Abstract
Purpose
Use of neoadjuvant chemotherapy (NAC) for locally advanced colon cancer (LACC) remains controversial. An integrated analysis of data from high-quality studies may inform the long-term safety of NAC for this cohort. Our aim was to perform a systematic review and meta-analysis of randomised clinical trials (RCTs) and propensity-matched studies to assess the oncological safety of NAC in patients with LACC.
Methods
A systematic review was performed as per preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. Survival was expressed as hazard ratios using time-to-effect generic inverse variance methodology, while surgical outcomes were expressed as odds ratios (ORs) using the Mantel-Haenszel method. Data analysis was performed using Review Manager version 5.4.
Results
Eight studies (4 RCTs and 4 retrospective studies) including 31,047 patients with LACC were included. Mean age was 61.0 years (range: 19–93 years) and mean follow-up was 47.6 months (range: 2–133 months). Of those receiving NAC, 4.6% achieved a pathological complete response and 90.6% achieved R0 resection (versus 85.9%, P < 0.001). At 3 years, patients receiving NAC had improved disease-free survival (DFS) (OR: 1.28, 95% confidence interval (CI): 1.02–1.60, P = 0.030) and overall survival (OS) (OR: 1.76, 95% CI: 1.10–2.81, P = 0.020). When using time-to-effect modelling, a non-significant difference was observed for DFS (HR: 0.79, 95% CI: 0.57–1.09, P = 0.150) while a significant difference in favour of NAC was observed for OS (HR: 0.75, 95% CI: 0.58–0.98, P = 0.030).
Conclusion
This study highlights the oncological safety of NAC for patients being treated with curative intent for LACC using RCT and propensity-matched studies only. These results refute current management guidelines which do not advocate for NAC to improve surgical and oncological outcomes in patients with LACC.
Trial registration
International Prospective Register of Systematic Review (PROSPERO) registration: CRD4202341723.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditionally, surgical resection combined with adjuvant chemotherapy (AC) was the cornerstone of managing locally advanced colonic cancer (LACC) [1]. The paradigm of several other gastrointestinal malignancies, including rectal, gastric, and esophageal cancers, has evolved to recognise the benefits of neoadjuvant chemotherapy (NAC) [2,3,4]. NAC is advantageous for several reasons: tumour downstaging to facilitate complete resection (R0) [5], reducing the theoretical risk of micrometastatic dissemination of cancer cells within human circulation [6], providing in vivo data with respect to sensitivity of the tumour to systemic therapies (recognised to carry prognostic significance) [7], and ensuring higher systemic treatment completion rates (with rationale that complications and postoperative morbidity following surgery may delay progression) [8]. Notwithstanding these perceived benefits of NAC, there remains hesitancy among expert consensus guidelines, such as the National Comprehensive Cancer Network (NCCN) [9], the European Society for Medical Oncology (ESMO) [10], and the National Institute for Health and Care Excellence (NICE) [11], to alter recommendation in support of NAC as the standard of care for LACC. This is likely due to concern regarding overtreatment. Toxicity associated with NAC has been shown to compromise fitness of certain patients due to proceed to surgical resection [12]. Risk of resistant tumours advancing while on NAC is of considerable concern to the multidisciplinary team (MDT) [13]. Thus, contemporary clinical guidelines do not currently advocate for NAC in the setting of LACC.
The FOXTROT trial (NCT00647530) is the largest prospective multicentre randomised clinical trial (RCT) which formally evaluates the value of NAC in radiologically confirmed T3 (≥ 5-mm invasion beyond the muscularis propria) or T4 (tumour penetrates to the surface of the visceral peritoneum and further) LACC [14, 15]. Participants were randomised to either neoadjuvant FOLFOX (5-fluorouracil, leucovorin and oxaliplatin with the addition of panitumumab made based on Ras status) followed by surgical resection and subsequent AC or surgical resection followed by 24 weeks of systemic therapy in the adjuvant setting. Preliminary data from FOXTROT illustrated the oncological safety of NAC for patients with LACC, through enhanced survival outcomes, increased R0 resection rates, with lower treatment toxicities, and perioperative morbidity observed for the majority [15].
While FOXTROT provides a degree of optimism surrounding NAC for LACC [14, 15], there remains a paucity of high-quality studies providing long-term data supporting this therapeutic strategy, with several previous analyses failing to randomise or match patients to reduce the natural risk of competing confounding, selection, and ascertainment biases influencing results observed [5, 16]. Accordingly, the aim of the current study was to perform a systematic review and meta-analysis of RCTs and propensity-matched studies to evaluate the oncological safety of NAC in patients being treated with curative intent for LACC.
Methods
This systematic review was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, as previously outlined by Moher et al. [17]. As this study used data from previously published studies, ethical approval was not sought from the local institutional review board. All authors contributed to formulating a predetermined review protocol which was then prospectively registered and published on the International Prospective Register of Systematic Reviews (PROSPERO: CRD42023417231).
Population, intervention, comparison, and outcome (PICO) tool
Applying the PICO framework [18], as previously described by Richardson et al., the research question the authors sought to address through this analysis was as follows:
-
Population: any patient diagnosed with radiologically confirmed T3 (defined as disease extending ≥ 5-mm invasion beyond the muscularis propria or similar) or T4 (defined as tumour penetrating to the surface of the visceral peritoneum and other adjacent organs or similar) colon cancers. Patients either had to be randomised (in the clinical trial setting) or indicated to undergo NAC and subsequently matched with similar patients who had undergone upfront surgery followed by AC (in the retrospective cohort studies where propensity matching has occurred).
-
Intervention: any patient randomised or indicated to undergo NAC for primary treatment of their LACC.
-
Comparison: any patient randomised or indicated to undergo surgery and AC for primary treatment of their LACC.
-
Outcomes: the primary outcome measures and study endpoints included the following:
-
Disease-free (DFS) and overall survival (OS) outcomes for patients who underwent NAC and OS, expressed as dichotomous outcomes at 2-year, 3-year, 5-year follow-up and for overall outcomes, or as time-to-effect models as hazard ratios (HRs), with associated 95% confidence intervals (95% CIs).
-
Complete resection (or R0) rates between patients who underwent NAC and AC, expressed as dichotomous outcomes.
-
Study definitions
-
Overall survival: freedom from mortality due to any cause following treatment for primary LACC [19]
-
Disease-free survival: freedom from invasive disease recurrence or mortality due to any cause following treatment of primary LACC [19]
Search strategy
A predetermined electronic search was performed by two independent reviewers of the PubMed, Scopus, and Cochrane Library databases on the 29th of December 2022 to assess for relevant RCTs and matched studies which would be suitable for inclusion. The search was performed of all fields under the following headings: (neoadjuvant therapies) AND (colon cancer), under medical subheadings (or MeSH Terms), which were linked by ‘AND’ which operated as a Boolean operator. Included studies were limited to those published in the English language, and the authors elected not to restrict included studies based on year of publication. For retrieved studies, their titles were initially screened, before the study abstracts and full texts were evaluated to identify studies which were deemed appropriate for inclusion.
Eligibility criteria
Studies were considered eligible if they met the following inclusion criteria: (1) studies to be of prospective randomised or retrospective propensity-matched design to be eligible for inclusion in this study, (2) studies had to compare outcomes in adult patients aged 18 years who were randomised (or indicated) to receive NAC and subsequent surgical resection or upfront surgical resection followed by AC following diagnosis with radiologically confirmed T3/T4 colon cancers, and (3) studies had to report oncological and survival outcomes for those in the NAC and AC groups, respectively (as outlined previously).
Studies were excluded if they satisfied any one of the following exclusion criteria: (1) studies reporting outcomes for diseases other than LACC, (2) studies evaluating outcomes in the setting of diseases other than T3 or T4 LACC, (3) studies not reporting clinical outcomes in relation to NAC versus AC, (4) studies including participants aged 17 years and younger, (5) studies where participants were not randomised or matched, (6) case reports or series with less than 5 patients, or (7) any previous review article.
Data extraction and quality assessment
Literature search was performed by two independent reviewers (M.G.D. and A.H.A.) using the predesigned search strategy, as outlined previously. Duplicate studies were manually removed. Each reviewer systematically reviewed titles, abstracts, and/or full texts before identifying studies which met inclusion criteria. Retrieved manuscripts then had data pertaining to the study information, study design, patient information, treatment details, survival, and oncological outcomes extracted. Risk of bias assessments of included studies was performed using the risk of bias (ROB) tool for RCTs and risk of bias in non-randomised studies - of interventions (ROBINS-I) for non-randomised studies as appropriate [20, 21], as recommended in the 6th edition of the Cochrane Handbook of Systematic Review of Interventions (version 6.3, 2022) [22]. GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) was also performed [23].
Statistical analysis
Descriptive statistics were primarily used to determine associations between treatment with NAC and AC with both DFS and OS outcomes using Fisher’s exact test (†), as appropriate [24]. Thereafter, treatment strategies and respective survival outcomes were expressed as dichotomous or binary outcomes, before estimation of survival outcomes using the Mantel-Haenszel method. These survival outcomes were expressed as ORs with corresponding 95% CIs, similar to surgical outcomes (i.e.: R0 resection rates) which were also expressed as odds ratios (ORs). The Mantel-Haenszel method is useful in such instances to demonstrate the overall probability of an outcome for two different treatment exposures (i.e.: NAC vs. AC); however, it is limited in that it fails to demonstrate the influence of such exposures over a period of time [25]. Thus, the impact of treatment strategies on respective survival outcomes was analysed using time-to-effect modelling using generic inverse variance method and expressed as hazard ratios (HRs), to demonstrate the influence of these treatments on survival over time [26]. Random effects modelling was applied to all studies on the basis that significant heterogeneity (I2) had to existed between studies included in analysis, with heterogeneity determined using I2 statistics. Symmetry funnel plots were used to assess publication bias. All tests of significance were two-tailed with P < 0.050 indicating statistical significance. Descriptive statistics were performed using the Statistical Package for Social Sciences (SPSS) version 26 (International Business Machines Corporation, Armonk, New York). Meta-analysis was performed using Review Manager (RevMan), version 5.4 (Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Literature search
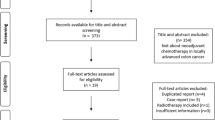
Systematic search strategy identified 2102 studies, of which 176 duplicates were manually removed. The remaining 1926 studies had titles screened for relevance, before 30 abstracts and 13 full texts were assessed for eligibility. In total, 8 studies fulfilled inclusion criteria [14, 27,28,29,30,31,32,33] (Fig. 1).
Study characteristics
Of the 8 included studies, 4 were prospective RCTs [14, 27, 29, 33] and 4 were retrospective studies where patients underwent propensity matching [28, 30,31,32] (both 50.0%). Four of the studies were from European research facilities (50.0%) [14, 28, 29, 32], 2 from China (25.0%) [7, 31], and 1 from both Japan [33] and the USA [30] (both 12.5%), respectively. Publication dates of included studies ranged over a 20-year period (2003–2023). Details and risk of bias assessments from the 8 included studies are outlined in detail in Table 1.
Patient characteristics
In total, 31,047 patients who were treated with primary curative intent for T3/T4 LACC were included. The mean age was 61.0 years (range: 19–93 years). Overall, 52.0% were male (16,137/31,047). Mean follow-up was 47.6 months (range: 2–133 months). Patient details from the 8 included studies are outlined in Table 2.
Neoadjuvant chemotherapy characteristics
Of the 31,047 patients included in this study, 8.8% were designated to undergo NAC (2729/31,047). In total, 4.6% of those undergoing NAC achieved a pathological complete response (pCR) (67/1457). Overall, 90.6% undergoing NAC (984/1086) and 85.9% in receipt of AC (801/933) achieved an R0 resection (P < 0.001, †). At meta-analysis, there was a non-significant difference in R0 resection rates when using overall data (OR: 1.14, 95% CI: 0.85–1.53, P = 0.370) (Fig. 2A), RCT data (OR: 1.23, 95% CI: 0.59–2.58, P = 0.580) (Fig. 2B), and matched data (OR: 1.04, 95% CI: 0.73–1.49, P = 0.810) (Fig. 2C). Details in relation to chemotherapy regimens are outlined in Table 1.
Disease-free survival
Overall, 81.4% of those undergoing NAC were free of recurrence or death at follow-up (1232/1514) compared to 78.4% of those undergoing AC (957/1221, P = 0.005, †) (Table 3). At meta-analysis, this non-significant difference was evident for DFS from overall (OR: 1.01, 95% CI: 0.75–1.36, P = 0.950) (Fig. 3A) and RCT data (OR: 1.08, 95% CI: 0.81–1.45, P = 0.590) (Fig. 3B). Zeng et al. was the sole study providing matched data for overall DFS and therefore was not analysed at meta-analysis [31].
At 2-year follow-up, 86.0% of those undergoing NAC were free of recurrence or death at follow-up (600/698) compared to 82.5% of those undergoing AC (292/354, P = 0.146, †). At 3-year follow-up, 81.2% of those undergoing NAC were free of recurrence or death at follow-up (910/1121) compared to 76.3% of those undergoing AC (594/779, P = 0.001, †) (Table 3). At meta-analysis, a significant difference in 3-year DFS was observed in favour of NAC from the overall data (OR: 1.28, 95% CI: 1.02–1.60, P = 0.030) (Supplementary Material 1A), which was comprised solely of RCT data.
At 5-year follow-up, 76.1% of those undergoing NAC were free of recurrence or death at follow-up (299/393) compared to 77.6% of those undergoing AC (343/442, P = 0.622, †) (Table 3). At meta-analysis, this non-significant difference was evident from the overall data (OR: 1.06, 95% CI: 0.47–2.41, P = 0.880) (Supplementary Material 1B).
When using a time-to-effect model at meta-analysis, a non-significant difference was observed from overall (HR: 0.79, 95% CI: 0.57–1.09, P = 0.150) (Supplementary Material 1C) and RCT data (HR: 0.82, 95% CI: 0.58–1.15, P = 0.250) (Supplementary Material 1D). Once again, Zeng et al. was the only study providing matched data for DFS using time-to-effect modelling and therefore was not analysed at meta-analysis [31].
Overall survival
Overall, 83.8% of those undergoing NAC were free of death at follow-up (2268/2706) compared to 79.4% of those undergoing AC (22,216/27,851, P < 0.001, †) (Table 3). At meta-analysis, this non-significant difference was evident from the overall (OR: 1.21, 95% CI: 0.97–1.52, P = 0.100) (Fig. 4A), RCT (OR: 1.38, 95% CI: 0.98–1.94, P = 0.060) (Fig. 4B), and matched data (OR: 1.02, 95% CI: 0.83–1.26, P = 0.820) (Fig. 4C), respectively.
At 3-year follow-up, 85.2% of the NAC group were free of death at follow-up (1720/2019) compared to 79.6% of those undergoing AC (21,449/26,966, P < 0.001, †) (Table 3). At meta-analysis, a non-significant difference in 3-year OS was observed from the overall (OR: 1.44, 95% CI: 0.94–2.22, P = 0.090) (Supplementary Material 2A); however, a significant difference was observed from the RCT data (OR: 1.76, 95% CI: 1.10–2.81, P = 0.020) (Supplementary Material 2B).
At 5-year follow-up, 78.3% of those undergoing NAC were free of death at follow-up (308/393) compared to 76.0% of those undergoing AC (336/442, P = 0.458, †) (Table 3). At meta-analysis, this non-significant difference was evident from the overall data (OR: 1.27, 95% CI: 0.72–2.22, P = 0.410) (Supplementary Material 2C).
When using a time-to-effect model at meta-analysis, a significant difference was observed from the overall (HR: 0.75, 95% CI: 0.58–0.98, P = 0.030) (Fig. 5A) data which was subsequently not apparent from RCT (OR: 0.77, 95% CI: 0.59–1.00, P = 0.050) (Fig. 5B) and matched data (HR: 0.90, 95% CI: 0.62–1.31, P = 0.580), respectively (Fig. 5C).
Discussion
This systematic review identified 8 high-quality randomised and propensity-matched studies which provide novel insights into the oncological safety of NAC relative to upfront surgery followed by AC for patients treated with curative intent for LACC. Outcomes from 31,047 patients were included representing some of the most meaningful data regarding NAC use in LACC, since publication of initial results of the seminal FOXTROT trial in 2012 [15]. The most important clinical finding in this analysis is the data supporting NAC as a safe treatment strategy in the setting of LACC, which remained consistent within sensitivity analyses performed using RCT data only. Thus, NAC seems a pragmatic therapeutic strategy which may be utilised in cases of LACC, where deemed feasible by the MDT.
As described, evidence from this study illustrates non-inferiority of NAC relative to AC in LACC, with comparable R0 resection rates and DFS and OS outcomes observed for both treatment arms at meta-analysis. In addition, when comparing raw data for survival between NAC and AC, outcomes tend to significantly favour NAC for both survival metrics (as outlined in detail in Table 3). These are important results, in particular when considered in tandem with 3-year survival outcomes which illustrate a significant improvement in DFS and OS at meta-analysis. Notwithstanding these results losing significance as follow-up progressed beyond the mean follow-up of almost 4 years, more caution should be exercised when interpreting data after this point surrounding true oncological safety of these therapeutic strategies.
Data supporting NAC in LACC is promising, particularly because the management paradigm for LACC has been subject to considerable lag behind other malignancies, where NAC has been adopted as a practical strategy for establishing locoregional control [34,35,36]. As previously described, the advantages of NAC include increased propensity to achieve tumour downstaging, increased R0 resection rates [5], and theoretical reduction of cancer cells disseminating within human circulation [6] and may be associated with enhanced outcomes [7]. This supports the current analysis where NAC improved surgical and oncological outcomes. Therefore, results of this study support long-term oncological benefit of NAC in LACC, notwithstanding surgical pragmatism of this strategy to improve R0 rates and increasing patient eligibility for local resection [13].
Importantly, the data from the current study and the FOXTROT trial support NAC in T3/T4 LACC [14, 15]. This evidence is not represented in current expert consensus guidelines: Recent NCCN guidelines acknowledge the perceived benefit of NAC in pT4b colon cancer from FOXTROT [9] but fails to endorse NAC in pT4a disease. Moreover, NAC is not recommended by the American Society of Colon and Rectal Surgeons (ASCRS) and European Society for Medical Oncology (ESMO) guidelines for the management of LACC [10, 37], with such therapies currently reserved solely for stage IV disease [38]. Accordingly, this study provides clinical data indicating that NAC is advantageous in LACC, therefore refuting recent recommendations of the aforementioned expert consensuses and guidelines.
Overall, the authors see the data proposed in the current study as being representative of patients with LACC in the ‘real-world’ setting. For example, the mean age of included patients in this study was 61 years, considerably lower than the typical patient with stage IV colon cancer [39], yet directly compared to previous studies where patients received NAC for LACC [5, 40]. Moreover, 52% of patients treated in the current study were male demonstrating consistency with global cancer statistics as reported by Siegel et al. in 2023 [41], where close to a 50:50 split in colon cancer diagnoses was observed among male and female patients with LACC. In addition, a pCR rate of 4.6% was observed in this study, which is similar to previously published study by Hasan et al. from North America which used patient data from the National Cancer Database [42]. Similarly, utility of NAC significantly increased R0 resection rates in this study to approximately 90%, again consistent with recent results of Huang et al. [43]. Accordingly, when considering the promising results of this study in tandem with the work of our colleagues globally, the authors believe this data provides a fair representation of the typical patient who may be subject to NAC as a therapeutic strategy in contemporary LACC management, making these results translatable to clinical practice.
The present systematic review and meta-analysis suffers from a number of limitations. Firstly, various neoadjuvant chemotherapeutic strategies have been evaluated in this study, some of which may be scrutinised in providing limited data within the context of current best practice guidelines. Secondly, while the raw data captured in this study seems to support NAC as a safe treatment in LACC, there is limited data surrounding tumour progression rates, with 10% of patients undergoing incomplete (R1 or R2) resections post NAC. Thirdly, inclusion of retrospective data inevitably renders results subject to unavoidable confounding and selection bias [44]. Fourthly, surgical techniques and concepts surrounding colonic resection have evolved in recent decades (e.g.: complete mesocolic resection) [45], which may have implications on the oncological and surgical outcomes observed for patients with LACC. Finally, it is imperative to highlight that the authors appreciate that under no circumstances, it is sensible to assume that propensity-matched studies are capable of replicating the insights provided by studies of a prospective, randomised design [46]. Thus, their analyses were performed in isolation in the current study as well as being pooled with the RCT data. Nevertheless, as described, the authors believe this data provides ‘real-world’ data with advocacy for use of NAC as a practical therapeutic strategy in LACC.
In conclusion, 8 randomised or propensity-matched studies were identified which, in tandem, highlight the oncological safety of NAC for patients being treated with curative intent for LACC. While this study may face criticism due to inclusion of non-randomised studies, these results certainly refute current management guidelines which do not advocate for NAC as a practical strategy to improve surgical and oncological outcomes in those with LACC.
Data availability
Data will be made available upon responsible request from the corresponding author.
References
Chakrabarti S, Peterson CY, Sriram D et al (2020) Early stage colon cancer: current treatment standards, evolving paradigms, and future directions. World J Gastrointest Oncol 12(8):808–832
Chan KKW, Saluja R, Delos Santos K et al (2018) Neoadjuvant treatments for locally advanced, resectable esophageal cancer: a network meta-analysis. Int J Cancer 143(2):430–437
Prasad P, Sivaharan A, Navidi M et al (2022) Significance of neoadjuvant downstaging in gastric adenocarcinoma. Surgery 172(2):593–601
Petrelli F, Trevisan F, Cabiddu M et al (2020) Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg 271(3):440–448
Gosavi R, Chia C, Michael M et al (2021) Neoadjuvant chemotherapy in locally advanced colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis 36(10):2063–2070
Roth MT, Eng C (3030) Neoadjuvant chemotherapy for colon cancer. Cancers (Basel) 12(9)
Hu Y, Hu D, Li W et al (2019) Neoadjuvant chemotherapy brings more survival benefits than postoperative chemotherapy for resectable gastric cancer: a meta-analysis of randomized controlled trials. J Buon 24(1):201–214
Imyanitov EN, Yanus GA (2018) Neoadjuvant therapy: theoretical, biological and medical consideration. Chin Clin Oncol 7(6):55
Cancer NCCNC (2022) Version 2. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
Argilés G, Tabernero J, Labianca R et al (2020) Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 31(10):1291–1305
Excellence NIfHaC (2020). https://www.nice.org.uk/guidance/ng151/chapter/Recommendations#management-of-local-disease
Kneuertz PJ, Chang GJ, Hu C-Y et al (2015) Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg 150(5):402–409
Body A, Prenen H, Latham S et al (2021) The role of neoadjuvant chemotherapy in locally advanced colon cancer. Cancer Manag Res 13:2567–2579
Morton D, Seymour M, Magill L et al (2023) Preoperative chemotherapy for operable colon cancer: mature results of an international randomized controlled trial. J Clin Oncol 41(8):1541–1552
Foxtrot CG (2012) Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 13(11):1152–1160
Yunlong W, Tongtong L, Hua Z (2023) The efficiency of neoadjuvant chemotherapy in colon cancer with mismatch repair deficiency. Cancer Med 12(3):2440–2452
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Richardson WS, Wilson MC, Nishikawa J et al (1995) The well-built clinical question: a key to evidence-based decisions. ACP J Club 123(3):A12–A13
Sargent DJ, Wieand HS, Haller DG et al (2005) Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 23(34):8664–8670
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898
Sterne JA, Hernán MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors) (2022) Cochrane handbook for systematic reviews of interventions 6.3. Available from: www.training.cochrane.org/handbook
Andrews JC, Schünemann HJ, Oxman AD et al (2013) GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol 66(7):726–35
Kim H-Y (2017) Statistical notes for clinical researchers: chi-squared test and Fisher’s exact test. Restor Dent Endod 42(2):152–155
Fidler V, Nagelkerke N (2013) The Mantel-Haenszel procedure revisited: models and generalizations. PLoS ONE 8(3):e58327
Lin L, Chu H (2020) Meta-analysis of proportions using generalized linear mixed models. Epidemiology 31(5):713–717
Hu H, Huang M, Li Y et al (2022) Perioperative chemotherapy with mFOLFOX6 or CAPOX for patients with locally advanced colon cancer (OPTICAL): a multicenter, randomized, phase 3 trial. J Clinical Oncol 40(16_suppl):3500
de Gooyer JM, Verstegen MG, t Lam-Boer J et al (2020) Neoadjuvant Chemotherapy for locally advanced T4 colon cancer: a nationwide propensity-score matched cohort analysis. Dig Surg 37(4):292–301
Karoui M, Rullier A, Piessen G et al (2020) Perioperative FOLFOX 4 versus FOLFOX 4 plus cetuximab versus immediate surgery for high-risk stage II and III colon cancers: a phase II multicenter randomized controlled trial (PRODIGE 22). Ann Surg 271(4):637–645
Dehal A, Graff-Baker AN, Vuong B et al (2018) Neoadjuvant chemotherapy improves survival in patients with clinical T4b colon cancer. J Gastrointest Surg 22(2):242–249
Zeng W, Liu Y, Wang C et al (2022) Efficacy and safety of neoadjuvant chemotherapy combined with adjuvant chemotherapy for locally advanced colon cancer: a propensity score-matching analysis. Medicina (Kaunas) 58(11)
Laursen M, Dohrn N, Gögenur I et al (2022) Neoadjuvant chemotherapy in patients undergoing colonic resection for locally advanced nonmetastatic colon cancer: a nationwide propensity score matched cohort study. Colorectal Dis 24(8):954–964
(2003) Results of a randomized trial with or without 5-FU-based preoperative chemotherapy followed by postoperative chemotherapy in resected colon and rectal carcinoma. Jpn J Clin Oncol 33(6):288–96
(2018) Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 19(1):27–39
Mansour JC, Tang L, Shah M et al (2007) Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann Surg Oncol 14(12):3412–3418
Shah RD, Cassano AD, Neifeld JP (2014) Neoadjuvant therapy for esophageal cancer. World J Gastrointest Oncol 6(10):403–406
Vogel JD, Eskicioglu C, Weiser MR et al (2017) The American Society of Colon and Rectal Surgeons clinical practice guidelines for the treatment of colon cancer. Dis Colon Rectum 60(10):999–1017
Van Cutsem E, Cervantes A, Nordlinger B et al (2014) Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25 Suppl 3:iii1–9
Davey MG, Feeney G, Annuk H et al (2022) MicroRNA expression profiling predicts nodal status and disease recurrence in patients treated with curative intent for colorectal cancer. Cancers (Basel) 14(9)
Esposito L, Allaix ME, Galosi B et al (2022) Should be a locally advanced colon cancer still considered a contraindication to laparoscopic resection? Surg Endosc 36(5):3039–3048
Siegel RL, Miller KD, Wagle NS et al (2023) Cancer statistics, 2023. CA Cancer J Clinic 73(1):17–48
Hasan S, Renz P, Wegner RE et al (2020) Microsatellite instability (MSI) as an independent predictor of pathologic complete response (PCR) in locally advanced rectal cancer: a National Cancer Database (NCDB) analysis. Ann Surg 271(4):716–723
Huang CM, Huang CW, Ma CJ et al (2020) Outcomes of neoadjuvant chemoradiotherapy followed by radical resection for T4 colorectal cancer. World J Gastrointest Oncol 12(12):1428–1442
Shafer SL, Dexter F (2012) Publication bias, retrospective bias, and reproducibility of significant results in observational studies. Anesth Analg 114(5):931–932
Dimitriou N, Griniatsos J (2015) Complete mesocolic excision: techniques and outcomes. World J Gastrointest Oncol 7(12):383–388
Stevens RJ, Oke JL (2022) Propensity scores in surgery: don’t believe the hype. Colorectal Dis 24(8):896–898
Funding
Open Access funding provided by the IReL Consortium.
Author information
Authors and Affiliations
Contributions
Study conceptualisation was performed by Matthew G. Davey. Matthew G. Davey, Odhrán K. Ryan., Mark Donnelly, and Amira H. Amir had access to raw data. Matthew G. Davey, Odhrán K. Ryan, Noel E. Donlon, Mark Donnelly, and Amira H. Amir performed statistical analyses. Matthew G. Davey, Odhrán K. Ryan, Mark Donnelly, Noel E. Donlon, and Amira H. Amir prepared study tables and figures. Analysis and interpretation of data were done by Matthew G. Davey, Mark Regan, Babak Meshkat, Emmeline Nugent, Myles Joyce, and Aisling M. Hogan. Matthew G. Davey drafted and wrote the manuscript, while all other authors were consulted with several drafts to appraise the intellectual content of the manuscript. All authors were involved in the preparation of this manuscript and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Davey, M.G., Amir, A.H., Ryan, O.K. et al. Evaluating the oncological safety of neoadjuvant chemotherapy in locally advanced colon carcinoma: a systematic review and meta-analysis of randomised clinical trials and propensity-matched studies. Int J Colorectal Dis 38, 193 (2023). https://doi.org/10.1007/s00384-023-04482-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s00384-023-04482-x