Abstract
Interstitial cells of Cajal (ICCs) are pacemaker cells of gastrointestinal motility that generate and transmit electrical slow waves to smooth muscle cells in the gut wall, thus inducing phasic contractions and coordinated peristalsis. Traditionally, tyrosine-protein kinase Kit (c-kit), also known as CD117 or mast/stem cell growth factor receptor, has been used as the primary marker of ICCs in pathology specimens. More recently, the Ca2+-activated chloride channel, anoctamin-1, has been introduced as a more specific marker of ICCs. Over the years, various gastrointestinal motility disorders have been described in infants and young children in which symptoms of functional bowel obstruction arise from ICC-related neuromuscular dysfunction of the colon and rectum. The current article provides a comprehensive overview of the embryonic origin, distribution, and functions of ICCs, while also illustrating the absence or deficiency of ICCs in pediatric patients with Hirschsprung disease intestinal neuronal dysplasia, isolated hypoganglionosis, internal anal sphincter achalasia, and congenital smooth muscle cell disorders such as megacystis microcolon intestinal hypoperistalsis syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interstitial cells of Cajal (ICCs) were first described in 1893 by the Spanish histopathologist and Nobel Prize laureate Santiago Ramón y Cajal as “fibroblast-like cells in the muscularis externa and villous stroma of the gastrointestinal tract” [1]. Later, light and electron microscopy studies demonstrated that ICCs are neither neurons nor Schwann cells [2,3,4], forming a unique class of cells that are distinct from the enteric nervous system (ENS) [5]. In the 1990s, the tyrosine-protein kinase Kit (c-kit), also known as CD117 or mast/stem cell growth factor receptor, has been identified as the primary marker of ICCs in pathology specimens [6, 7]. However, while loss of c-kit positivity is not necessarily indicative of loss of ICCs, it also follows that normal levels of c-kit positivity are not automatically suggestive of normal ICC distribution [8, 9]. This, in addition to the finding that c-kit also labels mast cells in the circular muscle layer [10], has led recently to the introduction of the Ca2+-activated chloride channel anoctamin-1 (Ano1) as more specific marker of ICCs [11,12,13].
Today, it is well established that ICCs are distributed throughout the entire alimentary tract, from the upper esophageal sphincter to the internal sphincter of the anus [14,15,16]. ICCs are located between the nerve endings of motor neurons and smooth muscle cells, modulating inhibitory and excitatory signals from the ENS [9, 17]. They play a major role in gastrointestinal motility by generating slow-wave electrical activity, which propagates throughout the smooth muscle layers of the gut, giving rise to peristaltic waves [8]. In 2013, it was reported that allotransplantation of ICCs could not only populate tissues but also establishes functional pacemaker activity, where they originally were absent [18]. Thus, further research in this field may provide the basis for a therapeutic treatment of gastrointestinal motility disorders in patients, where ICC networks have been disrupted or lost, for example due to genetic defects, pathophysiological insults or natural aging processes.
Embryonic origin and development of ICCs
In contrast to Cajal’s opinion, ICCs develop independently of neural crest-derived enteric neurons and glia, and originate mainly from c-kit-positive mesenchymal precursor cells [19,20,21,22]. Furthermore, it has been shown that the normal development of ICCs depends on the expression of c-kit [6, 7]. Kit signaling is essential for both the development and maintenance of functional ICCs in the embryonic gastrointestinal tract, with precursor ICCs expressing c-kit as early as embryonic day 11 (E11) in mice [20]. At E12, c-kit-positive cell clusters were found in the periphery of developing murine small intestine, just under the serosal surface. C-kit-positive cells occur from E15 onwards peripheral to developing myenteric ganglia [23]. The late gestational time between E15 and E18 seems to be a critical period during ICC development as the c-kit-positive precursors begin to develop toward a functional ICC phenotype. The pharmacological or genetic blockade of Kit signaling during late gestation results in the failure of ICC networks and pacemaker function to develop in the small intestine. However, the ICC network appears to have a certain plasticity, allowing for restorative changes and redevelopment of functional ICCs [21].
Various studies have investigated the fetal and postnatal development of ICCs in the human gastrointestinal tract, demonstrating c-kit-positive cells in the stomach from 9.5 weeks of gestation and in the small and large bowel from 12 to 13 weeks [24,25,26]. ICCs also undergo significant changes postnatally. The number of ICC cell bodies and volume of ICC within the human stomach and colon decrease with age at a rate of 13% per decade, with no differences according to sex or location in the gastrointestinal tract [27].
Functions and distribution of ICCs
Over a century ago, the Scottish anatomist Sir Arthur Keith had already suggested that ICCs might act as pacemaker cells of gastrointestinal motility, coordinating phasic contractile activity [28]. Later on, electron microscopical studies have shown a close association of ICCs with nerve terminals and gap junctions within smooth muscle cells [5, 29]. Today, numerous gastrointestinal functions are known that are affected by ICCs (e.g., generating and active propagation of electrical slow waves, depolarization into adjacent smooth musculature, etc.).
Extensive morphological and electrophysiological research has revealed multiple complex functions of ICCs (Box 1).
Several subtypes of ICCs have been distinguished according to their distinct distribution patterns and morphological features within the anatomical layers of the gastrointestinal tract [34]. Each type of ICCs is determined by the structure of their adjacent smooth muscle layer, their relation to neighboring nerve plexuses and the density of their connections with other ICCs (Table 1).
Identification and visualization of ICCs
Over the years, the distribution and morphology of ICCs have been analyzed by many methods. Historically, traditional histology stains such as methylene blue, silver or Golgi impregnation were used. These specific staining techniques led to the previous assumption that ICCs are primitive neurons, as they were unable to truly discriminate between neurons and ICCs. Later, electron microscopy was applied to further enable ultrastructural studies of ICCs [45,46,47,48]. To date, electron microscopy remains the method of choice for the examination of the typical ultrastructural features of ICCs, including their well-developed smooth endoplasmic reticulum, abundant intermediate filaments, lack of myosin filaments, numerous caveolae, dense bodies and bands as well as an oval indented nucleus [49,50,51,52,53,54,55,56,57]. Furthermore, ICCs are intercalated between neurons and smooth muscle cells and have been shown to form gap junctions with the latter cell type [58]. In addition to the characterization of single cells, the distribution and topography of ICCs in various tissues have been investigated in great detail. The identification of c-kit expression in ICCs was a major scientific breakthrough. This proto-oncogene that encodes the receptor tyrosine kinase kit is highly expressed in both ICCs and mast cells [7, 30, 31, 59, 60]. Consequently, many studies have been performed showing the expression of c-kit-positive ICCs in the gastrointestinal tract of several species, including humans [10, 26, 61, 62], mice [26], rats [63, 64], and guinea-pigs [65, 66]. These studies and further investigations have increased our understanding of the complex architecture of ICC networks in relation to the ENS and the intestinal smooth muscle layers [67]. Recently, Ano1 has been identified as a highly specific marker for all subtypes of ICCs within the gastrointestinal tract of mice and humans, and its expression has been associated with the generation of electric slow waves [12] (Figs. 1, 2 and 3).
The complex relationship of ICCs to the ENS and other surrounding structures was traditionally investigated in conventional thin histological sections. The development of whole-mount preparation has proven to be an effective technique for the visualization of the structure of the intrinsic networks (e.g., neurons and ICCs) and their patterns of branching and interconnection with each other and neighboring tissue layers (Fig. 4). It facilitates the three-dimensional study of the morphology of neuronal and ICC networks [68, 69], having some obvious advantages compared to conventional histological thin sections, as it enables a more detailed examination of the complex morphology of neurons, glial cells or ICC within the gut [70]. Whole-mount preparations can comprise several layers of bowel wall, including the longitudinal muscle layer and the adjacent myenteric plexus. They are made by separating the muscular layer from the submucosal layer, followed by removal of the circular muscle layer from the longitudinal muscle. Subsequently, the mucosa is removed from the submucosal layer to better visualize the submucosal plexus. The three-dimensional configuration of c-kit-positive cells was first described as typical for multipolar cells around the myenteric plexus and slender bipolar cells within the circular and longitudinal muscle layers [61]. Furthermore, close relationships between muscular ICCs and neurons with nitric-oxide synthase-like immunoreactivity, vesicular acetylcholine transporter and substance P-like immunoreactive axonal varicosities have been demonstrated in whole-mount preparations of guinea-pig small intestine [71]. Therefore, it has been assumed that enteric motor neurons, ICC, and smooth muscle cells form functional units [71, 72]. The close connections between ICCs and the intrinsic nitrergic innervation (e.g., NADPH-diaphorase-positive nerve fibers) have been shown in the human gut [68]. Several investigators have used the whole-mount preparation technique in specimens from the human gastrointestinal tract in combination with various other ENS staining methods, ranging from silver impregnation to enzyme histochemistry and immunohistochemistry [73,74,75].
ICCs in gastrointestinal motility disorders in childhood
Gastrointestinal motility disorders describe a heterogeneous group of conditions in infants and young children in which symptoms of functional bowel obstruction arise from a neuromuscular dysfunction of the colon and rectum (including ICCs or glial cells) [76]. In many cases, the exact pathogenesis remains poorly understood. There are a number of patients that present with clinical symptoms similar to Hirschsprung disease (HD) despite the presence of ganglion cells in rectal biopsies. Over the years, various terms such as “chronic idiopathic intestinal pseudo-obstruction”, “intestinal hypoperistalsis syndrome” or “pseudo-HD” have been used to describe these gastrointestinal motility disorders. In 1997, the pediatric surgeon Prem Puri suggested that “variant HD” may be a more appropriate description [77]. C-kit labeling has been used to study the pathological variations of ICCs in these conditions and absence or deficiency of ICC networks was identified.
Hirschsprung disease
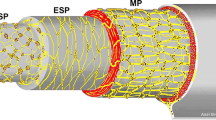
HD is one of the most common congenital gastrointestinal motility disorders. Clinical symptoms related to functional bowel obstruction, such as delayed first passage of meconium, abdominal distension, and bilious vomiting result from a congenital aganglionosis in the most distal part of the gastrointestinal tract. The distribution of ICCs has widely been studied in HD bowel using normal histology sections and whole-mount preparation techniques. The focus of these investigations has not been restricted to the aganglionic segment but has extended to the ganglionic segment in HD [61, 72, 78,79,80,81,82,83,84]. Most of these studies have shown a reduced number of c-kit-positive ICCs in the aganglionic bowel and also in the transition zone of HD patients [72, 78,79,80,81,82,83,84]. Major pathological features in HD included a reduced number of myenteric ICCs and disrupted myenteric ICC networks that are only sparsely distributed between hypertrophic nerve trunks (Fig. 5). Furthermore, muscular ICCs have been found to be markedly reduced in the HD bowel [79, 80]. Interestingly, a total reduction of ICCs in the proximal, ganglionic colon of HD patients has also been observed in comparison to healthy controls [85]. This observation has been contested by others, who did not find an overall difference in the distribution of c-kit-positive ICCs [80, 84, 86, 87]. However, a marked variability of ICC values in patients with HD has been noted, which may be a reflection of the heterogeneous character of this disease [86]. In addition, two studies linked poor clinical outcomes in HD patients to very low numbers of ICCs and a low ratio of ICCs to neural innervation [86, 88]. More recently, the use of c-kit has been replaced by the more specific ICC marker Ano1, showing a moderate reduction of ICC fibers in ganglionic HD colon, compared to the colon of non-HD patients [89]. These contradicting results are likely to arise from small study populations, but may have also been biased by the fact that the distribution of ICCs can vary with age and location in the gastrointestinal tract [8, 85, 89]. Moreover, an important question is whether the observed alterations of ICCs in HD are truly primary or whether they are secondary to long-lasting functional obstruction. Therefore, the role of structural ICC abnormalities in persistent bowel dysfunction in patients with HD is still to be elucidated.
Intestinal neuronal dysplasia
The pathologist William A. Meier-Ruge described intestinal neuronal dysplasia (IND) in 1971 as a hyperplastic malformation of the enteric plexus [90]. A few years later, a case of rectosigmoid aganglionosis associated with IND of the descending and transverse colon has been reported [91]. Nowadays, IND can be classified into two clinical and histological distinct subtypes [92]: IND type A (IND A), occurring in less than 5% of all IND cases, is characterized by congenital aplasia or hypoplasia of the sympathetic innervation. Normally, patients with IND A present in the neonatal period with episodes of abdominal distension, intestinal obstruction and diarrhea with bloody stools. Conversely, IND type B (IND B) is defined by hyperplasia of the parasympathetic submucosal and myenteric plexuses, accounting for over 95% of all IND cases. Typical histological features of IND B include hyperganglionosis, giant ganglia, ectopic ganglion cells, and increased activity of acetylcholinesterase (AChE) in the lamina propria and around submucosal blood vessels [93]. IND occurring in association with HD is invariably IND B. While some authors have found IND in up to 44% of their HD patients, others have rarely encountered IND in association with HD [94]. The existence of IND as a distinct histopathological entity remains controversial [95]. Hence, several researchers have suggested that the observed changes in IND may be either a variant of normal bowel development or a secondary acquired phenomenon caused by congenital obstruction or inflammation [96]. Nevertheless, a reduced number of c-kit-positive ICCs has been demonstrated in the myenteric plexus and muscle layers of IND cases [97].
Isolated hypoganglionosis
Isolated hypoganglionosis (HG) is a rare entity, which has been classified as a hypogenetic type of intestinal innervation disorders. The clinical presentation of patients with isolated HG is similar to those with classical HD with non-specific symptoms of severe constipation or bowel obstruction. It has been shown that congenital and acquired HG are two separate entities with different clinical features and histological findings [98]. At present, there are only a few cases in the published literature as isolated HG is one of the rarest types of gastrointestinal motility disorders and there remains controversy regarding it as a distinct isolated histopathological entity [96]. Some cases of isolated HG were reported to exhibit deficient expression of c-kit-positive ICCs within the myenteric plexus and the smooth muscle layer, which may contribute to the observed motility dysfunction in the hypoganglionic bowel segment. C-kit staining has been employed to investigate the expression of ICCs and, thus, intestinal pacemaker activity, which is markedly decreased or even absent in patients with isolated HG [99].
Internal anal sphincter achalasia
Internal anal sphincter achalasia (IASA) has a similar clinical presentation to HD, but with the presence of ganglion cells in rectal biopsies. Previously, IASA was referred to as ultrashort-segment HD, which is characterized by an aganglionic segment of 1–3 cm above the pectinate line, normal AChE activity in the lamina propria and increased AChE activity in the muscularis mucosae [100]. Thus, it has been suggested that IASA is a more accurate term for this pathological entity as many patients with absence of the rectosphincteric reflex on anorectal manometry actually showed presence of ganglion cells combined with normal AChE activity in rectal biopsies [101]. Despite attempts of numerous investigators to determine the pathophysiological mechanisms of IASA in more detail, the exact pathogenesis remains unknown. Age-related changes in the developing intramuscular innervation of the internal anal sphincter (IAS) most likely form the basis for the observed motility dysfunction [101]. Additionally, a reduced number of c-kit-positive ICCs has been found in the IAS of patients with IASA [97]. The deficiency in nitrergic innervation and ICCs may explain the impaired IAS relaxation in these cases.
Megacystis microcolon intestinal hypoperistalsis syndrome
Megacystis microcolon intestinal hypoperistalsis syndrome (MMIHS) is an extremely rare condition and the most severe form of functional bowel obstruction in the newborn, characterized by massive abdominal distension caused by a large-dilated non-obstructed bladder, microcolon with malrotation and decreased or absent intestinal peristalsis [102]. MMIHS was first observed in 1976 in five newborn girls [103]. Since then, various hypotheses have been proposed to explain the pathogenesis of MMIHS. Genetic, myogenic, neurogenic, and hormonal etiologies have been discussed. However, most of these theories were derived from case reports due to the rarity of this condition. Thus, the etiology remains poorly understood. Histological evaluation of myenteric and submucosal plexuses has revealed normal ganglion cells in 77% of the investigated bowel specimens from patients with MMIHS. The remaining 23% were shown to have various neuronal abnormalities including hyper-/hypoganglionosis and immature ganglia [100]. Furthermore, some authors found significant anomalies in smooth muscle cells from bowel and bladder specimens, such as vacuolar degeneration as well as thinning of the longitudinal muscle [104, 105]. Likewise, a decreased expression of ICCs in the bladder has been observed [97].
Conclusion and future directions
ICCs have a central function in the generation and propagation of gastrointestinal slow-wave activity and their loss might result in gastrointestinal motility dysfunction. Most of the available research studies have shown that HD and allied disorders are associated with either a loss of or deficiency in ICC networks. However, these findings require careful interpretation, as our current understanding of the nature of the relationships between the loss of ICCs and the development of clinical symptoms in humans is incomplete. It should be noted that all investigated specimens had previously been subjected to long-lasting functional obstruction. Thus, it is difficult to determine whether the loss or deficiency of ICCs is the consequence or the cause of the disease process. Consequently, further clinical and animal studies are necessary to improve our knowledge regarding the true importance of the impaired ICC function in infants and children with gastrointestinal motility disorders.
References
Cajal RS (1893) Sur les ganglions et plexus nerveux de I’intestin. CR Soc Biol (Paris) 45:217–223
Faussone Pellegrini MS (1985) Ultrastructural peculiarities of the inner portion of the circular layer of the colon. II. Research on the mouse. Acta Anat (Basel) 122:187–192. https://doi.org/10.1159/000146000
Taxi J (1965) Contribution á l’étude des connexions des neurones moteurs du systeme nerveux sutonome. Ann Sci Nat Zool Biol Anim 7:413–674
Taxi J (1952) Cellules de Schwann et “cellules interstitielles de Cajal” au niveau des plexus nerveux de la muscleuse intestinale du Cobaye: retour aux definitions. Arch Anat Micrsc Morphol Exp 41:281–304
Thuneberg L (1982) Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol 71:1–130
Komuro T, Tokui K, Zhou DS (1996) Identification of interstitial cells of Cajal. Histol Histopathol 11:769–786
Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A (1995) W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 26(373):347–349. https://doi.org/10.1038/373347a0
Huizinga JD, Chen JH (2014) Interstitial cells of Cajal: update on basic and clinical science. Curr Gastroenterol Rep 16:363. https://doi.org/10.1007/s11894-013-0363-z
Burns AJ (2007) Disorders of interstitial cells of Cajal. J Pediatr Gastroenterol Nutr 45:S103-106. https://doi.org/10.1097/MPG.0b013e31812e65e0
Rømert P, Mikkelsen HB (1998) c-kit immunoreactive interstitial cells of Cajal in the human small and large intestine. Histochem Cell Biol 109:195–202. https://doi.org/10.1007/s004180050218
Carmona R, Cano E, Mattiotti A, Gaztambide J, Muñoz-Chápuli R (2013) Cells derived from the coelomic epithelium contribute to multiple gastrointestinal tissues in mouse embryos. PLoS One 8:e55890. https://doi.org/10.1371/journal.pone.0055890
Sanders KM, Zhu MH, Britton F, Koh SD, Ward SM (2012) Anoctamins and gastrointestinal smooth muscle excitability. Exp Physiol 97:200–206. https://doi.org/10.1113/expphysiol.2011.058248
Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G (2009) Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 296:G1370-1381. https://doi.org/10.1152/ajpgi.00074.2009
Torihashi S, Horisawa M, Watanabe Y (1999) c-kit immunoreactive interstitial cells in the human gastrointestinal tract. J Auton Nerv Syst 75:38–50. https://doi.org/10.1016/s0165-1838(98)00174-x
Hagger R, Gharaie S, Finlayson C, Kumar D (1998) Distribution of the interstitial cells of Cajal in the human anorectum. J Auton Nerv Syst 73:75–79. https://doi.org/10.1016/s0165-1838(98)00038-1
Faussone-Pellegrini MS, Cortesini C (1985) Ultrastructural features and localization of the interstitial cells of Cajal in the smooth muscle coat of human esophagus. J Submicrosc Cytol 17:187–197
Benarroch EE (2007) Enteric nervous system: functional organization and neurologic implications. Neurology 69:1953–1957. https://doi.org/10.1212/01.wnl.0000281999.56102.b5
McCann CJ, Hwang SJ, Bayguinov Y, Colletti EJ, Sanders KM, Ward SM (2013) Establishment of pacemaker activity in tissues allotransplanted with interstitial cells of Cajal. Neurogastroenterol Motil 25:e418-428. https://doi.org/10.1111/nmo.12140
Klüppel M, Huizinga JD, Malysz J, Bernstein A (1998) Developmental origin and Kit-dependent development of the interstitial cells of Cajal in the mammalian small intestine. Dev Dyn 211:60–71. https://doi.org/10.1002/(SICI)1097-0177(199801)211:1%3c60::AID-AJA6%3e3.0.CO;2-5
Torihashi S, Ward SM, Sanders KM (1997) Development of c-kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology 112:144–155. https://doi.org/10.1016/s0016-5085(97)70229-4
Lecoin L, Gabella G, Le Douarin N (1996) Origin of the c-kit-positive interstitial cells in the avian bowel. Development 122:725–733. https://doi.org/10.1242/dev.122.3.725
Young HM, Ciampoli D, Southwell BR, Newgreen DF (1996) Origin of interstitial cells of Cajal in the mouse intestine. Dev Biol 180:97–107. https://doi.org/10.1006/dbio.1996.0287
Beckett EA, Ro S, Bayguinov Y, Sanders KM, Ward SM (2007) Kit signaling is essential for development and maintenance of interstitial cells of Cajal and electrical rhythmicity in the embryonic gastrointestinal tract. Dev Dyn 236:60–72. https://doi.org/10.1002/dvdy.20929
Kenny SE, Connell G, Woodward MN, Lloyd DA, Gosden CM, Edgar DH, Vaillant C (1999) Ontogeny of interstitial cells of Cajal in the human intestine. J Pediatr Surg 34(8):1241–1247. https://doi.org/10.1016/s0022-3468(99)90160-4
Ward SM, Ordög T, Bayguinov JR, Horowitz B, Epperson A, Shen L, Westphal H, Sanders KM (1999) Development of interstitial cells of Cajal and pacemaking in mice lacking enteric nerves. Gastroenterology 117:584–594. https://doi.org/10.1016/s0016-5085(99)70451-8
Wester T, Eriksson L, Olsson Y, Olsen L (1999) Interstitial cells of Cajal in the human fetal small bowel as shown by c-kit immunohistochemistry. Gut 44:65–71. https://doi.org/10.1136/gut.44.1.65
Gomez-Pinilla PJ, Gibbons SJ, Sarr MG, Kendrick ML, Shen KR, Cima RR, Dozois EJ, Larson DW, Ordog T, Pozo MJ, Farrugia G (2011) Changes in interstitial cells of cajal with age in the human stomach and colon. Neurogastroenterol Motil 23:36–44. https://doi.org/10.1111/j.1365-2982.2010.01590.x
Keith A (1915) The Cabendish lecture: on a new theory of the causation of enterostasis. Lancet 186:371–375. https://doi.org/10.1016/S0140-6736(01)53737-X
Yamamoto M (1977) Electron microscopic studies on the innervation of the smooth muscle and the interstitial cell of Cajal in the small intestine of the mouse and bat. Arch Histol Jpn 40:171–201. https://doi.org/10.1679/aohc1950.40.171
Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM (1995) c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res 280:97–111. https://doi.org/10.1007/BF00304515
Ward SM, Burns AJ, Torihashi S, Sanders KM (1994) Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol 480:91–97. https://doi.org/10.1113/jphysiol.1994.sp020343
Sanders KM, Ward SM (2006) Interstitial cells of Cajal: a new perspective on smooth muscle function. J Physiol 576:721–726. https://doi.org/10.1113/jphysiol.2006.115279
Komuro T (2006) Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol 576:653–658. https://doi.org/10.1113/jphysiol.2006.116624
Huizinga JD, Hussain A, Chen JH (2021) Interstitial cells of Cajal and human colon motility in health and disease. Am J Physiol Gastrointest Liver Physiol 321:G552–G575. https://doi.org/10.1152/ajpgi.00264.2021
Mitsui R, Komuro T (2003) Distribution and ultrastructure of interstitial cells of Cajal in the gastric antrum of wild-type and Ws/Ws rats. Anat Embryol (Berl) 206:453–460. https://doi.org/10.1007/s00429-003-0323-8
Seki K, Komuro T (2002) Distribution of interstitial cells of Cajal and gap junction protein, Cx 43 in the stomach of wild-type and W/Wv mutant mice. Anat Embryol (Berl) 206:57–65. https://doi.org/10.1007/s00429-002-0279-0
Horiguchi K, Semple GS, Sanders KM, Ward SM (2001) Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum. J Physiol 537:237–250. https://doi.org/10.1111/j.1469-7793.2001.0237k.x
Ishikawa K, Komuro T (1996) Characterization of the interstitial cells associated with the submuscular plexus of the guinea-pig colon. Anat Embryol (Berl) 194:49–55. https://doi.org/10.1007/BF00196314
Berezin I, Huizinga JD, Daniel EE (1988) Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. J Comp Neurol 273:42–51. https://doi.org/10.1002/cne.902730105
Mazet B, Raynier C (2004) Interstitial cells of Cajal in the guinea pig gastric antrum: distribution and regional density. Cell Tissue Res 316:23–34. https://doi.org/10.1007/s00441-003-0835-9
Rumessen JJ, Thuneberg L, Mikkelsen HB (1982) Plexus muscularis profundus and associated interstitial cells. II. Ultrastructural studies of mouse small intestine. Anat Rec 203:129–146. https://doi.org/10.1002/ar.1092030112
Blair PJ, Bayguinov Y, Sanders KM, Ward SM (2012) Interstitial cells in the primate gastrointestinal tract. Cell Tissue Res 350:199–213. https://doi.org/10.1007/s00441-012-1468-7
Zhou DS, Komuro T (1992) Interstitial cells associated with the deep muscular plexus of the guinea-pig small intestine, with special reference to the interstitial cells of Cajal. Cell Tissue Res 268:205–216. https://doi.org/10.1007/BF00318788
Vanderwinden JM, Rumessen JJ, Bernex F, Schiffmann SN, Panthier JJ (2000) Distribution and ultrastructure of interstitial cells of Cajal in the mouse colon, using antibodies to Kit and Kit(W-lacZ) mice. Cell Tissue Res 302:155–170. https://doi.org/10.1007/s004419900170
Rogers DC, Burnstock G (1966) The interstitial cell and its place in the concept of the autonomic ground plexus. J Comp Neurol 126:255–284. https://doi.org/10.1002/cne.901260207
Cook RD, Burnstock G (1976) The ultrastructure of Auerbach`s plexus in the guinea pig. II Non-neuronal elements J Neurocytol 5:195–206. https://doi.org/10.1007/BF01181656
Imaizumi M, Hama K (1969) An electron microscopic study on the interstitial cells of the gizzard in the love bird (Uronloncha domestica). Z Zellforsch Mikrosk Anat 97:351–357. https://doi.org/10.1007/BF00968841
Gabella G (1972) Fine structure of the myenteric plexus in the guinea-pig ileum. J Anat 111:69–97
Faussone-Pellegrini MS (1992) Histogenesis, structure and relationships of interstitial cells of Cajal (ICC): from morphology to functional interpretation. Eur J Morphol 30:137–148
Rumessen JJ, Mikkelsen HB, Qvortrup K, Thuneberg L (1993) Ultrastructure of interstitial cells of Cajal in circular muscle of human small intestine. Gastroenterology 104:343–350. https://doi.org/10.1016/0016-5085(93)90400-7
Faussone-Pellegrini MS, Thuneberg L (1999) Guide to the identification of interstitial cells of Cajal. Microsc Res Tech 47:248–266. https://doi.org/10.1002/(SICI)1097-0029(19991115)47:4%3c248::AID-JEMT4%3e3.0.CO;2-W
Mazzia C, Porcher C, Julé Y, Christen MO, Henry M (2000) Ultrastructural study of relationships between c-kit immunoreactive interstitial cells and other cellular elements in the human colon. Histochem Cell Biol 113:401–411. https://doi.org/10.1007/s004180000154
Hagger R, Finlayson C, Jeffrey I, Kumar D (1997) Role of the interstitial cells of Cajal in the control of gut motility. Brit J Surg 84:445–450
Rumessen JJ, Thuneberg L (1991) Interstitial cells of Cajal in the human small intestine. Ultrastructural identification and organization between the main smooth muscle layers. Gastroenterology 100:1417–1431
Rumessen JJ, Thuneberg L (1996) Pacemaker cells in the gastrointestinal tract: interstitial cells of Cajal. Scand J Gastroenterol 216:82–94. https://doi.org/10.3109/00365529609094564
Komuro T, Tokui K, Zhou DS (1996) Identification of the interstitial cells of Cajal. Histol Histopathol 11:769–786
Christensen J, Rick GA, Lowe LS (1992) Distributions of interstitial cells of Cajal in the stomach and colon of cat, dog, ferret, opossum, rat, guinea pig and rabbit. J Auton Nerv Syst 37:47–56. https://doi.org/10.1016/0165-1838(92)90144-6
Christensen J (1992) A commentary on the morphological identification of interstitial cells of Cajal in the gut. J Auton Nerv Syst 37:75–88. https://doi.org/10.1016/0165-1838(92)90236-a
Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa S (1992) Requirement of c-kit for development of intestinal pacemaker system. Development 116:369–375. https://doi.org/10.1242/dev.116.2.369
Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM (1995) Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol 269:C1577-1585. https://doi.org/10.1152/ajpcell.1995.269.6.C1577
Horisawa M, Watanabe Y, Torihashi S (1998) Distribution of c-kit immunopositive cells in normal human colon and in Hirschsprung’s disease. J Pediatr 33:1209–1214. https://doi.org/10.1016/s0022-3468(98)90152-x
Horie K, Fujita J, Takakura K, Kanzaki H, Suginami H, Iwai M, Nakayama H, Mori T (1993) The expression of c-kit protein in human adult and fetal tissue. Hum Reprod 8:1955–1962. https://doi.org/10.1093/oxfordjournals.humrep.a137967
Sanders KM (1996) A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 111:492–515. https://doi.org/10.1053/gast.1996.v111.pm8690216
Horiguchi K, Komuro T (1998) Ultrastructural characterization of interstitial cells of Cajal in the rat small intestine using control and Ws/Ws mutant rats. Cell Tissue Res 293:277–284. https://doi.org/10.1007/s004410051119
Isozaki K, Hirota S, Nakama A, Miyagawa J, Shinomura Y, Xu Z, Nomura S, Kitamura Y (1995) Disturbed intestinal movement, bile reflux to the stomach, and deficiency of c-kit-expressing cells in Ws/Ws mutant rats. Gastroenterology 109:456–464. https://doi.org/10.1016/0016-5085(95)90333-x
Burns AJ, Herbert TM, Ward SM, Sanders KM (1997) Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res 290:11–20. https://doi.org/10.1007/s004410050902
Komuro T, Zhou DS (1996) Anti-c-kit protein immunoreactive cells corresponding to the interstitial cells of Cajal in the guinea-pig small intestine. J Auton Nerv Syst 61:169–174. https://doi.org/10.1016/s0165-1838(96)00078-1
Nemeth L, Puri P (2001) Three-dimensional morphology of c-kit-positive cellular network and nitrergic innervation in the human gut. Arch Pathol Lab Med 125:899–904. https://doi.org/10.5858/2001-125-0899-TDMOCK
Fekete E, Resch BA, Benedeczky I (1995) Histochemical and ultrastructural features of the developing enteric nervous system of the human foetal small intestine. Histol Histopathol 10:127–134
Mebis J, Penninckx F, Geboes K, Eggermont E, Desmet V (1990) Neuropathology of Hirschsprung’s disease: en face study of microdissected intestine. Hepatogastroenterology 37:596–600
Wang XY, Sanders KM, Ward SM (1999) Intimate relationship between interstitial cells of Cajal and enteric nerves in the guinea-pig small intestine. Cell Tissue Res 295:247–256. https://doi.org/10.1007/s004410051231
Ward SM (2000) Interstitial cells of Cajal in enteric neurotransmission. Gut 47:iv40-43. https://doi.org/10.1136/gut.47.suppl_4.iv40
Rolle U, Nemeth L, Puri P (2002) Nitrergic innervation of the normal gut and in motility disorders of childhood. J Pediatr Surg 37:551–567. https://doi.org/10.1053/jpsu.2002.31610
Wedel T, Krammer HJ, Kühnel W, Sigge W (1998) Alterations of the enteric nervous system in neonatal necrotizing enterocolitis revealed by whole-mount immunohistochemistry. Pediatr Pathol Lab Med 18:57–70
Ferri GL, Botti PL, Vezzadini P (1982) Peptide-containing innervation of the human intestinal mucosa. An immunocytochemical study on whole-mount preparations. Histochemistry 76:413–420. https://doi.org/10.1007/BF00543961
Knowles CH, De Giorgio R, Kapur RP, Bruder E, Farrugia G, Geboes K, Lindberg G, Martin JE, Meier-Ruge WA, Milla PJ, Smith VV, Vandervinden JM, Veress B, Wedel T (2010) The London classification of gastrointestinal neuromuscular pathology: report on behalf of the gastro 2009 international working group. Gut 59:882–887. https://doi.org/10.1136/gut.2009.200444
Friedmacher F, Puri P (2013) Classification and diagnostic criteria of variants of Hirschsprung’s disease. Pediatr Surg Int 29:855–872. https://doi.org/10.1007/s00383-013-3351-3
Yamataka A, Kato Y, Tibboel D, Murata Y, Sueyoshi N, Fujimoto T, Nishiye H, Miyano T (1995) A lack of intestinal pacemaker (c-kit) in aganglionic bowel of patients with Hirschsprung’s disease. J Pediatr Surg 30:441–444. https://doi.org/10.1016/0022-3468(95)90051-9
Yamataka A, Ohshiro K, Kobayashi H, Fujiwara T, Sunagawa M, Miyano T (1997) Intestinal pacemaker C-KIT+ cells and synapses in allied Hirschsprung’s disorders. J Pediatr Surg 32:1069–1074. https://doi.org/10.1016/s0022-3468(97)90401-2
Vanderwinden JM, Rumessen JJ, Liu H, Descamps D, De Laet MH, Vanderhaeghen JJ (1996) Interstitial cells of Cajal in the colon and in the Hirschsprung’s disease. Gastroenterology 111:901–910. https://doi.org/10.1016/s0016-5085(96)70057-4
Rolle U, Piotrowska AP, Nemeth L, Puri P (2002) Altered distribution of interstitial cells of Cajal in Hirschsprung disease. Arch Pathol Lab Med 126:928–933. 10.5858/2002-126-0928-ADOICO
Piotrowska AP, Solari V, de Caluwe D, Puri P (2003) Immunocolocalization of the heme oxygenase-2 and interstitial cells of Cajal in normal and aganglionic colon. J Pediatr Surg 38:73–77. https://doi.org/10.1053/jpsu.2003.50014
Newgreen D, Young HM (2002) Enteric nervous system: development and developmental disturbances–part 2. Pediatr Dev Pathol 5:329–349. https://doi.org/10.1007/s10024-002-0002-4
Taguchi T, Suita S, Masumoto K, Nada O (2003) Universal distribution of c-kit-positive cells in different types of Hirschsprung’s disease. Pediatr Surg Int 19:273–279. https://doi.org/10.1007/s00383-002-0931-z
Gfroerer S, Rolle U (2013) Interstitial cells of Cajal in the normal human gut and in Hirschsprung disease. Pediatr Surg Int 29:889–897. https://doi.org/10.1007/s00383-013-3364-y
Bettolli M, De Carli C, Jolin-Dahel K, Bailey K, Khan HF, Sweeney B, Krantis A, Staines WA, Rubin S (2008) Colonic dysmotility in postsurgical patients with Hirschsprung’s disease. Potential significance of abnormalities in the interstitial cells of Cajal and the enteric nervous system. J Pediatr Surg 43:1433–1438. https://doi.org/10.1016/j.jpedsurg.2007.10.067
Newman CJ, Laurini RN, Lesbros Y, Reinberg O, Meyrat BJ (2003) Interstitial cells of Cajal are normally distributed in both ganglionated and aganglionic bowel in Hirschsprung’s disease. Pediatr Surg Int 19:662–668. https://doi.org/10.1007/s00383-003-1026-1
Taguchi T, Suita S, Masumoto K, Nagasaki A (2005) An abnormal distribution of C-kit positive cells in the normoganglionic segment can predict a poor clinical outcome in patients with Hirschsprung’s disease. Eur J Pediatr Surg 15:153–158. https://doi.org/10.1055/s-2005-837612
Coyle D, Kelly DA, O’Donnell AM, Gillick J, Puri P (2016) Use of anoctamin 1 (ANO1) to evaluate interstitial cells of Cajal in Hirschsprung’s disease. Pediatr Surg Int 32:125–133. https://doi.org/10.1007/s00383-015-3822-9
Meier-Ruge W (1971) Casuistic of colon disorder with symptoms of Hirschsprung’s disease. Verh Dtsch Ges Pathol 55:506–510
Puri P, Lake BD, Nixon HH, Mishalany H, Claireaux AE (1977) Neuronal colonic dysplasia: an unusual association of Hirschsprung’s disease. J Pediatr Surg 12:681–685. https://doi.org/10.1016/0022-3468(77)90393-1
Fadda B, Maier WA, Meier-Ruge W, Schärli A, Daum R (1983) Neuronal intestinal dysplasia. Critical 10-years’ analysis of clinical and biopsy diagnosis. Z Kinderchir 38:305–311. https://doi.org/10.1055/s-2008-1059994
Bruder E, Meier-Ruge WA (2007) Intestinal neuronal dysplasia type B: how do we understand it today? Pathologe 28:137–142. https://doi.org/10.1007/s00292-007-0894-x
Montedonico S, Cáceres P, Muñoz N, Yáñez H, Ramírez R, Fadda B (2011) Histochemical staining for intestinal dysganglionosis: over 30 years experience with more than 1,500 biopsies. Pediatr Surg Int 27:479–486. https://doi.org/10.1007/s00383-010-2849-1
Schäppi MG, Staiano A, Milla PJ, Smith VV, Dias JA, Heuschkel R, Husby S, Mearin ML, Papadopoulou A, Ruemmele FM, Vandenplas Y, Koletzko S (2013) A practical guide for the diagnosis of primary enteric nervous system disorders. J Pediatr Gastroenterol Nutr 57:677–686. https://doi.org/10.1097/MPG.0b013e3182a8bb50
Martucciello G, Pini Prato A, Puri P, Holschneider AM, Meier-Ruge W, Jasonni V, Tovar JA, Grosfeld JL (2005) Controversies concerning diagnostic guidelines for anomalies of the enteric nervous system: a report from the fourth International Symposium on Hirschsprung’s disease and related neurocristopathies. J Pediatr Surg 40:1527–1531. https://doi.org/10.1016/j.jpedsurg.2005.07.053
Rolle U, Piaseczna-Piotrowska A, Puri P (2007) Interstitial cells of Cajal in the normal gut and in intestinal motility disorders of childhood. Pediatr Surg Int 23:1139–1152. https://doi.org/10.1007/s00383-007-2022-7
Taguchi T, Masumoto K, Ieiri S, Nakatsuji T, Akiyoshi J (2006) New classification of hypoganglionosis: congenital and acquired hypoganglionosis. J Pediatr Surg 41:2046–2051. https://doi.org/10.1016/j.jpedsurg.2006.08.004
Rolle U, Yoneda A, Solari V, Nemeth L, Puri P (2002) Abnormalities of C-Kit-positive cellular network in isolated hypoganglionosis. J Pediatr Surg 37:709–714. https://doi.org/10.1053/jpsu.2002.32259
Friedmacher F, Puri P (2012) Comparison of posterior internal anal sphincter myectomy and intrasphincteric botulinum toxin injection for treatment of internal anal sphincter achalasia: a meta-analysis. Pediatr Surg Int 28:765–771. https://doi.org/10.1007/s00383-012-3123-5
Doodnath R, Puri P (2009) Internal anal sphincter achalasia. Semin Pediatr Surg 18:246–248. https://doi.org/10.1053/j.sempedsurg.2009.07.006
Gosemann JH, Puri P (2011) Megacystis microcolon intestinal hypoperistalsis syndrome: systematic review of outcome. Pediatr Surg Int 27:1041–1046. https://doi.org/10.1007/s00383-011-2954-9
Berdon WE, Baker DH, Blanc WA, Gay B, Santulli TV, Donovan C (1976) Megacystis-microcolon-intestinal hypoperistalsis syndrome: a new cause of intestinal obstruction in the newborn. Report of radiologic findings in five newborn girls. AJR Am J Roentgenol 126:957–964. https://doi.org/10.2214/ajr.126.5.957
Rolle U, O’Briain S, Pearl RH, Puri P (2002) Megacystis-microcolon-intestinal hypoperistalsis syndrome: evidence of intestinal myopathy. Pediatr Surg Int 18:2–5. https://doi.org/10.1007/s003830200001
Puri P, Lake BD, Gorman F, O’Donnell B, Nixon HH (1983) Megacystis-microcolon-intestinal hypoperistalsis syndrome: a visceral myopathy. J Pediatr Surg 18:64–69. https://doi.org/10.1016/s0022-3468(83)80275-9
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
FF and UR wrote the manuscript. UR provided the images. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Friedmacher, F., Rolle, U. Interstitial cells of Cajal: clinical relevance in pediatric gastrointestinal motility disorders. Pediatr Surg Int 39, 188 (2023). https://doi.org/10.1007/s00383-023-05467-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-023-05467-1