Abstract
Purpose
Theabrownin (TB)-containing Pu-erh tea has been shown to be hypolipidemic in rats fed a high-fat diet. Physical exercise such as swinging is also known to reduce obesity. We hypothesized that TB in combination with swinging can synergistically ameliorate obesity and insulin resistance in rats with metabolic syndrome.
Methods
TB, rosiglitazone, or lovastatin (controls) was administered by gavage to rats fed a diet high in fat, sugar, and salt. A subgroup of the rats was subjected to a 30-min daily swinging exercise regimen, whereas the other rats did not exercise.
Results
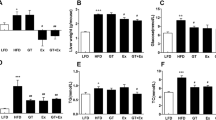
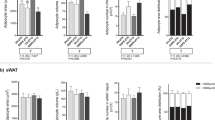
Theabrownin in combination with swinging was found to significantly improve serum lipid status and prevent development of obesity and insulin resistance in rats. Liver transcriptomics data suggested that theabrownin activated circadian rhythm, protein kinase A, the adenosine monophosphate-activated protein kinase, and insulin signaling pathways by enhancing cyclic adenosine monophosphate levels and, hence, accelerating nutrient metabolism and the consumption of sugar and fat. The serum dopamine levels in rats increased significantly after exercise. In parallel work, intraperitoneal dopamine injections were shown to significantly reduce weight gain and prevent the elevation in triglyceride levels that would otherwise be induced by the high fat-sugar–salt diet. Theabrownin prevented obesity and insulin resistance mainly by affecting the circadian rhythm, while swinging exercise stimulated the overproduction of dopamine to accelerate metabolism of glucose and lipid.
Conclusions
Theabrownin and exercise synergistically ameliorated metabolic syndrome in rats and effectively prevented obesity.





Similar content being viewed by others
References
Lv HP, Zhang YJ, Lin Z, Liang YR (2013) Processing and chemical constituents of Pu-erh tea: a review. Food Res Int 53:608–618. https://doi.org/10.1016/j.foodres.2013.02.043
Cao ZH, Gu DH, Lin QY, Xu ZQ, Huang QC, Rao H, Liu EW, Jia JJ, Ge CR (2011) Effect of Pu-erh tea on body fat and lipid profiles in rats with diet-induced obesity. Phytother Res 25:234–238. https://doi.org/10.1002/ptr.3247
Ding Y, Zou X, Jiang X, Wu J, Zhang Y, Chen D, Liang B (2015) Pu-erh tea down-regulates sterol regulatory element-binding protein and stearyol-COA desaturase to reduce fat storage in Caenorhabditis elegans. PLoS One 10:e0113815. https://doi.org/10.1371/journal.pone.0113815
Hou Y, Shao W, Xiao R, Xu K, Ma Z, Johnstone BH, Du Y (2009) Pu-erh tea aqueous extracts lower atherosclerotic risk factors in a rat hyperlipidemia model. Exp Gerontol 44:434–439. https://doi.org/10.1016/j.exger.2009.03.007
Hu WY, Ma XH, Zhou WY, Li XX, Sun TT, Sun H (2017) Preventive effect of Silibinin in combination with Pu-erh tea extract on non-alcoholic fatty liver disease in ob/ob mice. Food Funct 8:1105–1115. https://doi.org/10.1039/C6FO01591C
Zeng L, Yan J, Luo L, Zhang D (2015) Effects of Pu-erh tea aqueous extract (PTAE) on blood lipid metabolism enzymes. Food Funct 6:2008–2016. https://doi.org/10.1039/c5fo00362h
Gong JS, Tang C, Peng CX (2012) Characterization of the chemical differences between solvent extracts from Pu-erh tea and Dian Hong black tea by CP–Py–GC/MS. J Anal Appl Pyrol 95:189–197. https://doi.org/10.1016/j.jaap.2012.02.006
Gong J, Peng C, Chen T, Gao B, Zhou H (2010) Effects of theabrownin from pu-erh tea on the metabolism of serum lipids in rats: mechanism of action. J Food Sci 75:H182–H189. https://doi.org/10.1111/j.1750-3841.20
Peng CX, Liu J, Liu HR, Zhou HJ, Gong JS (2013) Influence of different fermentation raw materials on pyrolyzates of Pu-erh tea theabrownin by Curie-point pyrolysis-gas chromatography–mass spectroscopy. Int J Biol Macromol 54:197–203. https://doi.org/10.1016/j.ijbiomac.2012.12.021
Chen DX (2012) Psychological clairvoyance of traditional sports-swing. J Guizhou Norm Univ (Nat Sci) 30:26–29
Zhang XX, Tao ZB, Wang JB (2004) Folk sports in Chinese festivals. J Beijing Univ Phys Educ 27:1581–1583
Meyns P, Bruijn SM, Duysens J (2013) The how and why of arm swing during human walking. Gait Posture 38:555–562. https://doi.org/10.1016/j.gaitpost.2013.02.006
Modica JR, Kram R (2005) Metabolic energy and muscular activity required for leg swing in running. J Appl Physiol 98:2126–2131. https://doi.org/10.1152/japplphysiol.00511.2004
Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Alemãn JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G (2008) Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab 7:125–134. https://doi.org/10.1016/j.cmet.2007.11.013
Eckel RH, Grundy SM, Zimmet PZ (2005) The metabolic syndrome. Lancet 365:1415–1428. https://doi.org/10.1016/S0140-6736(05)67777-X
Liu J, Peng CX, Gao B, Gong JS (2016) Serum metabolomics analysis of rat after intragastric infusion of Pu-erh theabrownin. J Sci Food Agric 96:3708–3716. https://doi.org/10.1002/jsfa.7556
Wu EK, Wang QP, Gong JS, Zhang TT (2019) Effect of fermentation methods on theabrownin composition of Pu-erh tea. Food Sci 40:215–221. https://doi.org/10.7506/spkx1002-6630-20171201-006
United States Food and Drug Administration (2005) Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Center for Drug Evaluation and Research (CDER), Rockville
Matthews DR, Hosker JR, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419. https://doi.org/10.1007/BF00280883
Zhang TT, Wu EK, Peng CX, Tan C, Gong JS (2018) Effects of theabrownins extracted from Pu-erh tea on blood glucose and blood lipid indexes of rats with high sugar diet. Food Sci Technol 43:7. https://doi.org/10.13684/j.cnki.spkj.2018.07.011
Nieto-Vazquez I, Fernã N-VS, De AC, Lorenzo M (2008) Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes 57:3211–3221. https://doi.org/10.2337/db07-1062
Tilg H, Shapiro L, Atkins MB, Dinarello CA, Mier JW (1993) Induction of circulating and erythrocyte-bound IL-8 by IL-2 immunotherapy and suppression of its in vitro production by IL-1 receptor antagonist and soluble tumor necrosis factor receptor (p75) chimera. J Immunol 151(6):3299–3307
Grippo RM, Purohit AM, Zhang Q, Zweifel LS, Güler AD (2017) Direct midbrain dopamine input to the suprachiasmatic nucleus accelerates circadian entrainment. Curr Biol 27:2475–2645. https://doi.org/10.1016/j.cub.2017.06.084
Stauffer W, Lak A, Yang A, Borel M, Paulsen O, Boyden E, Schultz W (2016) Dopamine neuron-specific optogenetic stimulation in rhesus macaques. Cell 166:1564–1571. https://doi.org/10.1016/j.cell.2016.08.024
Kebabian JW (1978) Multiple classes of dopamine receptors in mammalian central nervous system: the involvement of dopamine-sensitive adenylyl cyclase. Life Sci 23:479–483. https://doi.org/10.1016/0024-3205(78)90157-1
Kohlie R, Perwitz N, Resch J, Schmid SM, Lehnert H, Klein J, Iwen KA (2016) Dopamine directly increases mitochondrial mass and thermogenesis in brown adipocytes. J Mol Endocrinol 58:57–66. https://doi.org/10.1530/JME-16-0159
Pappa KI, Gazouli M, Anastasiou E, Iliodromiti Z, Antsaklis A, Anagnou NP (2013) The major circadian pacemaker ARNT-like protein-1 (BMAL1) is associated with susceptibility to gestational diabetes mellitus. Diabetes Res Clin Pract 99:151–157. https://doi.org/10.1016/j.diabres.2012.10.015
Richards J, Diaz AN, Gumz ML (2014) Clock genes in hypertension: novel insights from rodent models. Blood Press Monit 19:249–254. https://doi.org/10.1097/MBP.0000000000000060
Hatanaka F, Matsubara C, Myung J, Yoritaka T, Kamimura N, Tsutsumi S, Kanai A, Suzuki Y, Sassone-Corsi P, Aburatani H (2010) Genome-wide profiling of the core clock protein bmal1 targets reveals a strict relationship with metabolism. Mol Cell Biol 30:5636–5648. https://doi.org/10.1016/j.neures.2011.07.226
Rudic RD, Mcnamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA (2004) BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2:e377. https://doi.org/10.1371/journal.pbio.0020377
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, Mcdearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR (2005) Obesity and metabolic syndrome in circadian clock mutant mice. Science 308:1043–1045. https://doi.org/10.1126/science.1108750
Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH (2010) Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinemia and diabetes. Nature 466:627–631. https://doi.org/10.1038/nature09253
Lee YJ, Han DH, Pak YK, Cho S (2012) Circadian regulation of low density lipoprotein receptor promoter activity by CLOCK/BMAL1, Hes1 and Hes6. Exp Mol Med 44:642–652. https://doi.org/10.3858/emm.2012.44.11.073
Finck BN, Kelly DP (2006) PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116:615–622. https://doi.org/10.1172/JCI27794
Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM (2003) Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell 113:159–170. https://doi.org/10.1016/S0092-8674(03)00269-1
Hubacek JA, Bobkova D (2006) Role of Cholesterol 7α-hydroxylase (CYP7A1) in nutrigenetics and pharmacogenetics of cholesterol lowering. Mol Diagn Ther 10:93–100. https://doi.org/10.1007/BF03256448
Shi Y, Liu C (2017) Study on the integration of circadian clock and energy metabolism. Chin J Cell Biol 39:261–270
Hatori M, Vollmers C, Zarrinpar A, Ditacchio L, Bushong E, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JJ (2012) Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15:848–860. https://doi.org/10.1016/j.cmet.2012.04.019
Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, Bradfield CA, Vaughan CH, Eiden M, Masoodi M (2012) Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med 18:1768–1777. https://doi.org/10.1038/nm.2979
Bass J, Takahashi JS (2010) Circadian integration of metabolism and energetics. Science 330:1349–1354. https://doi.org/10.1126/science.1195027
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31560456, 81860608) and Yunnan Agricultural Foundation Projects [2017FG001(-011) and 2017FG001(-089)].
Author information
Authors and Affiliations
Contributions
JSG obtained financial support, and designed and oversaw this study. EKW performed the swinging research and analyzed data. TTZ performed the non-swinging research and analyzed data. CT designed the experimental swing. QPW, CXP, and YC prepared, reviewed, and edited the manuscript. All authors contributed to the discussion, read the final manuscript and approved it. JSG is the guarantor of this work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Animal ethics statement
Experiments on animals were conducted in full compliance with the Yunnan Agricultural University institutional and Chinese national guidelines for care and use of laboratory animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, E., Zhang, T., Tan, C. et al. Theabrownin from Pu-erh tea together with swinging exercise synergistically ameliorates obesity and insulin resistance in rats. Eur J Nutr 59, 1937–1950 (2020). https://doi.org/10.1007/s00394-019-02044-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-02044-y