Abstract
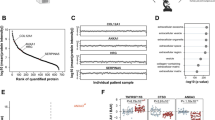
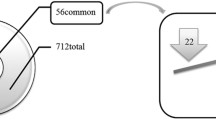
Calcific aortic valve disease (CAVD) is the most common heart valve disease requiring intervention. Most research on CAVD has focused on inflammation, ossification, and cellular phenotype transformation. To gain a broader picture into the wide range of cellular and molecular mechanisms involved in this disease, we compared the total protein profiles between calcified and non-calcified areas from 5 human valves resected during surgery. The 1413 positively identified proteins were filtered down to 248 proteins present in both calcified and non-calcified segments of at least 3 of the 5 valves, which were then analyzed using Ingenuity Pathway Analysis. Concurrently, the top 40 differentially abundant proteins were grouped according to their biological functions and shown in interactive networks. Finally, the abundance of selected osteogenic proteins (osteopontin, osteonectin, osteocalcin, osteoprotegerin, and RANK) was quantified using ELISA and/or immunohistochemistry. The top pathways identified were complement system, acute phase response signaling, metabolism, LXR/RXR and FXR/RXR activation, actin cytoskeleton, mineral binding, nucleic acid interaction, structural extracellular matrix (ECM), and angiogenesis. There was a greater abundance of osteopontin, osteonectin, osteocalcin, osteoprotegerin, and RANK in the calcified regions than the non-calcified ones. The osteogenic proteins also formed key connections between the biological signaling pathways in the network model. In conclusion, this proteomic analysis demonstrated the involvement of multiple signaling pathways in CAVD. The interconnectedness of these pathways provides new insights for the treatment of this disease.






Similar content being viewed by others
Abbreviations
- APP:
-
Amyloid-β A4 protein
- a-SMA:
-
A-Smooth muscle actin
- FXR/RXR:
-
Farnesoid X receptor/retinoid X receptor
- LPC:
-
Lysophosphatidylcholine
- LXR/RXR:
-
Liver X receptor/retinoid X receptor
- MAC:
-
Membrane attack complex
- MYC:
-
Myc proto-oncogene protein
- OSTCN*:
-
Osteocalcin
- OSTP*:
-
Osteopontin
- TNR11*:
-
RANK/CD265
- SNP:
-
Single nucleotide polymorphisms
- SPARC*:
-
Osteonectin
- TGFB1:
-
Transformation growth factor-β1
- TR11B*:
-
Osteoprotegerin
References
Demer LL, Tintut Y (2008) Vascular calcification: pathobiology of a multifaceted disease. Circulation 117:2938–2948
Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM (2011) Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the national heart and lung and blood institute aortic stenosis working group. executive summary: calcific aortic valve disease-2011 update. Circulation 124:1783–1791
Mohler ER 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS (2001) Bone formation and inflammation in cardiac valves. Circulation 103:1522–1528
New SE, Aikawa E (2011) Cardiovascular calcification: an inflammatory disease. Circulation J 75:1305–1313
Martin-Rojas T, Gil-Dones F, Lopez-Almodovar LF, Padial LR, Vivanco F, Barderas MG (2012) Proteomic profile of human aortic stenosis: insights into the degenerative process. J Proteome Res 11:1537–1550
Hinton RB Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE (2006) Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res 98:1431–1438
Martin-Rojas T, Mourino-Alvarez L, Alonso-Orgaz S, Rosello-Lleti E, Calvo E, Lopez-Almodovar LF, Rivera M, Padial LR, Lopez JA, de la Cuesta F, Barderas MG (2015) iTRAQ proteomic analysis of extracellular matrix remodeling in aortic valve disease. Sci Rep 5:17290
Stephens EH, Saltarrelli JG, Baggett LS, Nandi I, Kuo JJ, Davis AR, Olmsted-Davis EA, Reardon MJ, Morrisett JD, Grande-Allen KJ (2011) Differential proteoglycan and hyaluronan distribution in calcified aortic valves. Cardiovasc Pathol 20:334–342
Wirrig EE, Hinton RB, Yutzey KE (2011) Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J Mol Cell Cardiol 50:561–569
Schlotter F, Halu A, Goto S, Blaser MC, Body SC, Lee LH, Higashi H, DeLaughter DM, Hutcheson JD, Vyas P, Pham T, Rogers MA, Sharma A, Seidman CE, Loscalzo J, Seidman JG, Aikawa M, Singh SA, Aikawa E (2018) Spatiotemporal multi-omics mapping generates a molecular atlas of the aortic valve and reveals networks driving disease. Circulation 138:377–393
Di Vito A, Donato A, Presta I, Mancuso T, Brunetti FS, Mastroroberto P, Amorosi A, Malara N, Donato G (2021) Extracellular matrix in calcific aortic valve disease: architecture, dynamic and perspectives. Int J Mol Sci 22:913
Gomez-Stallons MV, Tretter JT, Hassel K, Gonzalez-Ramos O, Amofa D, Ollberding NJ, Mazur W, Choo JK, Smith JM, Kereiakes DJ, Yutzey KE (2019) Calcification and extracellular matrix dysregulation in human postmortem and surgical aortic valves. Heart 105:1616–1621
Hutson HN, Marohl T, Anderson M, Eliceiri K, Campagnola P, Masters KS (2016) Calcific aortic valve disease is associated with layer-specific alterations in collagen architecture. PLoS ONE 11:e0163858
Chen JH, Simmons CA (2011) Cell-matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circ Res 108:1510–1524
Bini A, Mann KG, Kudryk BJ, Schoen FJ (1999) Noncollagenous bone matrix proteins, calcification, and thrombosis in carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol 19:1852–1861
Shevchenko A, Loboda A, Ens W, Schraven B, Standing KG, Shevchenko A (2001) Archived polyacrylamide gels as a resource for proteome characterization by mass spectrometry. Electrophoresis 22:1194–1203
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645
Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, Jensen LJ, von Mering C (2017) The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res 45:D362–D368
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Freeman RV, Otto CM (2005) Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 111:3316–3326
Wiltz DC, Han RI, Wilson RL, Kumar A, Morrisett JD, Grande-Allen KJ (2014) Differential aortic and mitral valve interstitial cell mineralization and the induction of mineralization by lysophosphatidylcholine in vitro. Cardiovasc Eng Technol 5:371–383
White MP, Theodoris CV, Liu L, Collins WJ, Blue KW, Lee JH, Meng X, Robbins RC, Ivey KN, Srivastava D (2015) NOTCH1 regulates matrix gla protein and calcification gene networks in human valve endothelium. J Mol Cell Cardiol 84:13–23
Calkin AC, Tontonoz P (2010) Liver x receptor signaling pathways and atherosclerosis. Arterioscler Thromb Vasc Biol 30:1513–1518
Miyazaki-Anzai S, Levi M, Kratzer A, Ting TC, Lewis LB, Miyazaki M (2010) Farnesoid X receptor activation prevents the development of vascular calcification in ApoE-/- mice with chronic kidney disease. Circ Res 106:1807–1817
Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R (2007) Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation 115:377–386
Balachandran K, Sucosky P, Yoganathan AP (2011) Hemodynamics and mechanobiology of aortic valve inflammation and calcification. Int J Inflam 2011:263870
Liu AC, Joag VR, Gotlieb AI (2007) The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol 171(5):1407–1418
Mohler ER 3rd, Chawla MK, Chang AW, Vyavahare N, Levy RJ, Graham L, Gannon FH (1999) Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis 8:254–260
Lencinas A, Chhun DC, Dan KP, Ross KD, Hoover EA, Antin PB, Runyan RB (2013) Olfactomedin-1 activity identifies a cell invasion checkpoint during epithelial-mesenchymal transition in the chick embryonic heart. Dis Model Mech 6:632–642
Wirrig EE, Snarr BS, Chintalapudi MR, O’Neal JL, Phelps AL, Barth JL, Fresco VM, Kern CB, Mjaatvedt CH, Toole BP, Hoffman S, Trusk TC, Argraves WS, Wessels A (2007) Cartilage link protein 1 (Crtl1), an extracellular matrix component playing an important role in heart development. Dev Biol 310:291–303
Dahal S, Huang P, Murray BT, Mahler GJ (2017) Endothelial to mesenchymal transformation is induced by altered extracellular matrix in aortic valve endothelial cells. J Biomed Mater Res A 105:2729–2741
Wirrig EE, Yutzey KE (2014) Conserved transcriptional regulatory mechanisms in aortic valve development and disease. Arterioscler Thromb Vasc Biol 34:737–741
Guauque-Olarte S, Messika-Zeitoun D, Droit A, Lamontagne M, Tremblay-Marchand J, Lavoie-Charland E, Gaudreault N, Arsenault BJ, Dube MP, Tardif JC, Body SC, Seidman JG, Boileau C, Mathieu P, Pibarot P, Bosse Y (2015) Calcium signaling pathway genes RUNX2 and CACNA1C are associated with calcific aortic valve disease. Circ Cardiovasc Genet 8:812–822
Ferguson JF, Matthews GJ, Townsend RR, Raj DS, Kanetsky PA, Budoff M, Fischer MJ, Rosas SE, Kanthety R, Rahman M, Master SR, Qasim A, Li M, Mehta NN, Shen H, Mitchell BD, O’Connell JR, Shuldiner AR, Ho WK, Young R, Rasheed A, Danesh J, He J, Kusek JW, Ojo AO, Flack J, Go AS, Gadegbeku CA, Wright JT Jr, Saleheen D, Feldman HI, Rader DJ, Foulkes AS, Reilly MP, Investigators CSP (2013) Candidate gene association study of coronary artery calcification in chronic kidney disease: findings from the CRIC study (Chronic Renal Insufficiency Cohort). J Am Coll Cardiol 62:789–798
Sun Y, Mauerhan DR, Honeycutt PR, Kneisl JS, Norton JH, Hanley EN Jr, Gruber HE (2010) Analysis of meniscal degeneration and meniscal gene expression. BMC Musculoskelet Disord 11:19
Colgan SP, Eltzschig HK, Eckle T, Thompson LF (2006) Physiological roles for ecto-5’-nucleotidase (CD73). Purinergic Signal 2:351–360
St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, Carlson-Donohoe H, Lederman RJ, Chen MY, Yang D, Siegenthaler MP, Arduino C, Mancini C, Freudenthal B, Stanescu HC, Zdebik AA, Chaganti RK, Nussbaum RL, Kleta R, Gahl WA, Boehm M (2011) NT5E mutations and arterial calcifications. N Engl J Med 364:432–442
Kuninger DT, Izumi T, Papaconstantinou J, Mitra S (2002) Human AP-endonuclease 1 and hnRNP-L interact with a nCaRE-like repressor element in the AP-endonuclease 1 promoter. Nucleic Acids Res 30:823–829
Finnson KW, Tam BY, Liu K, Marcoux A, Lepage P, Roy S, Bizet AA, Philip A (2006) Identification of CD109 as part of the TGF-beta receptor system in human keratinocytes. FASEB J 20:1525–1527
Hunter GK, O’Young J, Grohe B, Karttunen M, Goldberg HA (2010) The flexible polyelectrolyte hypothesis of protein-biomineral interaction. Langmuir 26:18639–18646
Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS (2001) Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 107:1055–1061
Speer MY, Chien YC, Quan M, Yang HY, Vali H, McKee MD, Giachelli CM (2005) Smooth muscle cells deficient in osteopontin have enhanced susceptibility to calcification in vitro. Cardiovasc Res 66:324–333
Porter PL, Sage EH, Lane TF, Funk SE, Gown AM (1995) Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem 43:791–800
Born AK, Lischer S, Maniura-Weber K (2012) Watching osteogenesis: life monitoring of osteogenic differentiation using an osteocalcin reporter. J Cell Biochem 113:313–321
Han RI, Wheeler TM, Lumsden AB, Reardon MJ, Lawrie GM, Grande-Allen KJ, Morrisett JD, Brunner G (2016) Morphometric analysis of calcification and fibrous layer thickness in carotid endarterectomy tissues. Comput Biol Med 70:210–219
Abedin M, Tintut Y, Demer LL (2004) Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 24:1161–1170
Masjedi S, Lei Y, Patel J, Ferdous Z (2017) Sex-related differences in matrix remodeling and early osteogenic markers in aortic valvular interstitial cells. Heart Vessels 32:217–228
Porras AM, McCoy CM, Masters KS (2017) Calcific aortic valve disease: a battle of the sexes. Circ Res 120:604–606
Acknowledgements
The authors thank Katie Brown for blinded IHC scoring. This study was supported in part by NIH grants T32 HL07812 and R21 HL104377.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Reardon serves as a consultant for Medtronic, Boston Scientific, Abbott Medical, and Gore Medical; all fees for such are to his department and there is no overlap between the consulting work and the research presented here.
Ethical standards
Research use of the surgically-resected tissues was approved by the Institutional Review Boards at Houston Methodist Hospital and Rice University and was in accordance with the ethical standards of the IRBs and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained and details that might disclose the identity of patients were removed from samples and the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In Abbreviations: *Whenever possible, the UniProt (UniProt KB) protein name is used for consistency.
Rights and permissions
About this article
Cite this article
Han, R.I., Hu, C.W., Loose, D.S. et al. Differential proteome profile, biological pathways, and network relationships of osteogenic proteins in calcified human aortic valves. Heart Vessels 37, 347–358 (2022). https://doi.org/10.1007/s00380-021-01975-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-021-01975-z