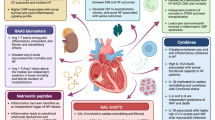
Abstract
Background
Oxidative stress may be a key pathophysiological mediator in the development and progression of heart failure (HF). The role of serum-free thiol concentrations, as a marker of systemic oxidative stress, in HF remains largely unknown.
Objective
The purpose of this study was to investigate associations between serum-free thiol concentrations and disease severity and clinical outcome in patients with new-onset or worsening HF.
Methods
Serum-free thiol concentrations were determined by colorimetric detection in 3802 patients from the BIOlogy Study to TAilored Treatment in Chronic Heart Failure (BIOSTAT-CHF). Associations between free thiol concentrations and clinical characteristics and outcomes, including all-cause mortality, cardiovascular mortality, and a composite of HF hospitalization and all-cause mortality during a 2-years follow-up, were reported.
Results
Lower serum-free thiol concentrations were associated with more advanced HF, as indicated by worse NYHA class, higher plasma NT-proBNP (P < 0.001 for both) and with higher rates of all-cause mortality (hazard ratio (HR) per standard deviation (SD) decrease in free thiols: 1.253, 95% confidence interval (CI): 1.171–1.341, P < 0.001), cardiovascular mortality (HR per SD: 1.182, 95% CI: 1.086–1.288, P < 0.001), and the composite outcome (HR per SD: 1.058, 95% CI: 1.001–1.118, P = 0.046).
Conclusions
In patients with new-onset or worsening HF, a lower serum-free thiol concentration, indicative of higher oxidative stress, is associated with increased HF severity and poorer prognosis. Our results do not prove causality, but our findings may be used as rationale for future (mechanistic) studies on serum-free thiol modulation in heart failure.
Graphical abstract
Associations of serum-free thiol concentrations with heart failure severity and outcomes

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress has been identified as an important pathophysiological mediator in the development and progression of heart failure (HF) [1, 2]. Oxidative stress reflects an imbalance between the production of reactive oxygen species (ROS) and the antioxidant capacity [3]. Although ROS provide important beneficial physiological functions at lower concentrations, excess of ROS can cause DNA damage and harmful modifications of proteins. This can result in cellular dysfunction, including changes in cardiomyocyte excitation–contraction coupling, calcium handling and energy metabolism [1]. Excess of ROS can also exert pro-fibrotic effects, leading to extracellular matrix remodeling and eventually worsening of diastolic and systolic function [4,5,6].
Free thiols, organosulfur compounds with an –SH group, act as one of the most potent and versatile endogenous defense mechanisms against oxidative stress. Extracellular, i.e. circulating, thiols are the sum of and high- and low-molecular-weight thiols and are referred to as total free thiols. Free thiols mainly comprise of high molecular weight proteins with an –SH group attached, of which albumin is the most relevant example, whereas circulating low-molecular weight thiols such as cysteine or glutathione only account for < 3–5% [7]. By forming stable disulfide bonds through ROS scavenging, free thiols prevent ROS from inflicting lipid and protein oxidation and subsequent myocardial structural damage [7]. Depletion of the antioxidant-free thiol pool reflects greater oxidative stress, and has been linked to the severity of oxidative stress-associated diseases [8, 9]. Increasing free thiol levels (e.g., by N-acetylcysteine) improved cardiac function in animal models of HF and cardiac injury [10,11,12,13]. In addition, clinical trials in small numbers of patients with myocardial infarction or HF suggested that N-acetylcysteine may reduce oxidative stress [14,15,16]. Hence, targeting thiol levels may hold promise as a potential therapeutic strategy in patients with HF. Accordingly, we investigated the associations between serum-free thiol concentrations and clinical characteristics and outcomes in a large cohort of patients with new-onset or worsening HF including a broad range of left ventricular phenotypes.
Methods
Study population
We measured the concentration of free thiols in archived serum samples of the multinational, prospective, observational BIOSTAT-CHF study. Study design and data collection have been described in full elsewhere [17]. In brief, in the BIOSTAT-CHF index cohort, 2516 patients with new-onset or worsening signs and/or symptoms of HF from 11 European countries were included between 2010 and 2012. Participants were documented with a left ventricular ejection fraction (LVEF) of ≤ 40% or plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) of > 2000 ng/L. In addition, participants were considered to be on suboptimal evidence-based treatment for HF before enrollment [18]. A comparable validation cohort of BIOSTAT-CHF included another 1738 patients from six centers in Scotland between 2010 and 2014, who had to have a previously documented admission for HF. No additional NT-proBNP criteria were used for patients with a LVEF > 40% in the validation cohort, which resulted in a higher percentage of patients with a LVEF > 45%: 34% in the validation cohort versus 7% in the index cohort. The BIOSTAT-CHF study was conducted according to the Declaration of Helsinki, approved by the ethics committee of each center and all participants provided written informed consent prior to any study-related procedures.
Detection of serum-free thiols
Blood samples were drawn upon enrollment in the BIOSTAT-CHF cohort. Serum samples were stored at − 80 °C until free thiol measurement. The free thiol concentration was detected as previously described, with minor modifications [19, 20]. In short, after thawing, 75 μl serum samples were diluted 1:4 with a 0.1 M Tris buffer (pH 8.2) and then transferred to a microplate. The background absorption was measured, using a Sunrise microplate reader (Tecan Trading AG, Männedorf, Switzerland) at 412 nm, with a reference filter at 630 nm. Subsequently, 10 μl 3.8 mM 5,5′-Dithio-bis(2-nitrobenzoic acid) (DTNB, CAS-number 69–78–3, Sigma Aldrich Corporation, Saint Louis, MO, USA) in a 0.1 M phosphate buffer (pH 7.0) was added to the samples. Following 20 min of incubation at room temperature, absorption was measured again and subtracted from background absorption. The concentration of free thiols in the samples was determined by parallel measurement of an l-cysteine (CAS-number 52–90–4, Fluka Biochemika, Buchs, Switzerland) calibration standard in the concentration range of 15.6–1000 μM in 0.1 M Tris and 10 mM EDTA (pH 8.2). All measurements were performed in duplo, where the mean of the free thiol value of both measurements was used for analyses. Measurements with a coefficient of variation > 20% were excluded from further analysis.
Clinical outcome parameters
The primary clinical outcome parameter of this study was a composite endpoint of all-cause mortality and HF-related hospitalizations at 2 years. Our secondary outcome was all-cause mortality at 2 years. As sensitivity analysis, associations with cardiovascular mortality were studied. The cause of death and hospitalization were determined by the individual site investigators. Clinical events were collected during the 9-months follow-up study visit (index cohort), standard clinical follow-up and by telephonic contacts every 6 months for at least 2 years or until the end of follow-up (2015).
Statistical analysis
For this study, both BIOSTAT-CHF cohorts were analyzed together. Normally distributed variables were displayed as mean with standard deviation (SD), non-normally distributed variables as median with interquartile range [IQR], and categorical variables as numbers with percentages (%). Distribution of continuous data was visually inspected using normal probability (Q–Q) plots. Baseline characteristics were presented according to tertiles of serum-free thiol levels. Between-group differences were compared using one-way analysis of variance (ANOVA), the Kruskal–Wallis test or the chi-square test, as appropriate. Clinical characteristics with a P-value < 0.1 were selected from the baseline table to investigate their associations with the serum-free thiol concentration using univariable, age- and sex-adjusted and multivariable linear regression analyses. All variables with P < 0.1 in age- and sex-adjusted analyses were included in multivariable analysis and subjected to backward elimination. Variables with P < 0.05 were retained in the final multivariable regression model. Prior to linear regression, normal distribution of residuals was checked, as well as presence of outliers. All variables were standardized and non-normally distributed variables were log-transformed before entry into regression analysis. To assess associations with clinical outcomes at 2-years follow-up, follow-up time and clinical events were truncated at 730.5 days. Kaplan–Meier survival curves were drawn for tertiles of serum-free thiols. The log-rank test was used to test for differences in outcomes between the tertiles. Subsequently, Cox proportional hazards regression analyses were performed to investigate associations between the free thiol concentration and disease outcome. The proportionality of hazards assumption was checked for all models to confirm absence of violation. Cox regression analyses were adjusted in a stepwise manner by first adjusting for age and sex and subsequently for sex and the previously published BIOSTAT-CHF risk models [21]. Variables in BIOSTAT-CHF risk model to predict the composite endpoint included age, HF hospitalization in the year before inclusion, edema, NT-proBNP, systolic blood pressure, hemoglobin, high-density lipoprotein levels, serum sodium concentration, and the failure to prescribe a beta-blocker. When investigating the associations between free thiols and cardiovascular mortality, non-cardiovascular mortality was used as competing risk. For the primary and secondary endpoint, pre-specified subgroup analyses were performed, testing for interactions for age (≤ 70 vs > 70), sex, HF groups (HF with reduced ejection fraction (HFrEF) vs HF with mildly reduced ejection fraction (HFmrEF) vs HFpEF), ischemic etiology, NYHA class (I–II vs III–IV) and history of chronic kidney disease (CKD). In this study, a P-value of < 0.05 was considered statistically significant. All analyses were conducted with R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Serum-free thiol levels were measured in 3802 participants of the BIOSTAT-CHF cohort. Mean free thiol concentration was 336 (SD 92) µmol/L. Baseline characteristics of the study population are presented according to tertiles of free thiol levels in Table 1. Patients within the lowest tertile were older (75 vs 69 years old, P < 0.001), more often female (36% vs 23%, P < 0.001), had less frequent an ischemic etiology of HF (56 vs 64%, P < 0.001) and shorter duration of HF diagnosis (median 10 vs 15 months, P = 0.003), compared with patients within the highest tertile of free thiol levels. Patients within the lowest tertile experienced more signs and symptoms of HF, had more advanced NYHA classes and higher NT-proBNP levels (P < 0.001 for all). Baseline characteristics are also presented for both cohorts separately (Supplementary Tables 1 and 2). The distribution of characteristics across the free thiols tertiles within the individual cohorts was quite comparable to the distribution in the combined study population.
Associations between free thiol concentration and age, sex, signs and symptoms of HF and NT-proBNP were also observed in regression analyses (Table 2). Moreover, lower free thiol levels were associated with lower systolic and diastolic blood pressure, enrollment in the BIOSTAT-CHF cohort as an inpatient (instead of at the outpatient clinic), and the absence of treatment with an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB). Furthermore, (laboratory parameters of) other diseases, including anemia and chronic kidney disease were associated with lower levels of serum-free thiols. Lower albumin, higher age, and higher urea and NT-proBNP were the strongest determinants of lower free thiol concentration (P < 0.001 for all; Table 2).
Serum-free thiols and follow-up outcomes
Median follow-up time of the combined study population was 545 [IQR 255–730] days. In the index cohort, median follow-up time was 553 [IQR 253–730] days, and for patients in the validation cohort, the median follow-up time was 517 [266–730] days. During 2 years of follow-up, 932 (24.5%) patients died, of which 594 (64%) due to a cardiovascular cause, and 908 (23.9%) patients were hospitalized for HF. The composite endpoint of all-cause mortality and HF hospitalization occurred in 1475 (38.8%) patients. Incidences of the composite outcome, as well as secondary outcome, differed significantly across tertiles (log-rank test: P < 0.001), and occurred most frequent in the lowest tertile of serum-free thiol levels (Fig. 1). In Cox regression analyses, lower levels of free thiols were associated with adverse disease outcome, also after adjustment for factors included in the BIOSTAT risk model [Hazard Ratio (HR) per SD decrease in free thiols: 1.058, 95%-confidence interval (CI): 1.001–1.118, P = 0.046 for the composite endpoint; Table 3]. Lower free thiol concentrations were also independently associated with higher rates of all-cause mortality [HR per SD decrease: 1.253, 95% CI: 1.171–1.341, P < 0.001] and cardiovascular mortality [HR per SD decrease: 1.182, 95% CI: 1.086–1.288, P < 0.001].
Subgroup analyses
Pre-defined subgroup analyses on the primary endpoint and on all-cause mortality were performed for age, sex, HF type, ischemic origin, NYHA class, and history of CKD. No significant subgroup interactions were observed (Fig. 2; Supplementary Table 3).
Forest plot depicting Hazard ratio’s for the composite endpoint of all-cause mortality or heart failure-related hospitalization (upper panel) and all-cause mortality alone (lower panel) during a 2-year follow-up, per SD decrease in serum-free thiols, across pre-specified subgroups. All Cox proportional hazards models were adjusted for the corresponding BIOSTAT-CHF risk model. Abbreviations: CKD chronic kidney disease, HF heart failure, HFmrEF heart failure with mildly reduced ejection fraction, HFpEF heart failure with preserved ejection fraction, HFrEF heart failure with reduced ejection fraction, NYHA New York Heart Association, SD standard deviation
Discussion
This study demonstrates that lower serum concentrations of free thiols, reflecting increased oxidative stress, are associated with greater HF severity and worse outcome for patients with new-onset or worsening HF. Our data provide rationale for future mechanistic studies on the role of free thiols in HF, and for studies evaluating effects of free thiol modulation for modifying disease outcomes for patients with HF.
Extracellular free thiols are critically involved in redox signaling, but also possess a strong antioxidant buffering capacity to a variety of reactive species [7]. Under conditions of oxidative stress, the circulating free thiol pool (reduced thiols with -SH group) scavenge ROS, leading to the oxidation of thiols and the formation of disulfide bonds. Therefore, measurement of the reduced, i.e. free, thiol pool is a simple, direct, and robust method to systemically assess the degree of oxidative stress [8].
Depletion of the free thiol pool has been observed and linked to disease severity in a variety of non-cardiovascular and cardiovascular diseases before, including anemia, diabetes mellitus, inflammatory bowel disease, kidney disease, and myocardial infarction [8, 9, 22,23,24], which was also investigated and confirmed for anemia and kidney disease in present study. In small exploratory studies including 120 and 101 patients with chronic HF, associations between serum-free thiol concentrations and increased HF severity [25, 26], and poorer clinical outcomes were suggested [26]. We substantiate previous work and extended upon this studies in several ways. Our sample size was almost 40 times larger reducing the risk of a type 1 error. Our study also generalized the link to HF by including patients with HFpEF, whereas the previous studies were limited to patients with a LVEF of < 45%. Furthermore, our patient population consisted of less-stable patients with new-onset or worsening HF on suboptimal pharmacological treatment, which were possibly more subjected to increased levels of oxidative stress. We observed robust and independent association between free thiol levels and disease outcomes, whereas the previous studies were underpowered to perform multivariable analyses on outcome. Our findings are consistent with the previous prognostic value of free thiols in a general population-based cohort [27, 28]. In the present study, we did not observe significant effect modifications between free thiols and disease outcomes by predefined subgroups.
Lower free thiol levels were consistently associated with signs of systemic or pulmonary congestion and natriuretic peptide levels. Other HF characteristics that were associated with lower free thiol levels were non-ischemic etiology, shorter duration of HF, inpatient study enrollment, and the absence of treatment with an ACEi or ARB. Differences in oxidative stress between ischemic vs dilated cardiomyopathy have been previously attributed to enhanced ROS-induced mitochondrial instability in the latter [29]. The activity of the antioxidant thioredoxin system was also higher in dilated cardiomyopathy, but it has been postulated this might be an indirect reflection of excessive oxidative stress [29, 30]. As for the time from HF diagnosis, we speculate that free thiols might be lower in the early phase of HF due to enhancement of ROS by catecholamines [31], and higher in a later phase due to the effect of pharmacological treatment. Participants that were enrolled as inpatients (acute HF) had likely more congestion and oxidative stress, resulting in lower free thiol levels. The association between the use of an ACEi or ARB might be associated with antioxidant effects of this class of therapy [32, 33], or might be coincidental due to the BIOSTAT-CHF design, which required participants to be on suboptimal pharmacological treatment. We cannot exclude that free thiol concentrations were also influenced by other factors, for example the production of thiol-containing molecules by the liver [7].
Therapeutic potential
Associations between oxidative stress markers with heart failure severity and outcome have been established before, for example for 8-OHdG, malondialdehyde and uric acid [34, 35]. However, compared with other oxidative stress markers, free thiols constitute the central hubs of inter-organ redox communication [36], whereas malondialdehyde is merely a damage marker representing oxidative stress-induced lipid peroxidation. Moreover, free thiols are amendable for therapeutic modulation, for example by N-acetylcysteine or glutathione administration [10, 37, 38]. Our findings support the notion that thiol supplementation may have therapeutic potential in HF if a causal role of free thiols in the pathophysiology of HF can be addressed. Prior experimental studies showed that administration of N-acetylcysteine resulted in reduced oxidative stress and improved cardiac function in models with HF or ischemic injury [10,11,12,13, 39]. Beneficial effects of such thiol-containing drugs were also observed in patients with COVID-19 [40]. Thiol modulating studies in patients with HF are scarce and predominantly small or restricted to nitrate tolerance [14, 16, 41]. It has been suggested that administration of thiol-targeted antioxidants should be reserved for individuals with profound thiol depletion and incautious thiol supplementation could potentially disturb physiological redox signaling processes [42]. Therefore, more mechanistic studies and careful evaluation of treatment effects in patients with lower free thiols, are warranted. In this respect, circulating free thiols may aid in patient stratification to restore the systemic and local redox balance following a redox precision medicine approach.
Strengths and limitations
Strengths of our study include the large sample size, the phenotypically well-characterized patient population, combined with a systematic follow-up. Limitations of our study include the geographic restrictions of our cohort recruiting mainly Caucasians since some studies suggest ethnic and geographic differences in oxidative stress-related gene expression and antioxidant status [43, 44]. We also did not have data on dietary intake or total serum protein levels to make further adjustment for serum-free thiol level [7, 36]. Moreover, participants were enrolled back in 2010–2012, and HF treatments have advanced since then. At last, our results do not prove causality or mechanisms of free thiol depletion.
Conclusions
In patients with new-onset or worsening HF lower serum-free thiol concentrations, indicative of increased oxidative stress, are associated with greater HF severity and a poorer prognosis. If future studies prove a causal role of free thiols in the progression of HF, it might be of interest to study whether free thiol modulation, especially in patients with poor redox status, might improve clinical outcomes.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
van der Pol A, van Gilst WH, Voors AA, van der Meer P (2019) Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail 21:425–435. https://doi.org/10.1002/ejhf.1320
van’t Erve TJ, Kadiiska MB, London SJ, Mason RP (2017) Classifying oxidative stress by F(2)-isoprostane levels across human diseases: a meta-analysis. Redox Biol 12:582–599. https://doi.org/10.1016/j.redox.2017.03.024
Sies H (2015) Oxidative stress: a concept in redox biology and medicine. Redox Biol 4:180–183. https://doi.org/10.1016/j.redox.2015.01.002
Takimoto E, Kass DA (2007) Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49:241–248. https://doi.org/10.1161/01.HYP.0000254415.31362.a7
Hage C, Löfgren L, Michopoulos F, Nilsson R, Davidsson P, Kumar C et al (2020) Metabolomic profile in patients with heart failure with preserved ejection fraction versus patients with heart failure with reduced ejection fraction. J Card Fail 26:1050–1059. https://doi.org/10.1016/j.cardfail.2020.07.010
Münzel T, Gori T, Keaney JFJ, Maack C, Daiber A (2015) Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J 36:2555–2564. https://doi.org/10.1093/eurheartj/ehv305
Turell L, Radi R, Alvarez B (2013) The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic Biol Med 65:244–253. https://doi.org/10.1016/j.freeradbiomed.2013.05.050
Banne AF, Amiri A, Pero RW (2003) Reduced level of serum thiols in patients with a diagnosis of active disease. J Anti Aging Med 6:327–334. https://doi.org/10.1089/109454503323028920
Bourgonje AR, Gabriëls RY, de Borst MH, Bulthuis MLC, Faber KN, van Goor H et al (2019) Serum free thiols are superior to fecal calprotectin in reflecting endoscopic disease activity in inflammatory bowel disease. Antioxidants 8:351. https://doi.org/10.3390/antiox8090351
Ferrari R, Ceconi C, Curello S, Cargnoni A, Alfieri O, Pardini A et al (1991) Oxygen free radicals and myocardial damage: protective role of thiol-containing agents. Am J Med 91:95S-105S. https://doi.org/10.1016/0002-9343(91)90291-5
Adamy C, Mulder P, Khouzami L, Andrieu-abadie N, Defer N, Candiani G et al (2007) Neutral sphingomyelinase inhibition participates to the benefits of N-acetylcysteine treatment in post-myocardial infarction failing heart rats. J Mol Cell Cardiol 43:344–353. https://doi.org/10.1016/j.yjmcc.2007.06.010
Bourraindeloup M, Adamy C, Candiani G, Cailleret M, Bourin M-C, Badoual T et al (2004) N-acetylcysteine treatment normalizes serum tumor necrosis factor-alpha level and hinders the progression of cardiac injury in hypertensive rats. Circulation 110:2003–2009. https://doi.org/10.1161/01.CIR.0000143630.14515.7C
Dludla PV, Dias SC, Obonye N, Johnson R, Louw J, Nkambule BB (2018) A systematic review on the protective effect of N-acetyl cysteine against diabetes-associated cardiovascular complications. Am J Cardiovasc Drugs 18:283–298. https://doi.org/10.1007/s40256-018-0275-2
Mehra A, Shotan A, Ostrzega E, Hsueh W, Vasquez-Johnson J, Elkayam U (1994) Potentiation of isosorbide dinitrate effects with N-acetylcysteine in patients with chronic heart failure. Circulation 89:2595–2600. https://doi.org/10.1161/01.cir.89.6.2595
Pasupathy S, Tavella R, Grover S, Raman B, Procter NEK, Du YT et al (2017) Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM trial). Circulation 136:894–903. https://doi.org/10.1161/CIRCULATIONAHA.117.027575
Camuglia AC, Maeder MT, Starr J, Farrington C, Kaye DM (2013) Impact of N-acetylcysteine on endothelial function, B-type natriuretic peptide and renal function in patients with the cardiorenal syndrome: a pilot cross over randomised controlled trial. Heart Lung Circ 22:256–259. https://doi.org/10.1016/j.hlc.2012.10.012
Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P et al (2016) A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure: rationale, design, and baseline characteristics of BIOSTAT-CHF. Eur J Heart Fail 18:716–726. https://doi.org/10.1002/ejhf.531
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J 37:2129–2200. https://doi.org/10.1093/eurheartj/ehw128
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Hu ML, Louie S, Cross CE, Motchnik P, Halliwell B (1993) Antioxidant protection against hypochlorous acid in human plasma. J Lab Clin Med 121:257–262
Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P et al (2017) Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail 19:627–634. https://doi.org/10.1002/ejhf.785
Boekhoud L, Koeze J, van der Slikke EC, Bourgonje AR, Moser J, Zijlstra JG et al (2020) Acute kidney injury is associated with lowered plasma-free thiol levels. Antioxidants 9:1135. https://doi.org/10.3390/antiox9111135
Kundi H, Ates I, Kiziltunc E, Cetin M, Cicekcioglu H, Neselioglu S et al (2015) A novel oxidative stress marker in acute myocardial infarction; thiol/disulphide homeostasis. Am J Emerg Med 33:1567–1571. https://doi.org/10.1016/j.ajem.2015.06.016
Bourgonje AR, Abdulle AE, Bourgonje MF, Binnenmars SH, Gordijn SJ, Bulthuis M et al (2021) Serum free sulfhydryl status associates with new-onset chronic kidney disease in the general population. Redox Biol 48:102211. https://doi.org/10.1016/j.redox.2021.102211
Radovanovic S, Savic-Radojevic A, Pljesa-Ercegovac M, Djukic T, Suvakov S, Krotin M et al (2012) Markers of oxidative damage and antioxidant enzyme activities as predictors of morbidity and mortality in patients with chronic heart failure. J Card Fail 18:493–501. https://doi.org/10.1016/j.cardfail.2012.04.003
Koning AM, Meijers WC, Pasch A, Leuvenink HGD, Frenay A-RS, Dekker MM et al (2016) Serum free thiols in chronic heart failure. Pharmacol Res 111:452–458. https://doi.org/10.1016/j.phrs.2016.06.027
Abdulle AE, Bourgonje AR, Kieneker LM, Koning AM, la Bastide-van GS, Bulthuis MLC et al (2020) Serum free thiols predict cardiovascular events and all-cause mortality in the general population: a prospective cohort study. BMC Med 18:130. https://doi.org/10.1186/s12916-020-01587-w
Schöttker B, Brenner H, Jansen EHJM, Gardiner J, Peasey A, Kubínová R et al (2015) Evidence for the free radical/oxidative stress theory of ageing from the CHANCES consortium: a meta-analysis of individual participant data. BMC Med 13:300. https://doi.org/10.1186/s12916-015-0537-7
Neidhardt S, Garbade J, Emrich F, Klaeske K, Borger MA, Lehmann S et al (2019) Ischemic cardiomyopathy affects the thioredoxin system in the human myocardium. J Card Fail 25:204–212. https://doi.org/10.1016/j.cardfail.2019.01.017
Kishimoto C, Shioji K, Nakamura H, Nakayama Y, Yodoi J, Sasayama S (2001) Serum thioredoxin (TRX) levels in patients with heart failure. Jpn Circ J 65:491–494. https://doi.org/10.1253/jcj.65.491
Costa VM, Carvalho F, Bastos ML, Carvalho RA, Carvalho M, Remião F (2011) Contribution of catecholamine reactive intermediates and oxidative stress to the pathologic features of heart diseases. Curr Med Chem 18:2272–2314. https://doi.org/10.2174/092986711795656081
Münzel T, Gori T, Bruno RM, Taddei S (2010) Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J 31:2741–2748. https://doi.org/10.1093/eurheartj/ehq396
Williams HC, Griendling KK (2007) NADPH oxidase inhibitors: new antihypertensive agents? J Cardiovasc Pharmacol 50:9–16. https://doi.org/10.1097/FJC.0b013e318063e820
Romuk E, Wojciechowska C, Jacheć W, Zemła-Woszek A, Momot A, Buczkowska M et al (2019) Malondialdehyde and uric acid as predictors of adverse outcome in patients with chronic heart failure. Oxid Med Cell Longev 2019:9246138. https://doi.org/10.1155/2019/9246138
Di Minno A, Turnu L, Porro B, Squellerio I, Cavalca V, Tremoli E et al (2017) 8-Hydroxy-2-deoxyguanosine levels and heart failure: a systematic review and meta-analysis of the literature. Nutr Metab Cardiovasc Dis 27:201–208. https://doi.org/10.1016/j.numecd.2016.10.009
Cortese-Krott MM, Koning A, Kuhnle GGC, Nagy P, Bianco CL, Pasch A et al (2017) The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid Redox Signal 27:684–712. https://doi.org/10.1089/ars.2017.7083
Deneke SM (2000) Thiol-based antioxidants. Curr Top Cell Regul 36:151–180. https://doi.org/10.1016/s0070-2137(01)80007-8
Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA (2007) N-Acetylcysteine–a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol 7:355–359. https://doi.org/10.1016/j.coph.2007.04.005
Parra-Flores P, Riquelme JA, Valenzuela-Bustamante P, Leiva-Navarrete S, Vivar R, Cayupi-Vivanco J et al (2019) The association of ascorbic acid, deferoxamine and N-acetylcysteine improves cardiac fibroblast viability and cellular function associated with tissue repair damaged by simulated ischemia/reperfusion. Antioxidants 8:614. https://doi.org/10.3390/antiox8120614
Cazzola M, Rogliani P, Salvi SS, Ora J, Matera MG (2021) Use of thiols in the treatment of COVID-19: current evidence. Lung 199:335–343. https://doi.org/10.1007/s00408-021-00465-3
Lang NN, Ahmad FA, Cleland JG, O’Connor CM, Teerlink JR, Voors AA et al (2021) Haemodynamic effects of the nitroxyl donor cimlanod (BMS-986231) in chronic heart failure: a randomized trial. Eur J Heart Fail 23:1147–1155. https://doi.org/10.1002/ejhf.2138
Andreadou I, Efentakis P, Frenis K, Daiber A, Schulz R (2021) Thiol-based redox-active proteins as cardioprotective therapeutic agents in cardiovascular diseases. Basic Res Cardiol 116:44. https://doi.org/10.1007/s00395-021-00885-5
Sayanthooran S, Magana-Arachchi DN, Gunerathne L, Abeysekera TDJ, Sooriyapathirana SS (2016) Upregulation of oxidative stress related genes in a chronic kidney disease attributed to specific geographical locations of Sri Lanka. Biomed Res Int 2016:7546265. https://doi.org/10.1155/2016/7546265
Gut A, Shiel N, Kay PM, Segal I, Braganza JM (1994) Heightened free radical activity in blacks with chronic pancreatitis at Johannesburg, South Africa. Clin Chim Acta 230:189–199. https://doi.org/10.1016/0009-8981(94)90271-2
Acknowledgements
We gratefully acknowledge Janny Takens, Martin Dokter, Silke Oberdorf-Maass and Manuela Wels for excellent technical assistance. The Graphic abstract was created with BioRender.com.
Funding
BIOSTAT-CHF was funded by a grant from the European Commission (FP7-242209-BIOSTAT-CHF; EudraCT 2010–020808–29).
Author information
Authors and Affiliations
Contributions
JGFC, NJS, LLN, CCL, MM, GSF, DJV, SDA, KD, AAV, PH, MSLYK, SA, EL were involved in the design of the study. ERH, HVG and PH were involved in collection of the samples and free thiol measurements. JEE and SMF analyzed the data. MLYK and ARB wrote the manuscript. MLYK, JEE, ERH, ARB, SA, SMF, JGFC, NJS, LLN, CCL, MM, GSF, DJV, SDA, KD, AAV, EL, HVG, and PH critically revised and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
S.D.A. reports receiving fees from Abbott, Actimed, Bayer, Boehringer Ingelheim, Cardiac Dimension, Cordio, Impulse Dynamics, Novartis, Occlutech, Servier, and Vifor Pharma, and grant support from Abbott and Vifor Pharma. The other authors declare that there is no relevant conflict of interest.
Ethical standards
This study has been approved by the appropriate ethics committees and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants gave their informed consent prior to inclusion in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Koning, MS.L.Y., Emmens, J.E., Romero-Hernández, E. et al. Systemic oxidative stress associates with disease severity and outcome in patients with new-onset or worsening heart failure. Clin Res Cardiol 112, 1056–1066 (2023). https://doi.org/10.1007/s00392-023-02171-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-023-02171-x