN and VG diets during growth.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Patelakis E, Lage Barbosa C, Haftenberger M, Brettschneider A-K, Lehmann F, Heide K, Frank M, Perlitz H, Richter A, Mensink G (2019) Prevalence of vegetarian diet among children and adolescents in Germany. Results from EsKiMo II Ernähr Umsch 66:85–91. https://doi.org/10.4455/eu.2019.018
Schürmann S, Kersting M, Alexy U (2017) Vegetarian diets in children: a systematic review. Eur J Nutr 56:1797–1817. https://doi.org/10.1007/s00394-017-1416-0
Keller M, Müller S (2015) Vegetarian and vegan diets in children—pre-study with preliminary data. 12th European Nutrition Conference (FENS), Berlin, Germany, October 20–23, 2015: Abstracts. Ann Nutr Metab. https://doi.org/10.1159/000440895
Alexy U, Fischer M, Weder S, Längler A, Michalsen A, Sputtek A, Keller M (2021) Nutrient intake and status of german children and adolescents consuming vegetarian, vegan or omnivore diets: results of the VeChi Youth Study. Nutrients 13:1707. https://doi.org/10.3390/nu13051707
Hovinen T, Korkalo L, Freese R, Skaffari E, Isohanni P, Niemi M, Nevalainen J, Gylling H, Zamboni N, Erkkola M, Suomalainen A (2021) Vegan diet in young children remodels metabolism and challenges the statuses of essential nutrients. EMBO Mol Med 13:e13492. https://doi.org/10.15252/emmm.202013492
Desmond MA, Sobiecki JG, Jaworski M, Płudowski P, Antoniewicz J, Shirley MK, Eaton S, Książyk J, Cortina-Borja M, de Stavola B, Fewtrell M, Wells JCK (2021) Growth, body composition, and cardiovascular and nutritional risk of 5- to 10-y-old children consuming vegetarian, vegan, or omnivore diets. Am J Clin Nutr 113:1565–1577. https://doi.org/10.1093/ajcn/nqaa445
Ambroszkiewicz J, Chełchowska M, Szamotulska K, Rowicka G, Klemarczyk W, Strucińska M, Gajewska J (2019) Bone status and adipokine levels in children on vegetarian and omnivorous diets. Clin Nutr 38:730–737. https://doi.org/10.1016/j.clnu.2018.03.010
Nathan I, Hackett AF, Kirby S (1996) The dietary intake of a group of vegetarian children aged 7–11 years compared with matched omnivores. Br J Nutr 75:533–544
Thane CW, Bates CJ (2000) Dietary intakes and nutrient status of vegetarian preschool children from a British national survey. J Hum Nutr Diet 13:149–162. https://doi.org/10.1046/j.1365-277x.2000.00227.x
Ambroszkiewicz J, Klemarczyk W, Chelchowska M, Gajewska J, Laskowska-Klita T (2006) Serum homocysteine, folate, vitamin B12 and total antioxidant status in vegetarian children. Adv Med Sci 51:265–268
Laskowska-Klita T, Chelchowska M, Ambroszkiewicz J, Gajewska J, Klemarczyk W (2011) The effect of vegetarian diet on selected essential nutrients in children. Med Wieku Rozwoj 15:318–325
Gorczyca D, Prescha A, Szeremeta K (2013) Impact of vegetarian diet on serum immunoglobulin levels in children. Clin Pediatr (Phila) 52:241–246. https://doi.org/10.1177/0009922812472250
Ambroszkiewicz J, Klemarczyk W, Gajewska J, Chelchowska M, Laskowska-Klita T (2007) Serum concentration of biochemical bone turnover markers in vegetarian children. Adv Med Sci 52:279–282
Taylor A, Redworth EW, Morgan JB (2004) Influence of diet on iron, copper, and zinc status in children under 24 months of age. Biol Trace Elem Res 97:197–214. https://doi.org/10.1385/BTER:97:3:197
Gorczyca D, Prescha A, Szeremeta K, Jankowski A (2013) Iron status and dietary iron intake of vegetarian children from Poland. Ann Nutr Metab 62:291–297. https://doi.org/10.1159/000348437
Fulton JR, Hutton CW, Stitt KR (1980) Preschool vegetarian children. Dietary and anthropometric data [Abstract]. J Am Diet Assoc 76:360–365
Sanders TA, Manning J (1992) The growth and development of vegan children. J Hum Nutr Diet 5:11–21
Sanders TA, Purves R (1981) An anthropometric and dietary assessment of the nutritional status of vegan preschool children [Abstract]. J Hum Nutr 35:349–357
Sanders TA (1988) Growth and development of British vegan children. Am J Clin Nutr 48:822–825. https://doi.org/10.1093/ajcn/48.3.822
Wallace TC, Reider C, Fulgoni VL (2013) Calcium and vitamin D disparities are related to gender, age, race, household income level, and weight classification but not vegetarian status in the United States: analysis of the NHANES 2001–2008 data set. J Am Coll Nutr 32:321–330. https://doi.org/10.1080/07315724.2013.839905
Nathan I, Hackett AF, Kirby S (1997) A longitudinal study of the growth of matched pairs of vegetarian and omnivorous children, aged 7–11 years, in the north-west of England. Eur J Clin Nutr 51:20–25
Ambroszkiewicz J, Klemarczyk W, Gajewska J, Chelchowska M, Rowicka G, Oltarzewski M, Laskowska-Klita T (2011) Serum concentration of adipocytokines in prepubertal vegetarian and omnivorous children. Med Wieku Rozwoj 15:326–334
Weder S, Hoffmann M, Becker K, Alexy U, Keller M (2019) Energy, macronutrient intake, and anthropometrics of vegetarian, vegan, and omnivorous children (1–3 years) in Germany (VeChi Diet Study). Nutrients 11:832. https://doi.org/10.3390/nu11040832
Hilbig A, Drossard C, Kersting M, Alexy U (2015) Nutrient adequacy and associated factors in a nationwide sample of german toddlers. J Pediatr Gastroenterol Nutr 61:130–137. https://doi.org/10.1097/MPG.0000000000000733
Sichert-Hellert W, Kersting M, Chahda C, Schäfer R, Kroke A (2007) German food composition database for dietary evaluations in children and adolescents. J Food Compost Anal 20:63–70. https://doi.org/10.1016/j.jfca.2006.05.004
Richter M, Boeing H, Grünewald-Funk D, Heseker H, Kroke a, Leschik-Bonnet E, Oberritter H, Strohm D, Watzl, B. for the German Nutrition Society (2016) Vegan diet. Position of the German nutrition society (DGE). Ernähr Umsch 63:92–102. https://doi.org/10.4455/eu.2016.021
Ferrara P, Corsello G, Quattrocchi E, Dell’Aquila L, Ehrich J, Giardino I, Pettoello-Mantovani M (2017) Caring for infants and children following alternative dietary patterns. J Pediatr 187:339-340.e1. https://doi.org/10.1016/j.jpeds.2017.04.053
Melina V, Craig W, Levin S (2016) Position of the academy of nutrition and dietetics: vegetarian diets. J Acad Nutr Diet 116:1970–1980. https://doi.org/10.1016/j.jand.2016.09.025
Agnoli C, Baroni L, Bertini I, Ciappellano S, Fabbri A, Papa M, Pellegrini N, Sbarbati R, Scarino ML, Siani V, Sieri S (2017) Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. Nutr Metab Cardiovasc Dis 27:1037–1052. https://doi.org/10.1016/j.numecd.2017.10.020
WHO (2011) Child growth standards. WHO Anthro (version 3.2.2, January 2011) and macros. http://www.who.int/childgrowth/software/en/. Accessed 06 Sep 2017
Winkler J, Stolzenberg H (2009) Adjustierung des sozialen-schicht-index für die anwendung im kinder- und jugendgesundheitssurvey (KiGGS) 2003/2006, vol 7. Wismarer Diskussionspapiere Hochschule, Wismar, Fakultät für Wirtschaftswissenschaften, Wismar, Germany
Allen LH, Carriquiry AL, Murphy SP (2020) Perspective: proposed harmonized nutrient reference values for populations. Adv Nutr 11:469–483. https://doi.org/10.1093/advances/nmz096
Pallant J (2007) SPSS survival manual: a step by step guide to data analysis using SPSS for windows, 3rd edn. Open University Press, Maidenhead
Trumbo P, Yates AA, Schlicker S, Poos M (2001) Dietary reference intakes. Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 101(3):294–301
Pawlak R, Lester SE, Babatunde T (2014) The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: a review of literature. Eur J Clin Nutr 68:541–548. https://doi.org/10.1038/ejcn.2014.46
Marczykowski F, Breidenassel C (2017) Vegan diet: Reaching the reference values for nutrient intake of critical nutrients. Assortment and necessity of fortified foods. Ernahrungs Umschau 64:2–10
Rubner-Institut M (2008) National verzehrsstudie II. Ergebnisbericht, Teil, 2, Karlsruhe
Alexy U, Fischer M, Weder S, Längler A, Michalsen A, Keller M (2021) Food group intake of children and adolescents (6–18 years) on a vegetarian, vegan, or omnivore diet: results of the VeChi Youth Study. Br J Nutr. https://doi.org/10.1017/S0007114521003603
Iguacel I, Miguel-Berges ML, Gómez-Bruton A, Moreno LA, Julián C (2019) Veganism, vegetarianism, bone mineral density, and fracture risk: a systematic review and meta-analysis. Nutr Rev 77:1–18. https://doi.org/10.1093/nutrit/nuy045
Appleby PN, Key TJA (2019) Letter: Veganism, vegetarianism, bone mineral density, and fracture risk: a systematic review and meta-analysis. Nutr Rev 77:451. https://doi.org/10.1093/nutrit/nuz011
Karavasiloglou N, Selinger E, Gojda J, Rohrmann S, Kühn T (2020) Differences in bone mineral density between adult vegetarians and nonvegetarians become marginal when accounting for differences in anthropometric factors. J Nutr. https://doi.org/10.1093/jn/nxaa018
Reinehr T, Schnabel D, Wabitsch M, Bechtold-Dalla Pozza S, Bührer C, Heidtmann B, Jochum F, Kauth T, Körner A, Mihatsch W, Prell C, Rudloff S, Tittel B, Woelfle J, Zimmer K-P, Koletzko B (2019) Vitamin D supplementation after the second year of life: joint position of the Committee on Nutrition, German Society for Pediatric and Adolescent Medicine (DGKJ e.V.), and the German Society for Pediatric Endocrinology and Diabetology (DGKED e.V.). Mol Cell Pediatr 6:3. https://doi.org/10.1186/s40348-019-0090-0
DGE, Öge, SGE, Sve (2020) Referenzwerte für die nährstoffzufuhr, 2nd edn. Neuer Umschau Buchverlag, Bonn (6., aktualisierte Ausgabe)
Johner SA, Thamm M, Nöthlings U, Remer T (2013) Iodine status in preschool children and evaluation of major dietary iodine sources: a German experience. Eur J Nutr 52:1711–1719. https://doi.org/10.1007/s00394-012-0474-6
Alexy U, Fischer M, Weder S, Längler A, Michalsen A, Keller M (2020) Vegetarische und vegane ernährung bei kindern und jugendlichen in Deutschland—die VeChi-Youth-Studie. Deutsche Gesellschaft für Ernährung (DGE) e.V. 14. Ernährungsbericht, Bonn, pp 289–354
Johner SA, Günther ALB, Remer T (2011) Current trends of 24-h urinary iodine excretion in German schoolchildren and the importance of iodised salt in processed foods. Br J Nutr 106:1749–1756. https://doi.org/10.1017/S0007114511005502
Alexy U, Drossard C, Kersting M, Remer T (2009) Iodine intake in the youngest: impact of commercial complementary food. Eur J Clin Nutr 63:1368–1370. https://doi.org/10.1038/ejcn.2009.62
Koletzko B, Bauer C-P, Cierpka M, Cremer M, Flothkötter M, Graf C, Heindl I, Hellmers C, Kersting M, Krawinkel M, Przyrembel H, Vetter K, Weißenborn A, Wöckel A (2016) Ernährung und bewegung von säuglingen und stillenden frauen. Monatsschr Kinderheilkd 164:771–798. https://doi.org/10.1007/s00112-016-0147-2
Gibson RS, Heath AM, Szymlek-Gay EA (2014) Is iron and zinc nutrition a concern for vegetarian infants and young children in industrialized countries? Am J Clin Nutr 100:459–468. https://doi.org/10.3945/ajcn.113.071241
Pawlak R, Bell K (2017) Iron status of vegetarian children: a review of literature. Ann Nutr Metab 70:88–99. https://doi.org/10.1159/000466706
Bundesministerium für Ernährung und Landwirtschaft (2017) Bundeslebensmittelschlüssel 3.02. https://www.blsdb.de/. Accessed 25 Aug 2021
Calder PC (2015) Functional roles of fatty acids and their effects on human health. JPEN J Parenter Enteral Nutr 39:18S-32S. https://doi.org/10.1177/0148607115595980
Huybrechts I, Himes JH, Ottevaere C, de Vriendt T, de Keyzer W, Cox B, van Trimpont I, de Bacquer D, de Henauw S (2011) Validity of parent-reported weight and height of preschool children measured at home or estimated without home measurement: a validation study. BMC Pediatr 11:63. https://doi.org/10.1186/1471-2431-11-63
Himes JH (2009) Challenges of accurately measuring and using BMI and other indicators of obesity in children. Pediatrics 124(Suppl 1):S3–S22. https://doi.org/10.1542/peds.2008-3586D
Burrows TL, Martin RJ, Collins CE (2010) A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J Am Diet Assoc 110:1501–1510. https://doi.org/10.1016/j.jada.2010.07.008
Sichert-Hellert W, Kersting M, Schöch G (1998) Underreporting of energy intake in 1 to 18 year old German children and adolescents. Z Ernahrungswiss 37:242–251. https://doi.org/10.1007/s003940050023
Kroke A, Manz F, Kersting M, Remer T, Sichert-Hellert W, Alexy U, Lentze MJ (2004) The DONALD study. History, current status and future perspectives. Eur J Nutr 43:45–54. https://doi.org/10.1007/s00394-004-0445-7
Hölling H, Schlack R, Kamtsiuris P, Butschalowsky H, Schlaud M, Kurth BM (2012) Die KiGGS-Studie. Bundesweit repräsentative längs- und querschnittstudie zur gesundheit von Kindern und jugendlichen im rahmen des gesundheitsmonitorings am Robert Koch-Institut (The KiGGS study. Nationwide representative longitudinal and cross-sectional study on the health of children and adolescents within the framework of health monitoring at the Robert Koch Institute). Bundesgesundheitsbl-Gesundheitsforsch-Gesundheitsschutz 55:836–842. https://doi.org/10.1007/s00103-012-1486-3
Acknowledgements
The authors would like to thank all children and their families for their participation in the VeChi Diet Study and the DONALD study. Furthermore, we are grateful to all members of the study staff for collecting and coding the dietary records, especially Nicole Janz, who was also involved in the study concept. We would also like to thank the Erna Graff Stiftung für Tierschutz (Erna Graff Foundation for Animal Protection), Berlin, which funded the VeChi Diet Study.
Funding
Open Access funding enabled and organized by Projekt DEAL. The Erna Graff Stiftung für Tierschutz (Erna Graff Foundation for Animal Protection), Berlin funded the VeChi Diet Study. The Ministry of Science and Research of North Rhine–Westphalia, Germany is financially supporting the DONALD study. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest. MK is an unsalaried member of the scientific advisory board of ProVeg Germany and received lecture fees from the Alpro Foundation.
Ethics approval
The observational and non-invasive VeChi Diet Study was conducted according to the guidelines of the Declaration of Helsinki. The ethics committee of the University of Bonn approved it (046/17).
Consent to participate
Written informed consent was obtained from the parents.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weder, S., Keller, M., Fischer, M. et al. Intake of micronutrients and fatty acids of vegetarian, vegan, and omnivorous children (1–3 years) in Germany (VeChi Diet Study). Eur J Nutr 61, 1507–1520 (2022). https://doi.org/10.1007/s00394-021-02753-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02753-3