Abstract
Purpose
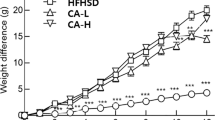
Rats fed a long-term sucrose-rich diet (SRD) developed adipose tissue dysfunction. In the adipose tissue of these SRD-fed rats, the present study analyzed the possible beneficial effects of dietary Salba (chia) seeds in improving or reversing the depletion of antioxidant defenses, changes in pro-inflammatory cytokines and ROS production.
Methods
Wistar rats were fed a SRD for 3 months. After that, half of the animals continued with the SRD until month 6, while in the other half, corn oil was replaced by chia seeds for 3 months (SRD + chia). A reference group consumed a control diet all the time.
Results
Compared with the SRD-fed rats, the animals fed a SRD + chia showed a reduction in epididymal fat pad weight; the activities of antioxidant enzymes CAT, SOD and GPx returned to control values, while GR significantly improved; mRNA GPx increased, and both mRNA SOD and the redox state of glutathione returned to control values; a significant increase in the expression of Nrf2 was recorded. These results were accompanied by a decrease in XO activity and ROS contents as well as plasma IL-6 and TNF-α levels. Chia seeds reversed the decrease in PPARγ protein mass level and increased the n-3/n-6 fatty acids ratio of membrane phospholipids. Besides, dyslipidemia and insulin sensitivity were normalized.
Conclusion
This study provides new information concerning some mechanisms related to the beneficial effects of dietary chia seeds in reversing adipose tissue oxidative stress and improving the adipose tissue dysfunction induced by a SRD.




Similar content being viewed by others
References
Bruce KD, Hanson MA (2010) The developmental origins, mechanisms, and implications of metabolic syndrome. J Nutr 140:648–652
Korkmaz GG, Altinoglu E, Civelek S, Sozer V, Erdenen F, Tabak O, Uzun H (2013) The association of oxidative stress markers with conventional risk factors in the metabolic syndrome. Metabolism 62:828–835
Chen GC, Huang CY, Chang MY, Chen CH, Chen SW, Huang CJ, Chao PM (2011) Two unhealthy dietary habits featuring a high fat content and a sucrose-containing beverage intake, alone or in combination, on inducing metabolic syndrome in Wistar rats and C57BL/6 J mice. Metabolism 60:155–164
Wei Y, Wang D, Topczewski F, Pagliassotti MJ (2007) Fructose-mediated stress signaling in the liver: implications for hepatic insulin resistance. J Nutr Biochem 18:1–9
Lombardo YB, Chicco AG (2006) Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. Rev J Nutr Biochem 17:1–13
Poudyal H, Panchal SK, Diwan V, Brown L (2011) Omega-3 fatty acids and metabolic syndrome: effects and emerging mechanisms of action. Prog Lipid Res 50:372–387
Barceló-Coblijn G, Murphy EJ (2009) Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog Lipid Res 48:355–374
da Silva Marineli R, Aguiar Moraes É, Alves Lenquiste S, Texeira Godoy A, Nogueira Eberlin M, Maróstica MR Jr (2014) Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispanica L.). LWT Food Sci Technol 59:1304–1310
Ayerza R, Coates W (2005) Ground chia seed and chia oil effects on plasma lipids and fatty acids in the rat. Nutr Res 25:995–1003
Poudyal H, Panchal SK, Waanders J, Ward L, Brown L (2012) Lipid redistribution by α-linolenic acid-rich chia seed inhibits stearoyl-CoA desaturase-1 and induces cardiac and hepatic protection in diet-induced obese rats. J Nutr Biochem 23:153–162
Chicco AG, D’Alessandro ME, Hein GJ, Oliva ME, Lombardo YB (2009) Dietary chia seed (Salvia hispanica L.) rich in α-linolenic acid improves adiposity and normalises hypertriacylglycerolaemia and insulin resistance in dyslipaemic rats. Br J Nutr 101:41–50
Oliva ME, Ferreira MR, Chicco A, Lombardo YB (2013) Dietary Salba (Salvia hispanica L.) seed rich in α-linolenic acid improves adipose tissue dysfunction and the altered skeletal muscle glucose and lipid metabolism in dyslipidemic insulin-resistant rats. Prostaglandins Leukot Essent Fatty Acids 89:279–289
Rossi AS, Oliva ME, Ferreira MR, Chicco A, Lombardo YB (2013) Dietary chia seed induced changes in hepatic transcription factors and their target lipogenic and oxidative enzyme activities in dyslipidaemic insulin-resistant rats. Br J Nutr 109:1617–1627
Piya MK, McTernan PG, Kumar S (2013) Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol 216:T1–T15
Lin Y, Berg AH, Iyengar P, Lam TKT, Giacca A, Combs TP, Rajala MW, Du X, Rollman B, Li W, Hawkins M, Barzilai N, Rhodes CJ, Fantus IG, Brownlee M, Scherer PE (2005) The hyperglycemia-induced inflammatory response in adipocytes. The role of reactive oxygen species. J Biol Chem 280:4617–4626
D’Alessandro ME, Selenscig D, Illesca P, Chicco A, Lombardo YB (2015) Time course of adipose tissue dysfunction associated with antioxidant defense, inflammatory cytokines and oxidative stress in dyslipemic insulin resistant rats. Food Funct 6:1299–1309
Vazquez Prieto MA, Bettaieb A, Rodriguez Lanzi C, Soto VC, Perdicaro DJ, Galmarini CR, Haj FG, Miatello RM, Oteiza PI (2015) Catechin and quercetin attenuate adipose inflammation in fructose-fed rats and 3T3-L1 adipocytes. Mol Nutr Food Res 59:622–633
Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M (2008) Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity 16:2081–2087
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
D’Alessandro ME, Chicco A, Lombardo YB (2013) Fish oil reverses the altered glucose transporter, phosphorylation, insulin receptor substrate-1 protein level and lipid contents in the skeletal muscle of sucrose-rich diet fed rats. Prostaglandins Leukot Essent Fatty Acids 88:171–177
Chicco A, D’Alessandro ME, Karabatas L, Pastorale C, Basabe JC, Lombardo YB (2003) Muscle lipid metabolism and insulin secretion are altered in insulin-resistant rats fed a high sucrose diet. J Nutr 133:127–133
Lee HS, Csallany AS (1987) Measurement of free and bound malondialdehyde in vitamin E-deficient and—supplemented rat liver tissues. Lipids 22:104–107
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Wang X, Che H, Zhang W, Wang J, Ke T, Cao R, Meng S, Li D, Weiming O, Chen J, Luo W (2015) Effects of mild chronic intermittent cold exposure on rat organs. Int J Biol Sci 11:1171–1180
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Alvarez SM, Gómez NN, Scardapane L, Zirulnik F, Martínez D, Giménez MS (2004) Morphological changes and oxidative stress in rat prostate exposed to a non-carcinogenic dose of cadmium. Toxicol Lett 153:365–376
Oliva ME, Selenscig D, D’Alessandro ME, Chicco A, Lombardo YB (2011) Soya protein ameliorates the metabolic abnormalities of dysfunctional adipose tissue of dyslipidaemic rats fed a sucrose-rich diet. Br J Nutr 105:1188–1198
Glantz SA (2005) Primer of biostatistics. McGraw-Hill Medical Pub, DivisionNew York
Snedecor GW, PCochran WG (1967) Factorial experiments. In: Ames IA (ed) Statistical methods applied to experimental agriculture and biology. Iowa State University Press, Ames, pp 339–350
Cummins TD, Holden CR, Sansbury BE, Gibb AA, Shah J, Zafar N, Tang Y, Hellmann J, Rai SN, Spite M, Bhatnagar A, Hill BG (2014) Metabolic remodeling of white adipose tissue in obesity. Am J Physiol Endocrinol Metab 307:E262–E277
Busserolles J, Zimowska W, Rock E, Rayssiguier Y, Mazur A (2002) Rats fed a high sucrose diet have altered heart antioxidant enzyme activity and gene expression. Life Sci 71:1303–1312
Yamakura F, Kawasaki H (2010) Post-translational modifications of superoxide dismutase. Biochim Biophys Acta 1804:318–325
Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, Shirakura T, Kato K, Imaizumi K, Takahashi H, Tamura M, Maeda N, Funahashi T, Shimomura I (2013) Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem 288:27138–27149
Flachs P, Rossmeisl M, Bryhn M, Kopecky J (2009) Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci (Lond) 116:1–16
Kalupahana NS, Claycombe KJ, Moustaid-Moussa N (2011) (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv Nutr 2:304–316
Baranowski M, Enns J, Blewett H, Yakandawala U, Zahradka P, Taylor CG (2012) Dietary flaxseed oil reduces adipocyte size, adipose monocyte chemoattractant protein-1 levels and T-cell infiltration in obese, insulin-resistant rats. Cytokine 59:382–391
Makni M, Fetoui H, Gargouri NK, EL Garoui M, Jaber H, Makni J, Boudawara T, Zeghal N (2008) Hypolipidemic and hepatoprotective effects of flax and pumpkin seed mixture rich in ω-3 and ω-6 fatty acids in hypercholesterolemic rats. Food Chem Toxicol 46:3714–3720
Schneider KS, Chan JY (2013) Emerging role of Nrf2 in adipocytes and adipose biology. Adv Nutr 4:62–66
Kusunoki C, Yang L, Yoshizaki T, Nakagawa F, Ishikado A, Kondo M, Morino K, Sekine O, Ugi S, Nishio Y, Kashiwagi A, Maegawa H (2013) Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes. Biochem Biophys Res Commun 430:225–230
Gao L, Wang J, Sekhar KR, Yin H, Yared NF, Schneider SN, Sasi S, Dalton TP, Anderson ME, Chan JY, Morrow JD, Freeman ML (2007) Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J Biol Chem 282:2529–2537
Zhao G, Etherton TD, Martin KR, Vanden Heuvel JP, Gillies PJ, West SG, Kris-Etherton PM (2005) Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem Biophys Res Commun 336:909–917
Vazquez-Prieto MA, Bettaieb A, Haj FG, Fraga CG, Oteiza PL (2012) (-)-Epicatechin prevents TNFα-induced activation of signaling cascades involved in inflammation and insulin sensitivity in 3T3-L1 adipocytes. Arch of Biochem and Biophysics 527:113–118
Qatanani M, Lazar MA (2007) Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev 21:1443–1455
Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW (1991) Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and ω-3 fatty acids in muscle phospholipid. Diabetes 40:280–289
González-Mañán D, Tapia G, Gormaz JG, D’ Espessailles A, Espinosa A, Masson L, Varela P, Valenzuela A, Valenzuela R (2012) Bioconversion of α-linolenic acid to n-3 LCPUFA and expression of PPAR-alpha, acyl Coenzyme A oxidase 1 and carnitine acyl transferase I are incremented after feeding rats with α-linolenic acid-rich oils. Food Funct 3:765–772
Vuksan V, Whitham D, Sievenpiper JL, Jenkins AL, Rogovik AL, Bazinet RP, Vidgen E, Hanna A (2007) Supplementation of conventional therapy with the novel grain Salba (Salvia hispanica L.) improves major and emerging cardiovascular risk factors in type 2 diabetes: results of a randomized controlled trial. Diabetes Care 30:2804–2810
Jin F, Nieman DC, Sha W, Xie G, Qiu Y, Jia W (2012) Supplementation of milled chia seeds increases plasma ALA and EPA in postmenopausal women. Plant Food Hum Nutr 67:105–110
Acknowledgments
The authors thank Dr. Carlos Marra INIBIOLP, Facultad de Ciencias Médicas, La Plata, Argentina for the evaluation of fatty acid composition of adipose tissue phospholipids. Thanks are also given to Silvia Rodríguez and Walter Da Ru for their skillful technical assistance. The present study was carried out with the financial support of Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT) (Grant PICT 945 BID OC /AR 2011) and University of Litoral (CAI+D 0058 LI-2012). The authors thank Agrisalba S.A, Buenos Aires, Argentina for providing the SALBA chia seeds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
M. R. Ferreira and S. M. Alvarez have contributed equally to the laboratory assays in the present study.
Rights and permissions
About this article
Cite this article
Ferreira, M.R., Alvarez, S.M., Illesca, P. et al. Dietary Salba (Salvia hispanica L.) ameliorates the adipose tissue dysfunction of dyslipemic insulin-resistant rats through mechanisms involving oxidative stress, inflammatory cytokines and peroxisome proliferator-activated receptor γ. Eur J Nutr 57, 83–94 (2018). https://doi.org/10.1007/s00394-016-1299-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1299-5