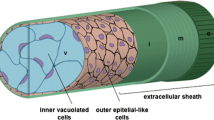
Abstract
Introduction
The caudal cell mass (CCM) is an aggregate of undifferentiated pluripotent cells and the main player in secondary neurulation. Previous studies have elucidated the dynamic fate of the multipotent cell lineages, with a recent interest in the neuromesodermal progenitors. However, a transcriptomic analysis of the CCM during secondary neurulation has not been performed yet.
Methods
We analyzed RNA sequencing data of CCM samples at three different developmental stages of chicken embryos; HH16 (largest CCM phase), HH20 (secondary neural tube formation phase), and HH28 (degeneration phase).
Results
The transcriptomic profiles were clearly distinguishable according to developmental stage, and HH20 was shown to have not only intermediate, but also unique properties in secondary neurulation. A total of 10,666 differentially expressed genes, including FGF18 and GDF11, were identified and enriched in several gene ontologies related to embryogenesis or organogenesis. We also found that genes encoding transcription factors, such as TWIST2, IRX4, HOXB4, HOXD13, LIN28A, CDX4, and Brachyury, were among the top-ranked differentially expressed genes.
Conclusion
Through transcriptomic profiling, we provided a picture of the developmental process of the CCM. We identified several key molecules or pathways involved in secondary neurulation and the pathogenesis of related diseases.






Similar content being viewed by others
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Griffith CM, Wiley MJ (1990) Distribution of cell surface glycoconjugates during secondary neurulation in the chick embryo. Anat Rec 226:81–90
Gajović S, Kostović-Knezević L, Svajger A (1989) Origin of the notochord in the rat embryo tail. Anat Embryol (Berl) 179:305–310
Švajger A, Kostović-Knežević L, Bradamante Ž, Wrischer M (1985) Tall gut formation in the rat embryo. Wilehm Roux Arch Dev Biol 194:429–432
Henrique D, Abranches E, Verrier L, Storey KG (2015) Neuromesodermal progenitors and the making of the spinal cord. Development 142:2864–2875
Garriock RJ, Chalamalasetty RB, Kennedy MW, Canizales LC, Lewandoski M, Yamaguchi TP (2015) Lineage tracing of neuromesodermal progenitors reveals novel Wnt-dependent roles in trunk progenitor cell maintenance and differentiation. Development 142:1628–1638
Shaker MR, Lee JH, Kim KH, Ban S, Kim VJ, Kim JY, Lee JY, Sun W (2021) Spatiotemporal contribution of neuromesodermal progenitor-derived neural cells in the elongation of developing mouse spinal cord. Life Sci 282:119393
Yang HJ, Wang KC, Chi JG, Lee MS, Lee YJ, Kim SK, Cho BK (2003) Neural differentiation of caudal cell mass (secondary neurulation) in chick embryos: Hamburger and Hamilton Stages 16–45. Brain Res Dev Brain Res 142:31–36
Schoenwolf GC (1981) Morphogenetic processes involved in the remodeling of the tail region of the chick embryo. Anat Embryol (Berl) 162:183–197
Choi S, Kim KH, Kim SK, Wang KC, Lee JY (2021) Three-dimensional visualization of secondary neurulation in chick embryos using microCT. Dev Dyn
Lee JY, Lee ES, Kim SP, Lee MS, Phi JH, Kim SK, Hwang YI, Wang KC (2017) Neurosphere formation potential resides not in the caudal cell mass, but in the secondary neural tube. Int J Dev Biol 61:545–550
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21
Anders S, Pyl PT, Huber W (2015) HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550
Anders S, Reyes A, Huber W (2012) Detecting differential usage of exons from RNA-seq data. Genome Res 22:2008–2017
da Huang W, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13
Ernst J, Bar-Joseph Z (2006) STEM: a tool for the analysis of short time series gene expression data. BMC Bioinformatics 7:191
Chae YK, Chang S, Ko T, Anker J, Agte S, Iams W, Choi WM, Lee K, Cruz M (2018) Epithelial-mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci Rep 8:2918
Tan TZ, Miow QH, Miki Y, Noda T, Mori S, Huang RY, Thiery JP (2014) Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol Med 6:1279–1293
Hu H, Miao YR, Jia LH, Yu QY, Zhang Q, Guo AY (2019) AnimalTFDB 3.0: a comprehensive resource for annotation and prediction of animal transcription factors. Nucleic Acids Res 47:D33–D38
Agopian AJ, Bhalla AD, Boerwinkle E, Finnell RH, Grove ML, Hixson JE, Shimmin LC, Sewda A, Stuart C, Zhong Y, Zhu H, Mitchell LE (2013) Exon sequencing of PAX3 and T (brachyury) in cases with spina bifida. Birth Defects Res A Clin Mol Teratol 97:597–601
Aguirre CE, Murgan S, Carrasco AE, López SL (2013) An intact brachyury function is necessary to prevent spurious axial development in Xenopus laevis. PLoS ONE 8:e54777
Fontanella F, van Maarle MC, Robles de Medina P, Oostra RJ, van Rijn RR, Pajkrt E, Bilardo CM (2016) Prenatal evidence of persistent notochord and absent sacrum caused by a mutation in the T (Brachyury) Gene. Case Rep Obstet Gynecol 2016:7625341
Zhang Q, Liu W, Zhang HM, Xie GY, Miao YR, Xia M, Guo AY (2020) hTFtarget: a comprehensive database for regulations of human transcription factors and their targets. Genomics Proteomics Bioinformatics 18:120–128
Ornitz DM, Itoh N (2001) Fibroblast growth factors. Genome Biol 2: Reviews3005
Takata N, Sakakura E, Eiraku M, Kasukawa T, Sasai Y (2017) Self-patterning of rostral-caudal neuroectoderm requires dual role of Fgf signaling for localized Wnt antagonism. Nat Commun 8:1339
Joshi P, Darr AJ, Skromne I (2019) CDX4 regulates the progression of neural maturation in the spinal cord. Dev Biol 449:132–142
Karabagli H, Karabagli P, Ladher RK, Schoenwolf GC (2002) Comparison of the expression patterns of several fibroblast growth factors during chick gastrulation and neurulation. Anat Embryol (Berl) 205:365–370
Smith CA, Sinclair AH (2001) Sex determination in the chicken embryo. J Exp Zool 290:691–699
Szumska D, Pieles G, Essalmani R, Bilski M, Mesnard D, Kaur K, Franklyn A, El Omari K, Jefferis J, Bentham J, Taylor JM, Schneider JE, Arnold SJ, Johnson P, Tymowska-Lalanne Z, Stammers D, Clarke K, Neubauer S, Morris A, Brown SD, Shaw-Smith C, Cama A, Capra V, Ragoussis J, Constam D, Seidah NG, Prat A, Bhattacharya S (2008) VACTERL/caudal regression/Currarino syndrome-like malformations in mice with mutation in the proprotein convertase Pcsk5. Genes Dev 22:1465–1477
Tsuda T, Iwai N, Deguchi E, Kimura O, Ono S, Furukawa T, Sasaki Y, Fumino S, Kubota Y (2011) PCSK5 and GDF11 expression in the hindgut region of mouse embryos with anorectal malformations. Eur J Pediatr Surg 21:238–241
Krumlauf R (1994) Hox genes in vertebrate development. Cell 78:191–201
Hostikka SL, Gong J, Carpenter EM (2009) Axial and appendicular skeletal transformations, ligament alterations, and motor neuron loss in Hoxc10 mutants. Int J Biol Sci 5:397–410
Mandeville I, Aubin J, LeBlanc M, Lalancette-Hébert M, Janelle MF, Tremblay GM, Jeannotte L (2006) Impact of the loss of Hoxa5 function on lung alveogenesis. Am J Pathol 169:1312–1327
de Santa BP, Roberts DJ (2002) Tail gut endoderm and gut/genitourinary/tail development: a new tissue-specific role for Hoxa13. Development 129:551–561
Aires R, de Lemos L, Nóvoa A, Jurberg AD, Mascrez B, Duboule D, Mallo M (2019) Tail bud progenitor activity relies on a network comprising Gdf11, Lin28, and Hox13 genes. Dev Cell 48:383-395.e388
Barak H, Preger-Ben Noon E, Reshef R (2012) Comparative spatiotemporal analysis of Hox gene expression in early stages of intermediate mesoderm formation. Dev Dyn 241:1637–1649
Gould A, Itasaki N, Krumlauf R (1998) Initiation of rhombomeric Hoxb4 expression requires induction by somites and a retinoid pathway. Neuron 21:39–51
Sharpe J, Nonchev S, Gould A, Whiting J, Krumlauf R (1998) Selectivity, sharing and competitive interactions in the regulation of Hoxb genes. EMBO J 17:1788–1798
Takemoto T, Uchikawa M, Yoshida M, Bell DM, Lovell-Badge R, Papaioannou VE, Kondoh H (2011) Tbx6-dependent Sox2 regulation determines neural or mesodermal fate in axial stem cells. Nature 470:394–398
Yang N, Wu N, Zhang L, Zhao Y, Liu J, Liang X, Ren X, Li W, Chen W, Dong S, Zhao S, Lin J, Xiang H, Xue H, Chen L, Sun H, Zhang J, Shi J, Zhang S, Lu D, Wu X, Jin L, Ding J, Qiu G, Wu Z, Lupski JR, Zhang F (2018) TBX6 compound inheritance leads to congenital vertebral malformations in humans and mice. Hum Mol Genet 28:539–547
Gentsch George E, Owens Nick DL, Martin Stephen R, Piccinelli P, Faial T, Trotter Matthew WB, Gilchrist Michael J, Smith James C (2013) In vivo T-Box transcription factor profiling reveals joint regulation of embryonic neuromesodermal bipotency. Cell Rep 4:1185–1196
Pennimpede T, Proske J, König A, Vidigal JA, Morkel M, Bramsen JB, Herrmann BG, Wittler L (2012) In vivo knockdown of Brachyury results in skeletal defects and urorectal malformations resembling caudal regression syndrome. Dev Biol 372:55–67
Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, Faux CH, Greene ND, Copp AJ (2007) Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development 134:789–799
Cai C, Shi O (2014) Genetic evidence in planar cell polarity signaling pathway in human neural tube defects. Front Med 8:68–78
Copp AJ, Stanier P, Greene ND (2013) Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol 12:799–810
Wen S, Zhu H, Lu W, Mitchell LE, Shaw GM, Lammer EJ, Finnell RH (2010) Planar cell polarity pathway genes and risk for spina bifida. Am J Med Genet A 152A:299–304
Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, Dedhar S, Derynck R, Ford HL, Fuxe J, García de Herreros A, Goodall GJ, Hadjantonakis A-K, Huang RYJ, Kalcheim C, Kalluri R, Kang Y, Khew-Goodall Y, Levine H, Liu J, Longmore GD, Mani SA, Massagué J, Mayor R, McClay D, Mostov KE, Newgreen DF, Nieto MA, Puisieux A, Runyan R, Savagner P, Stanger B, Stemmler MP, Takahashi Y, Takeichi M, Theveneau E, Thiery JP, Thompson EW, Weinberg RA, Williams ED, Xing J, Zhou BP, Sheng G, On behalf of the EMTIA (2020) Guidelines and definitions for research on epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 21:341–352
Gonzalez-Gobartt E, Allio G, Bénazéraf B, Martí E (2021) In vivo analysis of the mesenchymal-to-epithelial transition during chick secondary neurulation. In: Campbell K, Theveneau E (eds) The epithelial-to mesenchymal transition: methods and protocols. Springer, US, New York, NY, pp 183–197
Kim HY, Jackson TR, Davidson LA (2017) On the role of mechanics in driving mesenchymal-to-epithelial transitions. Semin Cell Dev Biol 67:113–122
Funding
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. 2021M3E5D9021884). This study was also supported by grant No. 04–20190430 from the Seoul National University Hospital Research Fund.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Eun Sun Lee, Veronica Jihyun Kim, Saet Pyoul Kim, and Saeli Ban. Data analysis was conducted by Seungbok Lee. The first draft of the manuscript was written by Seungbok Lee and Kyung Hyun Kim. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, S., Kim, K.H., Lee, E.S. et al. The transcriptomic landscape of caudal cell mass in different developmental stages of the chick embryo. Childs Nerv Syst 38, 2101–2111 (2022). https://doi.org/10.1007/s00381-022-05675-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-022-05675-5