Abstract
The association between fragmented QRS (fQRS) and autonomic nervous dysfunction, and major adverse cardiovascular events (MACE) is not fully clear in patients with acute myocardial infarction (AMI). This study aimed to observe whether combined assessment with fQRS and cardiac autonomic nervous function could enhance the predicting efficacy on outcome in AMI patients. A total of 153 consecutive hospitalized AMI patients were included in this retrospective study. Patients were divided into non-fQRS (nfQRS) group and fQRS group according to 12-lead electrocardiogram, into sHRV [severely depressed heart rate variability (HRV): standard deviation of NN intervals (SDNN) < 100 ms and very low frequency (VLF) < 26.7 ms] group and nsHRV (non-severely depressed HRV) group according to 24 h Holter monitoring, and into non-MACE (nMACE) group and MACE group according to 12 months’ follow-up results. The incidence of sHRV was significantly higher in the fQRS group than in the nfQRS group (71.9 vs. 39.3%, p < 0.05). The incidences of MACE were 7.4, 22.2, 25.7 and 56.5%, respectively, in nsHRV + nfQRS group, nsHRV + fQRS group, sHRV + nfQRS group and sHRV + fQRS group (p < 0.05). Multivariable Cox regression analysis showed that patients in the sHRV + fQRS group had a sixfold higher risk of MACE compared to patients in the nsHRV + nfQRS group (HR = 6.228, 95% CI 1.849–20.984, p = 0.003). The predicting sensitivity and specificity on MACE were 81.4 and 58.2% by sHRV, 69.8 and 69.1% by fQRS in these AMI patients. The specificity (81.8%) was the highest with the combination of sHRV and fQRS. Adding sHRV and fQRS to clinical data offered incremental prognostic value. Present results indicate that fQRS is closely related to sHRV, suggesting significant impairment of sympathetic nerve function in AMI patients with fQRS. Combined assessment with fQRS and sHRV enhances the predicting efficacy on outcome in AMI patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myocardial infarction (AMI) is the main pathogeny of sudden cardiac death in patients with ischemic cardiovascular diseases. Clinical adverse cardiovascular events are not rare despite successful percutaneous coronary intervention (PCI) in AMI patients [1]. Therefore, risk stratification among AMI patients is of crucial importance to define high-risk patients and make individualized decision-making aiming to improve the outcome of AMI patients. Fragmented QRS (fQRS) on electrocardiogram (ECG) is a response to abnormal ventricular myoelectric activity after AMI [2]. The presence of fQRS might indicate severe myocardial insult in AMI patients [3]. Previous studies demonstrated that fQRS was related to higher major adverse cardiovascular events (MACE) in patients with AMI [4,5,6,7,8]. Cardiac autonomic nerve function could be non-invasively assessed by heart rate variability (HRV) [9]. Significantly reduced HRV was evidenced and related to worse outcome in AMI patients [10,11,12,13,14]. Till now, the association between fQRS and autonomic nervous dysfunction is not fully clear in patients with AMI. The present study observed the correlation between fQRS and cardiac autonomic nervous dysfunction, and explored the predicting efficacy of combined assessment with fQRS and sHRV on outcome in AMI patients.
Materials and methods
Study population
A total of 156 consecutive hospitalized AMI patients, who were hospitalized in our department from January 2017 to December 2018, were included in this retrospective study. Three patients were lost to follow-up and data from 153 patients were analyzed in this study. Patients were divided into non-fQRS group (nfQRS group, n = 89) and fQRS group (n = 64) according to the 12-lead ECG at admission, into sHRV [severely depressed HRV: standard deviation of NN intervals (SDNN) < 100 ms [15] and very low frequency (VLF) < 26.7 ms, n = 81] group, and nsHRV (non-severely depressed HRV, n = 72) group according to 24 h Holter monitoring. Patients were also divided into non-MACE (nMACE) group (n = 110) and MACE group (n = 43) according to the 12 months’ follow-up results. Patients with cardiomyopathy, congenital heart disease, organic heart valvular disease, electrolyte disturbance, bundle branch block, Wolf–Parkinson–White syndrome, atrial fibrillation, pacemaker implantation, malignant tumor and digitalis medication were excluded. This study protocol was approved by the ethical committees of Wuhan Fourth Hospital, Puai Hospital affiliated to Tongji Medical College, Huazhong University of Science and Technology and conducted in compliance with the ethical principles of the Declaration of Helsinki. 24 h Holter monitoring results were obtained in the clinical medical records in this study and the requirement to obtain informed consent was waived by the ethical committees of Wuhan Fourth Hospital, Puai Hospital affiliated to Tongji Medical College, Huazhong University of Science and Technology.
ECG criteria for fQRS
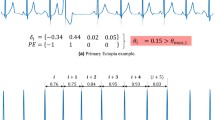
fQRS was first proposed by Das in 2006 [16]. The RSR pattern included the QRS interval (QRS duration < 120 ms) with or without the Q wave. It was defined by the presence of an additional R wave (R’) or notching in the nadir of the S wave, or the presence of R’ (fragmentation) in two contiguous leads, corresponding to a major coronary artery territory [16] (Fig. 1). The fQRS was recorded by 12-lead ECG (GE, Marquette, model Mac 800, filter range, 0.04–150 Hz, AC filter, 80 Hz, 25 mm/s, 10 mm/mV, General Electric Company, Boston, USA) and analyzed by two independent ECG diagnostician blinded to AMI patients.
HRV analysis
All patients received 24 h Holter monitoring (MARS Software and Seer Light recording box, General Electric Company, Boston, USA) within one week after infarction. 24 h mean heart rate and HRV parameters were analyzed according to the guidelines of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [17]. The main parameters of time domain are SDNN, standard deviation of all 5 min average NN intervals (SDANN), and square root of mean of the sum of squares of successive NN interval differences (rMSSD), number of successive NN interval differing by > 50 ms divided by the total number of successive NN intervals (pNN50). The frequency domain parameters are VLF at frequency between 0.0033 and 0.04 Hz, low frequency (LF) at frequency between 0.04 and 0.15 Hz, high frequency (HF) at frequency between 0.15 and 0.4 Hz, and ratio of low frequency/high frequency (LF/HF). Patients were classified according to the combination of time domain index SDNN and frequency domain index VLF. The sHRV was defined as both SDNN < 100 ms [15] and VLF < 26.7 ms (the optimal cutoff value identified by the Youden’s index from the ROC curve), and other patients were nsHRV.
Follow-up
All patients were followed up for 12 months by clinic visit or phone call. The main endpoint was MACE, which included all-cause mortality, non-fatal reinfarction, non-fatal stroke, heart failure, and urgent revascularization for unstable angina [18].
Statistical analysis
Kolmogorov–Smirnov test was used for normal distribution of all continuous variables. Continuous data with normal distribution were expressed as mean ± standard deviation and assessed by Student’s t test. Non-normal distribution data were expressed as median (inner-quartile distance) and assessed by two-tailed Mann–Whitney U test. Categorical variables were expressed as count and percentages, and compared using Chi-square test or Fischer’s exact test, as appropriate. Spearman correlation analysis was performed between fQRS and HRV parameters in the AMI patients. The cutoff value of VLF was derived from receiver operating characteristic curves (ROC) analysis by maximizing the sum of the sensitivity and specificity. The rates of MACE were compared by log-rank test of Kaplan–Meier curve. The hazard ratios (HRs) with 95% confidence intervals (CIs) of MACE were assessed by univariable and multivariable Cox proportional hazards regression analysis. Multivariable Cox regression models adjusting for clinical confounders with backward stepwise method (likelihood ratio) were used. Clinical confounders were identified as basic parameters (including age and sex) and parameters significantly associated all with fQRS, sHRV and MACE [including N-terminal pro-brain natriuretic peptide (NT-proBNP), serum creatinine (Scr) and left ventricular ejection fraction (LVEF)]. NT-proBNP was normalized prior to regression analysis using natural logarithm values (Ln). Incremental model performance was assessed by changes in the Chi-square value for the regression models with enter method. Statistical analyses were performed using IBM SPSS, version 22.0 for Windows (IBM Corp, New York, USA), with p < 0.05 (two-tailed test) as statistical significance.
Results
Clinical features of AMI patients in nfQRS group and fQRS group
Table 1 showed the clinical characteristics between nfQRS group and fQRS group. The proportion of patients with coronary artery lesion ≥ 3 and the blood biochemical indexes, such as cardiac troponin I (cTnI), creatine kinase (CK), creatine kinase isoenzyme-MB (CK-MB), total cholesterol (T-Chol), triglyceride (TG), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), Scr and NT-proBNP, were significantly higher in the fQRS group than in the nfQRS group (all p < 0.05). LVEF, SDNN, SDANN, VLF, LF and LF/HF values were significantly lower in the fQRS group than in the nfQRS group (p < 0.05). The percentage of sHRV was significantly higher in the fQRS group than in the nfQRS group (71.9 vs. 39.3%, p < 0.05).
Clinical features of AMI patients in nsHRV group and sHRV group
Age, history of hypertension and cerebral infarction, LDL-c, Scr, NT-proBNP and 24 h mean heart rate were significantly higher in the sHRV group than in the nsHRV group (all p < 0.05). Percentages of male, LVEF, SDNN, SDANN, rMSSD, pNN50, VLF, LF, HF and LF/HF values were significantly lower in the sHRV group than in the nsHRV group (all p < 0.05). The percentage of fQRS was significantly higher in the sHRV group compared to the nsHRV group (56.8 vs. 25.0%, p < 0.05) (Table 1).
Spearman correlation of fQRS and HRV parameters in AMI patients
Spearman correlation analysis showed that SDNN, SDANN, VLF, LF and LF/HF values were negatively correlated with fQRS, and sHRV was positively correlated with fQRS in AMI patients (r = 0.322, p < 0.001) (Table 2).
Follow-up results
Three patients were lost to follow-up, 1 in the nfQRS group and 2 in the fQRS group. There were 13 MACE in the nfQRS group: all-cause mortality (n = 2, both were cardiac mortality), re-hospitalization due to heart failure (n = 5), non-fatal reinfarction (n = 2), urgent revascularization for unstable angina (n = 1) and stroke (n = 3). There were 30 MACEs in the fQRS group: all-cause mortality (n = 5, all were cardiac mortality), rehospitalization due to heart failure (n = 8), non-fatal reinfarction (n = 5), urgent revascularization for unstable angina (n = 4) and stroke (n = 8).
According to the HRV classification, 1 patient in the nsHRV group and 2 patients in the sHRV group were lost to follow-up. There were 8 MACEs in the nsHRV group: all-cause mortality (n = 1, cardiac mortality), rehospitalization due to non-fatal reinfarction (n = 2), urgent revascularization for unstable angina (n = 1) and stroke (n = 4). There were 35 MACEs in the sHRV group: all-cause mortality (n = 6, all were cardiac mortality), rehospitalization due to heart failure (n = 13), non-fatal reinfarction (n = 5), urgent revascularization for unstable angina (n = 4) and stroke (n = 7).
Predicting value of different SDNN for MACE in AMI patients
The predicting values of different SDNN for MACE in AMI patients were as follows: the sensitivity, specificity, positive predictive value and negative predictive value of SDNN < 100 ms were 86.1, 36.4, 34.6 and 87.0%, respectively, and that of SDNN < 80 ms were 65.1, 66.4, 43.1 and 83.0%, respectively, and that of SDNN < 70 ms were 44.2, 78.2, 44.2, 78.2%, respectively. The sensitivity and negative predictive values of SDNN < 100 ms as cutoff value were the highest (Supplementary Table 1).
Risk factors of MACE in AMI patients
Age, history of hypertension and cerebral infarction, Scr, NT-proBNP and 24 h mean heart rate were significantly higher in the MACE group than in the nMACE group (p < 0.05). Percentages of STEMI and LVEF were significantly lower in the MACE group than in the nMACE group (p < 0.05). The SDNN, SDANN, LF, VLF, LF/HF values were significantly lower in the MACE group compared to the nMACE group (p < 0.05). The percentages of sHRV (81.4 vs. 41.8%) and fQRS (69.8 vs. 30.9%) were significantly higher in the MACE group compared to the nMACE group (both p < 0.001) (Table 3).
During 12 months’ follow-up, Kaplan–Meier curves demonstrated that the risk of MACE was significantly higher in the sHRV group compared to the nsHRV group (log-rank test, χ2 = 20.422, p < 0.001) (Fig. 2A), and in the fQRS group compared to the nfQRS group (log-rank test, χ2 = 21.557, p < 0.001) (Fig. 2B).
Kaplan–Meier curves comparing MACE of AMI patients with sHRV, fQRS, and combination of fQRS and sHRV. Kaplan–Meier curves demonstrated that the risk of MACE was significantly higher in the sHRV group compared to nsHRV group (log-rank test, χ2 = 20.422, p < 0.001, Fig. 2A), and in the fQRS group compared to nfQRS group (log-rank test, χ2 = 21.557, p < 0.001, Fig. 2B). The risk of MACE was significantly different among the four groups (log-rank test, χ2 = 36.007, p < 0.001). The risk of MACE was similar between the nsHRV + fQRS group and the nsHRV + nfQRS group (log-rank test, χ2 = 3.179, p = 0.075), and the risk of MACE was significantly higher in the sHRV + fQRS group compared to the sHRV + nfQRS group (log-rank test, χ2 = 8.376, p = 0.004), and sHRV + fQRS group was associated with significantly increased risk of MACE compared to nsHRV + nfQRS group (log-rank test, χ2 = 31.607, p < 0.001) during 12 months’ follow-up (Fig. 2C). AMI acute myocardial infarction, fQRS fragmented QRS, HRV heart rate variability, MACE major adverse cardiovascular events, nfQRS non-fragmented QRS, nsHRV non-severely depressed heart rate variability, sHRV severely depressed heart rate variability
The results of univariable Cox proportional hazards regression analysis showed that older age, higher Scr, NT-proBNP and 24 h mean heart rate, lower LVEF, sHRV and fQRS were risk factors of MACE, and SDNN, SDANN, VLF, LF, LF/HF values were inversely associated with MACE in AMI patients. The results of multivariable Cox proportional hazards regression analysis showed that SDNN and VLF values were inversely associated with MACE, sHRV and fQRS were independent risk factors of MACE after adjusting for age, sex, NT-proBNP, Scr and LVEF with backward elimination (Likelihood Ratio) method (Table 4).
The results of Chi-square test showed that there was significantly correlation between fQRS and MACE, as well as sHRV and MACE (both p < 0.001). The sHRV and fQRS were combined and the patients were further divided into nsHRV + nfQRS group (n = 54), nsHRV + fQRS group (n = 18), sHRV + nfQRS group (n = 35) and sHRV + fQRS group (n = 46). The incidences of MACE were 7.4, 22.2, 25.7 and 56.5%, respectively (p < 0.05). After adjusted for age, sex, Ln NT-proBNP, Scr and LVEF, sHRV + fQRS was an independent predictor (p = 0.021), using nsHRV + nfQRS as reference group, the risk of MACE was significantly increased in the other three groups, and patients in the sHRV + fQRS group had a sixfold higher risk of MACE (HR = 6.228, 95% CI 1.849–20.984, p = 0.003). As the whole cohort was divided into subgroups of LVEF ≥ 50% and LVEF < 50%, Cox regression analysis showed that sHRV + fQRS was also an independent predictor on MACE in LVEF ≥ 50% subgroup (p = 0.008). Using nsHRV + nfQRS as reference group, patients in the sHRV + fQRS group had a ninefold higher risk of MACE (HR = 9.149, 95% CI 2.417–34.638, p = 0.001) (Table 5).
Kaplan–Meier curves demonstrated that the risk of MACE was significantly different among the four subgroups (log-rank test, χ2 = 36.007, p < 0.001). The risk of MACE was similar between the nsHRV + fQRS group and the nsHRV + nfQRS group (log-rank test, χ2 = 3.179, p = 0.075), and the risk of MACE was significantly higher in the sHRV + fQRS group compared to the sHRV + nfQRS group (log-rank test, χ2 = 8.376, p = 0.004), and the sHRV + fQRS group was associated with the highest increased risk of MACE compared to other groups (p < 0.001) (Fig. 2C).
Predicting value of sHRV and fQRS for MACE in AMI patients
The predicting sensitivity, specificity and accuracy of sHRV for MACE in the AMI patients were 81.4, 58.2 and 64.7%, respectively. With fQRS as predicting parameter, the sensitivity, specificity and accuracy were 69.8, 69.1 and 69.3%, respectively. Combination of sHRV and fQRS as predicting parameters, the sensitivity, specificity and accuracy were 60.5, 81.8 and 75.8%, respectively. The specificity and accuracy were the highest with combined sHRV and fQRS as compared to sHRV or fQRS alone (Table 6).
Incremental predictive efficacy of clinical, sHRV and fQRS in AMI patients
Clinical variables (model I) including age, sex, NT-proBNP, Scr and LVEF were entered in the first step of a multivariable Cox model to predict MACE (Chi-square 77.54, p < 0.001). In model II, adding sHRV to the model I enhanced the explanatory power (Chi-square 79.56, p = 0.010 vs. model I). Adding fQRS (model III) further improved the prognostic performance of the model II (chi-square 84.57, p = 0.008 vs. model II) (Fig. 3).
Incremental model performance for predicting prognosis assessed by starting with the clinical variables (model I: age, sex, NT-proBNP, Scr and LVEF), followed by sHRV (model II: adding sHRV to model I), and finally by adding fQRS (model III). fQRS fragmented QRS, LVEF left ventricular ejection fraction, NT-proBNP N-terminal pro-brain natriuretic peptide, Scr serum creatinine, sHRV severely depressed heart rate variability
Discussion
The major findings of the present study were as follows: (1) fQRS is closely related to sHRV, suggesting the presence of significant autonomic nerve dysfunction in AMI patients with fQRS. (2) Combined assessment with fQRS and sHRV enhances the predicting efficacy on outcome in AMI patients. To the best of our knowledge, this study is the first report describing the association between fQRS and cardiac autonomic function defined by sHRV, and the prediction efficacy of combined assessment of fQRS and sHRV on outcome of patients with AMI.
Association between fQRS and sHRV in AMI patients
The fQRS is caused by the inhomogeneous conduction of electrocardiographic activities in the infarcted and ischemic regions, reflecting the inhomogeneous electrical activity of ventricular depolarization after AMI [16]. HRV is the result of fine regulation of cardiovascular system by neurohumoral factors, which could reflect the cardiac autonomic nervous function [19, 20]. Although HRV and fQRS are essentially different in nature, fQRS and HRV could jointly hint the pathological nature in AMI patients. Our results showed that NT-proBNP was significantly higher, and LVEF was significantly lower in the fQRS group than in the nfQRS group and in the sHRV group than in the nsHRV group. The presence of fQRS might indicate more severe myocardial ischemia injury in patients with AMI, which might also lead to more severe cardiac autonomic nerve dysfunction as expressed by sHRV. The sHRV could further aggravate cardiac dysfunction in AMI patients and form vicious circle in AMI patients.
SDNN is a HRV index reflecting the total activity of the sympathetic and vagus nerves. VLF is a controversial indicator. Most studies showed that VLF was related to the sympathetic nerve [21, 22], while some study demonstrated that VLF also reflected the parasympathetic nerve activity [23]. In present study, the values of rMSSD, pNN50 and HF (all reflecting vagus nerve function) were similar between the fQRS group and the nfQRS group, while the values of SDNN and VLF were significantly lower in the fQRS group than in the nfQRS group. Our results thus hinted that there was sympathetic nerve and vagus nerve dysfunction, especially the sympathetic nerve dysfunction in AMI patients with fQRS. Previous studies showed that SDNN and VLF in HRV were the most advantageous indicators for predicting the prognosis of AMI patients [23, 24]. Therefore, SDNN and VLF, which reflect sympathetic nerve and vagus nerve function, were used to divide AMI patients into nsHRV and sHRV groups in this study.
SDNN with various cutoffs was used as a risk stratification factor in studies of MI patients. In some studies, SDNN < 70 ms was used as a prognostic factor to predict all-cause death or cardiac death in AMI patients [11, 25]. In other studies, SDNN was classified into three categories, with SDNN < 50 ms as a high-risk factor, and 50 ≤ SDNN < 100 ms as the moderate risk factor. SDNN < 100 ms was considered as a moderate- to high-risk factor of defining autonomic nervous dysfunction in previous studies [17, 26, 27]. In another study, Hayano J. et al. [28] reported that the median SDNN was 90 ms for survivors and 65 ms for non-survivors in AMI group with an ejection fraction > 35%. In our study, the median SDNN of AMI patients in the nMACE group and MACE group was 88 and 72 ms, respectively. The sensitivity, specificity, positive predictive value and negative predictive value of SDNN < 100 ms were 86.1, 36.4, 34.6 and 87.0%, respectively, and that of SDNN < 80 ms were 65.1, 66.4, 43.1 and 83.0%, respectively, and that of SDNN < 70 ms were 44.2, 78.2, 44.2, 78.2%, respectively. The sensitivity and negative predictive value of SDNN < 100 ms as cutoff value were the highest and the incidence of missed diagnosis was the lowest. In addition, the results of multivariate Cox regression analysis showed that sHRV was an independent predictor of MACE after adjusting for age, gender, Ln-NT-proBNP, Scr and LVEF; however, with SDNN < 70 ms as cutoff value, sHRV was not an independent predictor of MACE. SDNN < 100 ms was thus used as the cutoff value in this study.
Enhanced predicting efficacy of combined assessment in AMI patients
To verify if fQRS and sHRV were confounding factors, we performed Chi-square test and the results showed that there was significantly correlation between fQRS and MACE, as well as sHRV and MACE (both p < 0.001), thus fQRS and sHRV were confounding factors for the development of MACE. The predictive effect of fQRS was weakened when sHRV was added to the Cox regression model (p = 0.052), and the predictive effect of sHRV was weakened when fQRS was added to the Cox regression model (p = 0.097) (Table 5). Thus, there was interaction between fQRS and sHRV even if the correlation coefficient between fQRS and sHRV was not high (r = 0.322) (Table 2). Therefore, we tested the predicting value of combined sHRV and fQRS, and multivariable Cox regression analysis showed that sHRV + fQRS was an independent predictor for MACE (p = 0.021), and patients in the sHRV + fQRS group had a sixfold higher risk of MACE (HR = 6.228, 95% CI 1.849–20.984, p = 0.003). Cox regression analysis showed that sHRV + fQRS was also an independent predictor on MACE in LVEF ≥ 50% subgroup (p = 0.008) (Table 5). The above results indicated that coexistence of sHRV and fQRS was an alarming sign for the worse outcome in all AMI patients or AMI patients with LVEF ≥ 50%. Combined assessment with sHRV and fQRS might thus be helpful on the risk stratification of AMI patients. Individualized therapy plan should thus be applied to these patients at the highest risk. Detailed secondary preventive strategies aiming to reduce the incidence of heart failure, reinfarction, target vessel revascularization and stroke are of importance.
As expected, adding sHRV and fQRS significantly increased the predicting performance of established clinical variables including age, sex, NT-proBNP, Scr and LVEF (Fig. 3). Future clinical studies with larger patient cohort are thus warranted to validate present results and studies are also required to compare the outcome among AMI patients with sHRV and fQRS receiving usual care or intensive individualized care. Clinicians should be urged to include fQRS and sHRV in the risk stratification of AMI patients. Individualized secondary preventive strategies should be applied to patients with fQRS or sHRV, especially, patients with both fQRS and sHRV.
Study limitations
This study is a retrospective study with a small patient cohort based on a single-center database. A larger patient cohort with multi-center database is essential to validate present results.
Conclusion
fQRS is closely related to sHRV, suggesting significant impairment of sympathetic nerve function in AMI patients with fQRS. Combined assessment of fQRS and sHRV enhances the predicting efficacy on outcome in AMI patients.
References
Akgul O, Uyarel H, Pusuroglu H, Surgit O, Turen S, Erturk M, Ayhan E, Bulut U, Baycan OF, Demir AR, Uslu N (2015) Predictive value of a fragmented QRS complex in patients undergoing primary angioplasty for ST elevation myocardial infarction. Ann Noninvasive Electrocardiol 20:263–272
Cho HJ, Yoon JY, Kim N, Jang SY, Bae MH, Lee JH, Yang DH, Park HS, Cho Y, Chae SC (2019) Predictive value of a fragmented QRS complex in diagnosing patients with myocardial ischemia. Clin Cardiol 42:379–384
Kocaman SA, Cetin M, Kiris T, Erdogan T, Canga A, Durakoglugil E, Satiroglu O, Sahinarslan A, Cicek Y, Sahin I, Bostan M (2012) The importance of fragmented QRS complexes in prediction of myocardial infarction and reperfusion parameters in patients undergoing primary percutaneous coronary intervention. Turk Kardiyol Dern Ars 40:213–222
Xia W, Feng XY (2018) Fragmented QRS (fQRS) complex predicts adverse cardiac events of ST-segment elevation myocardial infarction patients undergoing percutaneous coronary intervention and thrombolysis. Med Sci Monit 24:4634–4640
Gong B, Li Z (2016) Total mortality, major adverse cardiac events, and echocardiographic-derived cardiac parameters with fragmented QRS complex. Ann Noninvasive Electrocardiol 21:404–412
Lorgis L, Jourda F, Hachet O, Zeller M, Gudjoncik A, Dentan G, Stamboul K, Guenancia C, Mock L, Cottin Y, Group RSW (2013) Prognostic value of fragmented QRS on a 12-lead ECG in patients with acute myocardial infarction. Heart Lung 42:326–331
Gungor B, Ozcan KS, Karatas MB, Sahin I, Ozturk R, Bolca O (2016) Prognostic value of QRS fragmentation in patients with acute myocardial infarction: a meta-analysis. Ann Noninvasive Electrocardiol 21:604–612
Stavileci B, Cimci M, Ikitimur B, Barman HA, Ozcan S, Ataoglu E, Enar R (2014) Significance and usefulness of narrow fragmented QRS complex on 12-lead electrocardiogram in acute ST-segment elevation myocardial infarction for prediction of early mortality and morbidity. Ann Noninvasive Electrocardiol 19:338–344
Yu Y, Xu Y, Zhang M, Wang Y, Zou W, Gu Y (2018) Value of assessing autonomic nervous function by heart rate variability and heart rate turbulence in hypertensive patients. Int J Hypertens 2018:4067601. https://doi.org/10.1155/2018/4067601
Casolo GC, Stroder P, Signorini C, Calzolari F, Zucchini M, Balli E, Sulla A, Lazzerini S (1992) Heart rate variability during the acute phase of myocardial infarction. Circulation 85:2073–2079
La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ (1998) Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Atrami (autonomic tone and reflexes after myocardial infarction) investigators. Lancet 351:478–484
Coviello I, Pinnacchio G, Laurito M, Stazi A, Battipaglia I, Barone L, Mollo R, Russo G, Villano A, Sestito A, Lanza GA, Crea F (2013) Prognostic role of heart rate variability in patients with ST-segment elevation acute myocardial infarction treated by primary angioplasty. Cardiology 124:63–70
Huikuri HV, Stein PK (2013) Heart rate variability in risk stratification of cardiac patients. Prog Cardiovasc Dis 56:153–159
Bigger JT Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN (1992) Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 85:164–171
Ponikowski P, Anker SD, Chua TP, Szelemej R, Piepoli M, Adamopoulos S, Webb-Peploe K, Harrington D, Banasiak W, Wrabec K, Coats AJ (1997) Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 79:1645–1650
Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J (2006) Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 113:2495–2501
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 93:1043–1065
Okuno T, Aoki J, Tanabe K, Nakao K, Ozaki Y, Kimura K, Ako J, Noguchi T, Yasuda S, Suwa S, Fujimoto K, Nakama Y, Morita T, Shimizu W, Saito Y, Hirohata A, Morita Y, Inoue T, Okamura A, Mano T, Hirata K, Shibata Y, Owa M, Tsujita K, Funayama H, Kokubu N, Kozuma K, Uemura S, Tobaru T, Saku K, Ohshima S, Nishimura K, Miyamoto Y, Ogawa H, Ishihara M (2019) Association of onset-season with characteristics and long-term outcomes in acute myocardial infarction patients: results from the Japanese registry of acute myocardial infarction diagnosed by universal definition (J-MINUET) substudy. Heart vessels 34:1899–1908
Kamath MV, Ghista DN, Fallen EL, Fitchett D, Miller D, McKelvie R (1987) Heart rate variability power spectrogram as a potential noninvasive signature of cardiac regulatory system response, mechanisms, and disorders. Heart vessels 3:33–41
Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL (2010) Improvements in heart rate variability with exercise therapy. Can J Cardiol 26:303–312
Vinik AI, Ziegler D (2007) Diabetic cardiovascular autonomic neuropathy. Circulation 115:387–397
Perini R, Orizio C, Baselli G, Cerutti S, Veicsteinas A (1990) The influence of exercise intensity on the power spectrum of heart rate variability. Eur J Appl Physiol Occup Physiol 61:143–148
Taylor JA, Carr DL, Myers ChW, Eckberg DL (1998) Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation 98:547–555
Fang SC, Wu YL, Tsai PS (2020) Heart rate variability and risk of all-cause death and cardiovascular events in patients with cardiovascular disease: a meta-analysis of cohort studies. Biol Res Nurs 22:45–56
La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT Jr, Camm AJ, Schwartz PJ, Investigators ATRAMI (2001) Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation 103:2072–2077
Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ (1987) Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59:256–262
Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, Baig W, Flapan AD, Cowley A, Prescott RJ, Neilson JM, Fox KA (1998) Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation 98:1510–1516
Hayano J, Ueda N, Kisohara M, Yuda E, Carney RM, Blumenthal JA (2021) Survival predictors of heart rate variability after myocardial infarction with and without low left ventricular ejection fraction. Front Neurosci. https://doi.org/10.3389/fnins.2021.610955
Funding
This study was funded by the health and family planning commission of Wuhan municipality (WX18Q36 and WX15A07).
Author information
Authors and Affiliations
Contributions
Conceptualization: YX, methodology: YY, formal analysis and investigation: YW, writing—original draft preparation: YX, writing—review and editing: YY, funding acquisition: YX and LH, supervision: YG.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
This study protocol was approved by the ethical committees of Wuhan Fourth Hospital, Puai Hospital affiliated to Tongji Medical College, Huazhong University of Science and Technology and conducted in compliance with the ethical principles of the Declaration of Helsinki. 24 h Holter monitoring results were obtained in the clinical medical records in this study and the requirement to obtain informed consent was waived by the ethical committees of Wuhan Fourth Hospital, Puai Hospital affiliated to Tongji Medical College, Huazhong University of Science and Technology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, Y., Yu, Y., He, L. et al. Predicting efficacy of combined assessment with fragmented QRS and severely depressed heart rate variability on outcome of patients with acute myocardial infarction. Heart Vessels 37, 239–249 (2022). https://doi.org/10.1007/s00380-021-01930-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-021-01930-y