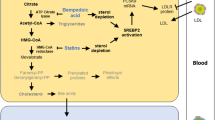
Abstract
This study examined the effects of pemafibrate, a selective peroxisome proliferator-activated receptor α agonist, on the serum biochemical parameters of male patients with coronary artery disease and metabolic syndrome (MetS). This was a post hoc analysis of a randomized, crossover study that treated hypertriglyceridemia with pemafibrate or bezafibrate for 24 weeks, followed by a crossover of another 24 weeks. Of the 60 patients enrolled in the study, 55 were male. Forty-one of 55 male patients were found to have MetS. In this sub-analysis, male patients with MetS (MetS group, n = 41) and those without MetS (non-MetS group, n = 14) were compared. The primary endpoint was a change in fasting serum triglyceride (TG) levels during pemafibrate therapy, and the secondary endpoints were changes in insulin resistance-related markers and liver function parameters. Serum TG levels significantly decreased (MetS group, from 266.6 to 148.0 mg/dL, p < 0.001; non-MetS group, from 203.9 to 97.6 mg/dL, p < 0.001); however, a percent change (%Change) was not significantly different between the groups (− 44.1% vs. − 51.6%, p = 0.084). Serum insulin levels and homeostasis model assessment of insulin resistance significantly decreased in the MetS group but not in the non-MetS group. %Change in liver enzyme levels was markedly decreased in the MetS group compared with that in the non-MetS group (alanine aminotransferase, − 25.1% vs. − 11.3%, p = 0.027; gamma-glutamyl transferase, − 45.8% vs. − 36.2%, p = 0.020). In conclusion, pemafibrate can effectively decrease TG levels in patients with MetS, and it may be a more efficient drug for improving insulin resistance and liver function in such patients.

Similar content being viewed by others
Data availability
The data that support the findings of this study cannot be shared publicly to protect the privacy of the study patients. The data will be shared by the corresponding author upon reasonable request.
References
Tall AR, Thomas DG, Conzales-Cabodevilla AG, Goldberg IJ (2022) Addressing dyslipidemic risk beyond LDL-cholesterol. J Clin Invest 132:e148559
Budoff M (2016) Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol 118:138–145
Matsuzawa Y (2005) Metabolic syndrome: definition and diagnosis criteria in Japan. J Jpn Soc Intern Med 94:188–203
Reaven GM (1988) Banting lecture 1988: role of insulin resistance in human disease. Diabetes 37:1595–1607
Yun JE, Won S, Sung J, Jee SH (2012) Impact of metabolic syndrome independent of insulin resistance on the development of cardiovascular disease. Circ J 76:2443–2448
Yamashita S, Masuda D, Mstsuzaka Y (2020) Pemafibrate, a new selective PPARα modulator: drug concept and its clinical applications for dyslipidemia and metabolic diseases. Curr Atheroscler Rep 22:5
Arai H, Yamashita S, Yokote K, Araki E, Suganami H, Ishibashi S, and K-877 Study Group (2018) Efficacy and safety of pemafibrate versus fenofibrate in patients with high triglyceride and low HDL cholesterol levels: a multicenter, placebo-controlled, double-blind, randomized trial. J Atheroscler Thromb 25:521–538
Nakamura A, Kagaya Y, Saito H, Kanazawa M, Sato K, Miura M, Kondo M, Endo H (2023) Efficacy and safety of pemafibrate versus bezafibrate to treat patients with hypertriglyceridemia: a randomized crossover study. J Atheroscler Thromb 30:443–454
Ishibashi S, Arai H, Yokote K, Araki E, Suganami H, Yamashita S; K-877 Study Group (2018) Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor α modulator, in patients with dyslipidemia: results from a 24-week, randomized, double blind, active-controlled, phase 3 trial. J Clin Lipidol 12:173–184
Araki E, Yamashita S, Arai H, Yokote K, Satoh J, Inoguchi T, Nakamura J, Maegawa H, Yoshioka N, Tanizawa Y, Watada H, Suganami H, Ishibashi S (2018) Effects of pemafibrate, a novel selective PPARα modulator, on lipid and glucose metabolism in patients with type 2 diabetes and hypertriglyceridemia: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 41:538–546
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM (1995) The nuclear receptor superfamily: the second decade. Cell 83:835–839
Issemann I, Green S (1990) Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347:645–650
Lefebvre P, Chinetti G, Fruchart JC, Staels B (2006) Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. J Clin Invest 116:571–580
Hu Y, Chen Y, Ding L, He X, Takahashi Y, Gao Y, Shen W, Cheng R, Chen Q, Qi X, Boulton ME, Ma JX (2013) Pathogenic role of diabetes-induced PPAR-α down-regulation in microvascular dysfunction. Proc Natl Acad Sci U S A 110:15401–15406
Bayoumy NMK, EI-Shabrawi MM, Younes S, Omar HH (2018) PPARα receptor expression in patients with metabolic syndrome. Diabetes Metab Syndr 12:711–714
Shao Y, Chen J, Dong LJ, He X, Cheng R, Zhou K, Liu J, Qiu F, Li XR, Ma JX (2019) A protective effect of PPARα in endothelial progenitor cells through regulating metabolism. Diabetes 68:2131–2142
Francque S, Verrijken A, Caron S, Prawitt J, Paumelle R, Derudas B, Lefebvre P, Taskinen MR, Hul WV, Mertens I, Hubens G, Marck EV, Michielsen P, Gaal LV, Staels B (2015) PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J Hepatol 63:164–173
Ju J, Huang Q, Sun J, Jin Y, Ma W, Song X, Sun H, Wang W (2018) Correlation between PPAR-α methylation level in peripheral blood and atherosclerosis of NAFLD patients with DM. Exp Ther Med 15:1474–1478
Yamagishi K, Iso H (2017) The criteria for metabolic syndrome and the national health screening and education system in Japan. Epidemiol Health 39:e2017003
Rallidis LS, Katsimardos A, Kosmas N, Rallidi T, Zapantiotis D, Varounis C, Kountouri A (2022) Differential prognostic value of resistin for cardiac death in patients with coronary artery disease according to the presence of metabolic syndrome. Heart Vessels 37:713–719
American Diabetes Association (2010) Standards of medical care in diabetes. Diabetes Care 33:S11–S61
Zhou J, Yuan Y, Li X (2023) The association between C-peptide and atrial cardiomyopathy in nondiabetic adults: results from NHANES III. Heart Vessels 38:1049–1055
Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV, American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons (2012) 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task force on practice guidelines, and the American College of Physicians, American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol 60:e44–e164
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A (2009) Collaborators developing the japanese equation for estimated GFR: revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Soejima H, Ogawa H, Morimoto T, Okada S, Matsumoto C, Nakayama M, Masuda I, Jinnouchi H, Waki M, Saito Y, the JPAD Trial Investigators (2022) Kidney function deterioration is dependent on blood pressure levels: 11.2 year follow-up in diabetic patients. Heart Vessels 37:1873–1881
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S (2013) Collaborators developing the Japanese equation for estimated GFR: GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis 61:197–203
Haffner SM, Miettinen H, Stern MP (1997) The homeostasis model in the San Antonio heart study. Diabetes Care 20:1087–1092
Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR (1992) Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 340:925–929
Sekiya M, Suzuki J, Watanabe K, Funada J, Otani T, Akutsu H (2001) Beneficial effect of troglitazone, an insulin-sensitizing antidiabetic agent, on coronary circulation in patients with non-insulin-dependent diabetes mellitus. Jpn Circ J 65:487–490
DREAM (Diabetes Reduction Assessment with ramipril and rosiglitazone Medication) troal Investigators, Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR (2006) Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomized controlled trial. Lancet 368:1096–1105
Tenenbaum A, Motro M, Fisman EZ, Schwammenthal E, Adler Y, Goldenberg I, Leor J, Boyko V, Mandelzweig L, Behar S (2004) Peroxisome proliferator-activated receptor ligand bezafibrate for prevention of type 2 diabetes mellitus in patients with coronary artery disease. Circulation 109:2197–2202
Vega GL, Cater NB, Hadizadeh DR 3rd, Meguro S, Grundy SM (2003) Free fatty acid metabolism during fenofibrate treatment of the metabolic syndrome. Clin Pharmacol Ther 74:236–244
Bajaj M, Suraamornkul S, Hardies LJ, Glass L, Musi N, DeFronzo RA (2007) Effects of peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ agonists on glucose and lipid metabolism in patients with type 2 diabetes mellitus. Diabetologia 50:1723–1731
Black RN, Ennis CN, Young IS, Hunter SJ, Atkinson AB, Bell PM (2014) The peroxisome proliferator-activated receptor alpha agonist fenofibrate has no effect on insulin sensitivity compared to atorvastatin in type 2 diabetes mellitus; a randomized, double-blind controlled trial. J Diabetes Complicat 28:323–327
Idzior-Walus B, Sieradzki J, Rostworowski W, Zdzienicka A, Kawalec E, Wόjcik J, Żarnecki A, Blane G (2000) Effects of comicronised fenofibrate on lipid and insulin sensitivity in patients with polymetabolic syndrome X. Eur J Clin Invest 30:871–878
Ishibashi S, Yamashita S, Arai H, Araki E, Yokote K, Suganami H, Fruchart JC, Kodama T, K-877-04 Study Group (2016) Effects of K-877, a novel selective PPARalpha modulator (SPPARMalpha), in dyslipidaemic patients: a randomized, double blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis 249:36–43
Yokote K, Yamashita S, Arai H, Araki E, Matsushita M, Nojima T, Suganami H, Ishibashi S (2021) Effects of pemafibrate on glucose metabolism markers and liver function tests in patients of six phase 2 and phase 3 randomized double-blind placebo-controlled clinical trials. Cardiovasc Diabetol 20:96
Dong T, Lyu J, Imachi H, Kobayashi T, Fukunaga K, Sato S, Ibata T, Yoshimoto T, Yonezaki K, Iwama H, Zhang G, Murao K (2018) Selective peroxisome proliferator-activated receptor-α modulator K-877 regulates the expression of ATP-binding cassette transporter A1 in pancreatic beta cells. Eur J Pharmacol 838:78–84
Matsuba I, Matsuba R, Ishibashi S, Yamashita S, Arai H, Yokote K, Suganami H, Araki E (2018) Effects of a novel selective peroxisome proliferator-activated receptor-α modulator, pemafibrate, on hepatic and peripheral glucose uptake in patients with hypertriglyceridemia and insulin resistance. J Diabetes Investig 9:1323–1332
Tripathy D, Almgren P, Tuomi T, Groop L (2004) Contribution of insulin-stimulated glucose measures of insulin sensitivity. Diabetes Care 27:2204–2210
Hoffman RP (2008) Indices of insulin action calculated from fasting glucose and insulin reflect hepatic, not peripheral, insulin sensitivity in African–American and Caucasian adolescents. Pediatr Diabetes 9:57–61
Shinozaki S, Tahara T, Lefor AK, Ogura M (2020) Pemafibrate decreases markers of hepatic inflammation in patients with non-alcoholic fatty liver disease. Clin Exp Hepatol 6:270–274
Nakajima A, Eguchi Y, Yoneda M, Imajo K, Tamaki N, Suganami H, Nojima T, Tanigawa R, Iizuka M, Iida Y, Loomba R (2021) Randomised clinical trial: pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator (SPPARMalpha), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 54:1263–1277
Son C, Hosoda K, Matsuda J, Fujikura J, Yonemitsu S, Iwakura H, Masuzaki H, Ogawa Y, Hayashi T, Itoh H, Nishimura H, Inoue G, Yoshimasa Y, Yamori Y, Nakao K (2001) Up-regulation of uncoupling protein 3 gene expression by fatty acids and agonists for PPARs in L6 myotubes. Endocrinology 142:4189–4194
Kusminski CM, Scherer PE (2009) The road from discovery to clinic: adiponectin as a biomarker of metabolic status. Clin Pharmacol Ther 86:592–595
Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y (2001) PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50:2094–2099
Bruder-Nascimento T, Faulkner JL, Haigh S, Kennard S, Antonova G, Patel VS, Fulton DJR, Chen W, Belin de Chantemèle EJ (2019) Leptin restores endothelial function via endothelial PPARγ-Nox1-mediated mechanisms in a mouse model of congenital generalized lipodystrophy. Hypertension 74:1399–1408
Suzuki A, Okamoto S, Lee S, Saito K, Shiuchi T, Minokoshi Y (2007) Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol Cell Biol 27:4317–4327
Chitturi S, Farrell G, Frost L, Kriketos A, Lin R, Liddle C, Samarasinghe D, George J (2002) Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology 36:403–409
Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS (2013) Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 369:932–943
Acknowledgements
We would like to thank Enago (www.enago.jp) for their assistance with English language editing.
Funding
This study received no grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AN, YK, KS, and HE conducted the study and performed clinical data analysis. AN, HS, MK, MM, and MK performed clinical work and data analysis. AN, HS, and HE designed the study. AN wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The present study was conducted in accordance with the principles of the Declaration of Helsinki, and the protocol was approved by the local ethics committee (Approval no. 540).
Informed consent
Written informed consent was obtained from all patients before the initiation of the PEBE study, and also informed consent for this study was obtained in the form of opt-out.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakamura, A., Kagaya, Y., Saito, H. et al. Impact of pemafibrate on lipid profile and insulin resistance in hypertriglyceridemic patients with coronary artery disease and metabolic syndrome. Heart Vessels 39, 486–495 (2024). https://doi.org/10.1007/s00380-024-02363-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-024-02363-z