Abstract
Soil pH is one of the main drivers of soil microbial functions, including carbon use efficiency (CUE), the efficiency of microorganisms in converting substrate C into biomass, a key parameter for C sequestration. We evaluated liming effects after maize-litter addition on total CUE (including microbial residues), CUE of microbial biomass (CUEMB), and fungal biomass on an acidic Acrisol with a low C. We established a 6-week incubation experiment to compare limed and unlimed Acrisol treatments and a reference soil, a neighboring Nitisol with optimal pH. Fungal biomass (ergosterol) increased ~ 10 times after litter addition compared with soils without litter, and the final amount was greater in the limed Acrisol than the Nitisol. Litter addition induced a positive priming effect that increased with increasing pH. The increases in soil pH also led to increases in litter-derived CO2C and decreases in particulate organic matter (POM)C. Thus, in spite of increasing microbial biomass C, CUE decreased with increasing pH and CUEMB was similar across the three soils. CUEMB was positively associated with saprotrophic fungi, implying that fungi are more efficient in incorporating litter-derived C into microbial, especially fungal biomass after 42 days. By including undecomposed maize litter and microbial residues, CUE provided a more comprehensive interpretation of pH and liming effects than CUEMB. Nevertheless, longer-term studies may provide further information on substrate-C turnover and the persistence of liming and pH effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil functions, soil microbial communities and their activity, are largely controlled by soil reaction (Canini et al. 2019; Malik et al. 2018; Wang et al. 2019). A low pH reduces microbial indicators of soil quality such as fungal, bacterial, and microbial biomass and, to a lesser extent, microbial activity (Rousk et al. 2009), without affecting the metabolic quotient (Moran-Rodas et al. 2022). On the other hand, a pH increase above 6.2 in low-pH soils of intensified systems can create a shift toward alkalinity, reducing soil organic carbon (SOC) sequestration through increased decomposition, following alleviation of acid retardation of microbial growth (Malik et al. 2018).
The most representative indicator of the role of microbial communities on SOC sequestration is microbial C use efficiency (CUE), which is usually defined as the relation between the amount of C used for anabolic and catabolic processes in the microbial community (Horn et al. 2021; Jones et al. 2018, 2019). CUE is a major regulator of SOC cycling at the local and global scale (Allison et al. 2010; Li et al. 2019; Wang et al. 2021). However, many soil factors such as nutrient availability, initial SOC levels, and pH can affect SOC sequestration directly and additionally alter CUE, generating co-varying or interactive effects on soil C-sequestration potential (Malik et al. 2018; Oliver et al. 2021). Some studies have found correlations between CUE and SOC contents (Oliver et al. 2021; Wang et al. 2021).
Soil pH is one of the most important variables to affect CUE, with increasing CUE up to a threshold of ~ 6.2 pH (Horn et al. 2021; Jones et al. 2019; Malik et al. 2018; Oliver et al. 2021; Pei et al. 2021; Silva-Sánchez et al. 2019; Xiao et al. 2021). The most common way to assess pH effects on CUE has been through existent geographical pH gradients, while a few studies have used the same soils, manipulating the pH through liming, which reduces the variability from other factors (Horn et al. 2021; Silva-Sánchez et al. 2019). Microbial anabolic and catabolic processes are important for predicting microbial stabilization of SOC (Liang et al. 2017), and CUE aims to represent both. However, its measurement is still ambiguous and different methods are used, with each of them influenced by different factors (Geyer et al. 2016). To assess the role of CUE in SOC dynamics, the CUE approach frequently used is the CUE of microbial biomass (CUEMB) (Manzoni et al. 2012; Sinsabaugh et al. 2013; Spohn et al. 2016). However, CUEMB has the disadvantage of excluding the role of microbial residues, non-biomass microbial metabolites, which are not mineralized during the incubation period. This fraction has been recognized for its relevant contribution to organic matter accumulation (Cotrufo et al. 2015; Geyer et al. 2020; Kallenbach et al. 2016; Liang et al. 2019; Miltner et al. 2012). Therefore, microbial residues add up to the C-fractions of microbial biomass and CO2 that are produced during metabolization of added substrate. If this is taken into consideration, CUE is increased three- to fivefold, compared with CUEMB deduced from microbial biomass growth and CO2C evolution alone (Börger et al. 2022; Schroeder et al. 2020). An additional difference between these CUE approaches is the fact that experimental studies on CUEMB from incubation experiments have been performed using low molecular weight substrates that are easy to assimilate, such as sugars, amino- and organic acids (Jones et al. 2018), making it difficult to translate to field conditions, where the ultimate substrate is plant residues.
Increasingly low SOC levels and acidic conditions are common in Indian agricultural soils (Lal 2004; Sathish et al. 2016). This study addresses an Acrisol with low pH and low SOC levels, in comparison with a Nitisol in South India (Moran-Rodas et al. 2022). The Acrisol had lower levels of microbial soil-quality indicators, such as microbial biomass, fungal biomass, and microbial residues, compared with irrigated conditions under improved pH (Moran-Rodas et al. 2022). However, other studies have shown different results on fungi, where acidic conditions (above a threshold of pH 4.5) favored their growth compared with bacteria (Rousk et al. 2009), or where bacterial growth and CUEMB were promoted by liming, while fungi remained unaffected (Silva-Sanchez et al. 2019).
To evaluate the effect of lime on both CUE and fungal biomass of an Acrisol, we performed an incubation experiment using limed and unlimed treatments of the Acrisol and a neighboring soil with an optimal pH (Nitisol) and applied both CUE methods. We hypothesize that (1) the constraints related to pH for the microbial community of the Acrisol are alleviated by liming, improving its CUE; thus, (2) the CUE is positively associated with pH, and (3) fungal biomass increases with litter addition but not with liming, and it positively affects CUE.
Materials and methods
Experimental design
The soils studied were a drip-irrigated (4 mm depth) Nitisol and a rainfed Acrisol (IUSS Working Group WRB 2015) from two experimental fields located at the GKVK campus, University of Agricultural Sciences, Bangalore (12°58′20.79′′N, 77°34′50.31′′E) at an altitude of 920 m above sea level. Mean annual temperature is 29.2 °C (Prasad et al. 2016) and mean annual rainfall is 902 mm (Murugan et al. 2019).
Four replicate plots under maize cultivation were located in each field. The plots contained subplots with high and low N fertilization levels. From each subplot, three soil cores were randomly collected from the topsoil (0–10 cm depth, diameter: 4.2 cm) and combined to a composite sample just before the harvest period in October 2018. These soil samples were sieved (< 2 mm) and stored frozen (− 18 °C) until analysis. The incubation experiment took place in November and December 2020 in Witzenhausen, Germany. Samples were thawed and corresponding low and high N level replicates were combined to four general samples per soil type. This was done to optimize the use of the soil as there were no relevant differences between low and high N level in terms of microbial and SOC-related parameters in either of the two fields, except for microbial respiration, which was a little higher under high N level in the Acrisol (Moran-Rodas et al. 2022).
The Nitisol had a higher soil pH and more clay, SOC, and total N and S, while the Acrisol contained more total P (Moran-Rodas et al. 2022). The Nitisol had a pH-CaCl2 of 6.32; the Acrisol, 4.39. To evaluate the effects of improved pH conditions and liming in the Acrisol, each of its four replicates was divided into four sub-replicates for a two-factorial experiment with the factors lime (limed and unlimed treatments) and maize-litter addition (with and without treatments), resulting in four replicates per treatment and 16 in total. In addition, the Nitisol remained unlimed with a neutral pH, but was subject to the litter treatment (with and without).
The maize litter used as substrate from the corresponding fields had a δ13C of − 12.38 ± 0.1‰, a C/N ratio of 47 ± 5.6, and a total C of 426.5 ± 5.2 mg g−1 DM at the Nitisol; at the Acrisol, δ13C of − 12.15 ± 0.1‰, a C/N ratio of 64 ± 7.5, and a total C of 443.6 ± 7 mg C g−1 DM. The litter was applied to soil samples corresponding to each field.
Soil pH-adjustment experiment
To achieve an optimal pH in limed soil replicates, we tested previous soil samples (from 2016) from the Acrisol with different amounts of lime as commercially available CaO, using three replicates per treatment. The pH changes after application were monitored to obtain the stabilization period required before litter addition. The pH was stabilized after 8 days of lime application. We used the final values to draw a regression model of required lime amounts to achieve a specific pH. The resulting equation for the regression model was y = 518.303 − 167.43x + 13.97x2, where y = milligrams of CaO in 50 g soil and x = target pH. For a pH of 6–7 similar to that of the Nitisol, this resulted in 0.62 mg CaO g−1 soil or 1.066 t ha−1.
Having determined the lime concentration, the next step of the pre-experiment was to find out whether the CO2 emissions and δ13C signature of CO2C of limed and unlimed soils differed without adding litter. It was assumed that the lime in contact with the soil CO2 trapped from the air and H2O would generate direct CO2 emissions with a slightly different δ13C signature than that of microbial respiration derived from SOC decomposition. δ13C was measured after the first week and resulted in a slight difference between the δ13C of limed and unlimed soil (− 19.8 and − 20.73 δ13C, respectively); however, the CO2 emissions were only different after the first 3 days, and this difference disappeared over time, with no difference by the end of the first week, this trend completely disappearing in the second week. We assumed that the δ13C difference between limed and unlimed soils would also completely disappear from the second week onwards. Thus, we established a pre-incubation period of 2 weeks for the main experiment, after which pH and CO2 emissions of limed and unlimed soils were stabilized before substrate addition. We also adjusted the soil water holding capacity (WHC) to 50%. After lime addition (0.62 mg CaO g−1 soil) and the subsequent two pre-incubation weeks, we measured the new pH in the limed samples before dividing them into the two subsamples for substrate addition (with and without litter) and incubation was started.
Incubation and CO2 analysis
Each treatment replicate consisted of 150 g of fresh soil in 200-ml glass beakers. For the substrate addition replicates, the soil was mixed with maize litter (5-mm cuttings), corresponding to 2 mg C g−1 soil. The beakers were placed into Mason jars, equipped with sealing rings, together with plastic containers with 0.5 M NaOH solution to trap the CO2 evolved during the incubation period. The vials were incubated at 25 °C for tropical soils. CO2 evolution was measured after 3 and 7 days and then on a weekly basis for 6 weeks. Water content was monitored gravimetrically every 2 weeks, but no adjustments were necessary over the 6 weeks.
We removed the initial CO2 in the jars with compressed oxygen to have a CO2-free atmosphere at the beginning of incubation. This compressed oxygen-ventilation procedure was repeated every time that isotopic analysis of CO2 samples was done. During weeks 3 and 5, compressed air was used instead of oxygen. To measure the respired CO2 trapped in the NaOH solution, we used precipitation with 5 ml of saturated BaCl2 solution, followed by back titration with 0.5 M HCl using a TITRONIC 500 (Xylem Analytics, Weilheim, Germany) system to the transition point of phenolphthalein at a pH of 8.3. The titration precipitates were centrifuged (3000 × g for 10 min at 20 °C), rinsed with H2O to remove excess ions, and freeze-dried for isotopic analysis to obtain the amount of litter-derived CO2C. This was done after 3, 7, 14, 28, and 42 days. The results from the third and fifth weeks were calculated by linear interpolation. At the end of the incubation period, we measured the final pH for all treatments.
Total microbial and fungal biomass
Total microbial biomass C (MBC) was determined by chloroform fumigation extraction (Vance et al. 1987), using soil samples adjusted to 50% of their WHC after thawing for 5 days at 4 °C. Fumigated and non-fumigated samples were extracted from 5 g moist soil with 20 ml 0.5 M K2SO4, followed by measuring organic C in the extracts with a multi C/N 2100S automatic analyzer (Analytik Jena, Germany). MBC was calculated as EC/kEC, where EC = (organic C extracted from fumigated soil) − (organic C extracted from non-fumigated soil) and kEC = 0.45 (Wu et al. 1990).
The fungal-cell membrane component ergosterol was extracted from 2 g moist soil with 100 ml ethanol by 30-min oscillating shaking at 250 rpm, followed by reversed-phase high-performance liquid chromatography with 100% methanol as the mobile phase and detection at 282 nm (Djajakirana et al. 1996).
Particulate organic matter
Particulate organic matter (POM) was obtained at the end of the incubation experiment from 100 g of fresh soil by wet sieving and flotation-decantation (Magid and Kjærgaard 2001; Muhammad et al. 2006), using a 400-μm sieve. POM was dried at 40 °C until constant weight, weighed, and ground for the analysis of total C and δ13C. The recovery rates of this method at day 0 were 95% and more (Börger et al. 2022; Schroeder et al. 2020).
Analysis of maize litter–derived C
The presence of litter-derived C through isotopic analysis of δ13C was measured on MBC, CO2C, and POMC. The δ13C in K2SO4 extracts (for MBC) as well as δ13C of BaCO3 (for CO2C) were analyzed in freeze-dried samples, while POM was analyzed on milled-dry samples. Isotope values were measured by elemental analyzer–isotope ratio mass spectrometry. The fraction of litter-derived C in the K2SO4 extracts of fumigated and non-fumigated samples, in CO2C as well as in POMC in each treatment replicate was calculated from the δ13C data according to a two-pool-mixing model (Balesdent and Mariotti 1996) using the following equation:
where δ13Csample represents the samples with litter-amended treatments, δ13Ccontrol the treatments without litter at six incubation weeks, and δ13Cmaize is the average signature of the substrate, i.e., pure maize litter.
The litter-induced priming effect was calculated as the difference between native soil-derived CO2C of the litter-amended soils and that of soils without litter for each corresponding soil and lime treatment.
CUE and CUEMB calculations
CUE values of maize litter were calculated according to Joergensen and Wichern (2018) considering all microbial metabolites, i.e., litter-derived microbial residue C (MRCmaize):
where litter-derived C is considered as a percentage of the added substrate in MBC, POMC, and CO2C, abbreviated as MBCmaize, POMCmaize, and CO2Cmaize, respectively. CUE was additionally calculated in the classical way that considers the incorporation of litter-derived C into the MBC but not that into MRC (Manzoni et al. 2012; Sinsabaugh et al. 2013; Spohn et al. 2016) and is therefore abbreviated in this study as CUEMB:
Statistical analysis
All statistical analyses were performed in the R environment (R Core Team 2019). Results are presented as arithmetic means on a soil dry mass basis. Variance homogeneity and normal distribution of the residuals were tested with the Levene test and Shapiro–Wilk test, respectively. One-way ANOVA was performed to test differences between Nitisol and limed and unlimed Acrisol treatments in litter-amended soils and in soils without litter separately, followed by Tukey test. To generate regression model equations for the relationships between pH ~ CaO (pH-adjustment experiment), priming effect ~ pH, CUE ~ pH, and CUEMB ~ fungal biomass, the “lm” function in the “stats” R package v. 3.5.3 was used after testing for their significant relationships using Pearson correlation (for normally distributed data) and Spearman rank correlation (for non-normally distributed data).
Results
Initially, the pH of the limed Acrisol was in the desired range of the reference Nitisol (Table 1), but this pH dropped compared with that of the Nitisol during the 6 incubation weeks. However, when comparing individual treatments, no significant changes occurred from initial to final pH.
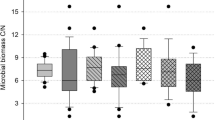
Ergosterol showed an approximate tenfold increase in litter-amended soils compared with soils without litter (Table 1), whereas that of total MBC (MBCmaize + MBCsoc; Tables 2 and 3, respectively) was just a threefold increase. The change in fungal biomass due to litter addition was more drastic in the Acrisol treatments than in the Nitisol. Liming had no significant effect on ergosterol content in the Acrisol, but the ergosterol content of the limed Acrisol surpassed that of the Nitisol.
Maize litter decomposition decreased with decreasing pH (Table 2, Fig. 1) according to the positive correlation between CO2Cmaize and soil pH (rs = 0.85, P < 0.05). This was confirmed by the negative correlation between recovered POMCmaize and soil pH (rs = − 0.70, P < 0.05). Total CO2C (CO2Cmaize + CO2Csoc) in litter-amended soils were six- to eightfold larger compared with soils without litter. In the soils without litter, soil respiration generally remained low (Supplementary Fig. 1). However, soil-derived CO2Csoc in litter-amended soils were doubled compared with soils without litter addition (Table 3), indicating a positive priming effect. CO2Csoc decreased in the order Nitisol > limed Acrisol > unlimed Acrisol, i.e., soil pH positively affected priming (Fig. 3A). In spite of greater SOC mineralization in litter-amended treatments, a greater amount of soil-derived POMCsoc was recovered by the end of the incubation compared with the total POMC recovered in their corresponding soils without litter (Table 2).
CUE was much greater than CUEMB and was greater in the limed and unlimed treatments compared with the Nitisol (Fig. 2A) due to greater values in terms of remaining POMC and smaller values in accumulated CO2C (Fig. 1). CUE was negatively affected by pH (Fig. 3B). The positive effect of fungi was only evident on CUEMB (Fig. 3C). The distributions of litter-derived C in some fractions differed among soils; however, they resulted in similar CUEMB values (Fig. 2A).
Linear relationships between A the priming effect of litter decomposition and final soil pH-CaCl2 (y = − 14 + 34.49x, R2 = 0.51, P < 0.01), B CUE and final soil pH-CaCl2 (y = 0.84 − 0.03x, R2 = 0.6, P < 0.01), and C CUEMB and ergosterol (y = 0.07 + 0.04x, R2 = 0.5, P = 0.02) in an unlimed Nitisol as well as a limed and unlimed Acrisol after 6 weeks of incubation at 25 °C
Discussion
Liming effect on pH and its general implications
The model prediction to achieve a desired initial pH using CaO was very accurate, despite the potential risk of model-prediction effects associated with quality and origin of the lime (Bailey et al. 1991) or with varying soil factors such as nutrient availability, buffering capacity, and aluminum saturation (Islam et al. 2004; Nelson and Su 2010; Olego et al. 2014). The drop in pH of the limed Acrisol treatment compared with the Nitisol after the 6 incubation weeks was probably due to the buffer capacity of the soils. Stabilizing soil properties such SOC or clay content were both higher in the Nitisol and positively associated with its buffer capacity (Aitken et al. 1990). Furthermore, substrate addition per se can differentially influence the liming effect on pH in different soils across time (Bramble et al. 2021).
Priming effect of litter addition on SOC
The current priming effect increased with increasing soil pH. Therefore, we do not discount the possibility that SOC priming is caused by the energy-induced synthesis of SOM-degrading exoenzymes. This was probably combined with accelerated turnover of the microbial biomass and a correlation between priming and mineralization of the added substrate (Mason-Jones et al. 2018), as indicated by the correlation between CO2Cmaize and CO2Csoc (r = 0.7, P < 0.01). An increase in pH may cause the increases in extracellular enzyme production and enzyme activity because the optimal pH value of the enzyme is reached. This may generally promote microbial activity and microbial biomass formation, followed by increased mineralization and priming. This is particularly true when growth of less efficient groups is promoted, suggested by the negative relationship between the contribution of fungal ergosterol to MBC and priming (r = − 0.6, P < 0.05). The negative association between more efficient microorganisms and priming is consistent with previous research that has found negative relationships between CUE and priming after straw addition (Mo et al. 2021). The apparently lower POMCsoc mineralization in litter-amended soils compared with soils without litter may be explained by humified SOC particles adhering to litter-derived POM, altering the sample’s δ13C and confounding the results of apparently recovered POMCsoc, with POMCmaize containing humified SOC.
The role of pH and liming in CUE
Low soil pH in the Acrisol resulted in less MBCsoc and MBCmaize as well as less CO2Cmaize but more POMCmaize, indicating general negative effects on the decomposition of fresh plant residues. Liming already alleviated some of this stress (Jones et al. 2019; Liu et al. 2018; Malik et al. 2018). However, at the same time, liming promoted microbial turnover and increased substrate mineralization, resulting in a similar CUE for limed and unlimed treatments. Thus, the increase in CUE with decreasing soil pH implies the accumulation of SOM, due to acidity constraints of microbial growth and activity (Malik et al. 2018; Zhang et al. 2020). On the other hand, and in agreement with previous studies, the trend observed on CUEMB in this study shows that liming may be a positive contributor to CUE of microbial biomass (CUEMB), as compared with substrate quality (i.e., litter C/N ratio). No difference in CUEMB was found in a study that compared two soils differing in POM-C/N ratios (Schroeder et al. 2020), which corresponds to our results, as the limed Acrisol and Nitisol did not differ, despite distinct litter C/N ratios.
The role of fungi in CUE
Fungi remained unaffected by liming or pH in our study, in agreement with others (Silva-Sánchez et al. 2019). This suggests that the pH is not a direct limiting factor for saprotrophic fungi in the current study, as similarly observed by Rousk et al. (2009) for pH-H2O < 4.5. In this case, the pH effects previously identified by Moran-Rodas et al. (2022) may rather indicate the indirect effects of lower plant productivity, lower fresh-C inputs, and competitive interactions with bacteria in the long term. The increases in fungal biomass promoted by litter addition were related to a higher CUEMB. Furthermore, the less MBCsoc, the higher CUEMB. Apparently, fungi that preferentially utilize fresh substrate inputs incorporate the litter-derived C into MBC, making the community more efficient. This greater capability of fungi to incorporate litter-derived C into their biomass has already been observed (Wei et al. 2022). Other groups that preferentially feed on the original SOC may be the main reason for the increases in priming and the decrease in CUE. The competitive interaction between these distinct groups that form microbial biomass is reflected by a negative correlation between fungal biomass and MBCsoc. These findings are in agreement with studies that suggest community characteristics (composition, diversity) as major drivers of CUE (Domeignoz-Horta et al. 2020; Kallenbach et al. 2016) and/or priming (Nottingham et al. 2009).
Carbon use efficiency measurements and their implications
The similar CUEMB values between the soils are the result of quite different combinations in the proportions of Cmaize recovered in the different pools. In the Nitisol, microbial communities assimilated more litter-derived C, but also respired more, resulting in a larger CO2Cmaize fraction, whereas in the unlimed Acrisol microbial communities assimilated less Cmaize and respired less. Hence, more Cmaize was recovered in the POM pool of the latter. Thus, the results of the CUE indicate that, from a broader perspective, the Acrisol is more efficient, as it produces a similar number of microbial residues (~ 47%) while consuming less POMC, compared with the reference Nitisol. The proportion of microbial residues found is consistent with recent findings within a similar timeframe (Geyer et al. 2020).
Our CUEMB values lay in the range of 15–20%, which is similar to those of Schroeder et al. (2020) of ~ 15% and of Börger et al. (2022) of ~ 17%, using the same approach as that applied in this study. CUE values were greater than CUEMB values in this study. This was very much in line with results found by Geyer et al. (2020), who used the concept of carbon stabilization efficiency “CSE” to compare it with CUEMB from several studies. Even if CUEMB values were obtained by short-term incubations with glucose addition in their case, their ranges of CSE and CUEMB resemble ours. This highlights the importance of the fractions included for the calculation of CUE values and their interpretation. Most studies evaluating CUE used CUEMB approaches based on short incubation periods and labile substrates. Our CUE can provide an insight into additional pools such as microbial residues for an intermediate period, as well as intermediate trends on SOC pathways.
Conclusions
Our 42-day incubation study revealed decreases in CUE, increases in litter mineralization, and increases in priming of SOC as a function of soil pH, refuting our first and second hypotheses. The higher CUE in the Acrisol compared with the Nitisol was mainly due to lower maize-derived CO2C production from reduced litter decomposition by the microbial community under lower pH. The fungal biomass was not affected by pH but was associated with a more efficient microbial community, confirming our third hypothesis. Saprotrophic fungi were responsible for increases in CUEMB by the incorporation of maize litter into microbial biomass. These results suggest that the low SOC content in the Acrisol is due to a low input of plant residues in the field and not to a lower CUE, while liming only moderately increased SOC mineralization and litter consumption. Furthermore, our CUE–CUEMB comparison confirms that not accounting for undecomposed maize and microbial residues underestimates CUE of litter-amended soils. Longer-term studies may provide further information on substrate-C turnover and the persistence of the observed effects on CUE and priming.
References
Aitken R, Moody P, Mckinley P (1990) Lime requirement of acidic Queensland soils I Relationships between soil properties and pH buffer capacity. Soil Res 28:695. https://doi.org/10.1071/SR9900695
Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nature Geosci 3:336–340. https://doi.org/10.1038/ngeo846
Bailey JS, Stevens RJ, Kilpatrick DJ (1991) A rapid method for predicting the lime requirement of acidic temperate soils with widely varying organic matter contents. In: Wright RJ, Baligar VC, Murrmann RP (eds) Plant-soil interactions at low pH. Springer, Dordrecht, Netherlands, pp 253–262
Balesdent J, Mariotti A (1996) Measurement of soil organic matter turn-over using 13C natural abundance. In: Boutton TW, Yamasaki SI (eds) Mass spectrometry of soils. Marcel Dekker, New York, pp 83–111
Börger M, Bublitz T, Dyckmans J, Wachendorf C, Joergensen RG (2022) Microbial carbon use efficiency of litter with distinct C/N ratios in soil at different temperatures, including microbial necromass as growth component. Biol Fertil Soils 58:761–770. https://doi.org/10.1007/s00374-022-01656-7
Bramble DSE, Gouveia GA, Ramnarine R, Farrell RE (2021) Organic residue and agricultural lime interactions on CO2 emissions from two contrasting soils: implications for carbon management in acid soils. J Soils Sediments 21:172–188. https://doi.org/10.1007/s11368-020-02736-7
Canini F, Zucconi L, Pacelli C, Selbmann L, Onofri S, Geml J (2019) Vegetation, pH and water content as main factors for shaping fungal richness, community composition and functional guilds distribution in soils of Western Greenland. Front Microbiol 10:2348. https://doi.org/10.3389/fmicb.2019.02348
Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix ML, Wall DH, Parton WJ (2015) Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nature Geosci 8:776–779. https://doi.org/10.1038/ngeo2520
Djajakirana G, Joergensen RG, Meyer B (1996) Ergosterol and microbial biomass relationship in soil. Biol Fertil Soils 22:299–304
Domeignoz-Horta LA, Pold G, Liu XJ, Frey SD, Melillo JM, DeAngelis KM (2020) Microbial diversity drives carbon use efficiency in a model soil. Nat Commun 11:3684. https://doi.org/10.1038/s41467-020-17502-z
Geyer KM, Kyker-Snowman E, Grandy AS, Frey SD (2016) Microbial carbon use efficiency: accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry 127:173–188. https://doi.org/10.1007/s10533-016-0191-y
Geyer K, Schnecker J, Grandy AS, Richter A, Frey S (2020) Assessing microbial residues in soil as a potential carbon sink and moderator of carbon use efficiency. Biogeochemistry 151:237–249. https://doi.org/10.1007/s10533-020-00720-4
Horn EL, Cooledge EC, Jones DL, Hoyle FC, Brailsford FL, Murphy DV (2021) Addition of base cations increases microbial carbon use efficiency and biomass in acidic soils. Soil Biol Biochem 161:108392. https://doi.org/10.1016/j.soilbio.2021.108392
Islam MA, Milham PJ, Dowling PM, Jacobs BC, Garden DL (2004) Improved procedures for adjusting soil pH for pot experiments. Commun Soil Sci Plant Anal 35:25–37. https://doi.org/10.1081/CSS-120027632
IUSS Working Group WRB (2015) World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps. FAO, Rome
Joergensen RG, Wichern F (2018) Alive and kicking: why dormant soil microorganisms matter. Soil Biol Biochem 116:419–430. https://doi.org/10.1016/j.soilbio.2017.10.022
Jones DL, Hill PW, Smith AR, Farrell M, Ge T, Banning NC, Murphy DV (2018) Role of substrate supply on microbial carbon use efficiency and its role in interpreting soil microbial community-level physiological profiles (CLPP). Soil Biol Biochem 123:1–6. https://doi.org/10.1016/j.soilbio.2018.04.014
Jones DL, Cooledge EC, Hoyle FC, Griffiths RI, Murphy DV (2019) pH and exchangeable aluminum are major regulators of microbial energy flow and carbon use efficiency in soil microbial communities. Soil Biol Biochem 138:107584. https://doi.org/10.1016/j.soilbio.2019.107584
Kallenbach CM, Frey SD, Grandy AS (2016) Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat Commun 7:13630. https://doi.org/10.1038/ncomms13630
Lal R (2004) Soil carbon sequestration in India. Clim Change 65:20
Li J, Wang G, Mayes MA, Allison SD, Frey SD, Shi Z, Hu XM, Luo Y, Melillo JM (2019) Reduced carbon use efficiency and increased microbial turnover with soil warming. Glob Change Biol 25:900–910. https://doi.org/10.1111/gcb.14517
Liang C, Schimel JP, Jastrow JD (2017) The importance of anabolism in microbial control over soil carbon storage. Nat Microbiol 2:17105. https://doi.org/10.1038/nmicrobiol.2017.105
Liang C, Amelung W, Lehmann J, Kästner M (2019) Quantitative assessment of microbial necromass contribution to soil organic matter. Glob Change Biol 25:3578–3590. https://doi.org/10.1111/gcb.14781
Liu W, Qiao C, Yang S, Bai W, Liu L (2018) Microbial carbon use efficiency and priming effect regulate soil carbon storage under nitrogen deposition by slowing soil organic matter decomposition. Geoderma 332:37–44. https://doi.org/10.1016/j.geoderma.2018.07.008
Magid J, Kjærgaard C (2001) Recovering decomposing plant residues from the particulate soil organic matter fraction: size versus density separation. Biol Fertil Soils 33:252–257. https://doi.org/10.1007/s003740000316
Malik AA, Puissant J, Buckeridge KM, Goodall T, Jehmlich N, Chowdhury S, Gweon HS, Peyton JM, Mason KE, van Agtmaal M, Blaud A (2018) Land use driven change in soil pH affects microbial carbon cycling processes. Nat Commun 9:3591. https://doi.org/10.1038/s41467-018-05980-1
Manzoni S, Taylor P, Richter A, Porporato A, Ågren GI (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196:79–91. https://doi.org/10.1111/j.1469-8137.2012.04225.x
Mason-Jones K, Schmücker N, Kuzyakov Y (2018) Contrasting effects of organic and mineral nitrogen challenge the N-Mining Hypothesis for soil organic matter priming. Soil Biol Biochem 124:38–46. https://doi.org/10.1016/j.soilbio.2018.05.024
Miltner A, Bombach P, Schmidt-Brücken B, Kästner M (2012) SOM genesis: microbial biomass as a significant source. Biogeochemistry 111:41–55. https://doi.org/10.1007/s10533-011-9658-z
Mo F, Zhang YY, Liu Y, Liao YC (2021) Microbial carbon-use efficiency and straw-induced priming effect within soil aggregates are regulated by tillage history and balanced nutrient supply. Biol Fertil Soils 57:409–420. https://doi.org/10.1007/s00374-021-01540-w
Moran-Rodas VE, Chavannavar SV, Joergensen RG, Wachendorf C (2022) Microbial response of distinct soil types to land-use intensification at a South-Indian rural-urban interface. Plant Soil 473:389–405. https://doi.org/10.1007/s11104-021-05292-2
Muhammad S, Müller T, Joergensen RG (2006) Decomposition of pea and maize straw in Pakistani soils along a gradient in salinity. Biol Fertil Soils 43:93–101. https://doi.org/10.1007/s00374-005-0068-z
Murugan R, Parama VRR, Madan B, Muthuraju R, Ludwig B (2019) Short-term effect of nitrogen intensification on aggregate size distribution, microbial biomass and enzyme activities in a semi-arid soil under different crop types. Pedosphere 29:483–491. https://doi.org/10.1016/S1002-0160(19)60802-7
Nelson PN, Su N (2010) Soil pH buffering capacity: a descriptive function and its application to some acidic tropical soils. Soil Res 48:201. https://doi.org/10.1071/SR09150
Nottingham AT, Griffiths H, Chamberlain PM, Stott AW, Tanner EV (2009) Soil priming by sugar and leaf-litter substrates: a link to microbial groups. Appl Soil Ecol 42:183–190. https://doi.org/10.1016/j.apsoil.2009.03.003
Olego MÁ, De Paz JM, Visconti F, Garzón JE (2014) Predictive modelling of soil aluminium saturation as a basis for liming recommendations in vineyard acid soils under Mediterranean conditions. J Soil Sci Plant Nutr 60:695–707. https://doi.org/10.1080/00380768.2014.930333
Oliver EE, Houlton BZ, Lipson DA (2021) Controls on soil microbial carbon use efficiency over long-term ecosystem development. Biogeochemistry 152:309–325. https://doi.org/10.1007/s10533-021-00758-y
Pei J, Li J, Mia S, Singh B, Wu J, Dijkstra FA (2021) Biochar aging increased microbial carbon use efficiency but decreased biomass turnover time. Geoderma 382:114710. https://doi.org/10.1016/j.geoderma.2020.114710
Prasad JVNS, Rao ChS, Srinivas K, Jyothi CN, Venkateswarlu B, Ramachandrappa BK, Dhanapal GN, Ravichandra K, Mishra PK (2016) Effect of ten years of reduced tillage and recycling of organic matter on crop yields, soil organic carbon and its fractions in Alfisols of semi arid tropics of southern India. Soil Tillage Res 156:131–139. https://doi.org/10.1016/j.still.2015.10.013
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rousk J, Brookes PC, Bååth E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol 75:1589–1596. https://doi.org/10.1128/AEM.02775-08
Sathish A, Ramachandrappa BK, Shankar MA, Srikanth Babu PN, Srinivasarao CH, Sharma KL (2016) Long-term effects of organic manure and manufactured fertilizer additions on soil quality and sustainable productivity of finger millet under a finger millet-groundnut cropping system in southern India. Soil Use Manag 32:311–321. https://doi.org/10.1111/sum.12277
Schroeder J, Jannoura R, Beuschel R, Pfeiffer B, Dyckmans J, Murugan R, Chavannavar S, Wachendorf C, Joergensen RG (2020) Carbon use efficiency and microbial functional diversity in a temperate Luvisol and a tropical Nitisol after millet litter and N addition. Biol Fertil Soils 56:1139–1150. https://doi.org/10.1007/s00374-020-01487-4
Silva-Sánchez A, Soares M, Rousk J (2019) Testing the dependence of microbial growth and carbon use efficiency on nitrogen availability, pH, and organic matter quality. Soil Biol Biochem 134:25–35. https://doi.org/10.1016/j.soilbio.2019.03.008
Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A (2013) Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol Lett 16:930–939. https://doi.org/10.1111/ele.12113
Spohn M, Pötsch EM, Eichorst SA, Woebken D, Wanek W, Richter A (2016) Soil microbial carbon use efficiency and biomass turnover in a long-term fertilization experiment in a temperate grassland. Soil Biol Biochem 97:168–175. https://doi.org/10.1016/j.soilbio.2016.03.008
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Wang C, Zhou X, Guo D, Zhao JH, Yan L, Feng GZ, Gao Q, Yu H, Zhao LP (2019) Soil pH is the primary factor driving the distribution and function of microorganisms in farmland soils in northeastern China. Ann Microbiol 69:1461–1473. https://doi.org/10.1007/s13213-019-01529-9
Wang C, Qu L, Yang L, Liu D, Morrissey E, Miao R, Liu Z, Wang Q, Fang Y, Bai E (2021) Large-scale importance of microbial carbon use efficiency and necromass to soil organic carbon. Glob Change Biol 27:2039–2048. https://doi.org/10.1111/gcb.15550
Wei Y, Xiong X, Ryo M, Badgery WB, Bi Y, Yang G, Zhang Y, Liu N (2022) Repeated litter inputs promoted stable soil organic carbon formation by increasing fungal dominance and carbon use efficiency. Biol Fertil Soils 58:619–631. https://doi.org/10.1007/s00374-022-01647-8
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction—an automated procedure. Soil Biol Biochem 22:1167–1169. https://doi.org/10.1016/0038-0717(90)90046-3
Xiao Q, Huang Y, Wu L, Tian Y, Wang Q, Wang B, Xu M, Zhang W (2021) Long-term manuring increases microbial carbon use efficiency and mitigates priming effect via alleviated soil acidification and resource limitation. Biol Fertil Soils 57:925–934. https://doi.org/10.1007/s00374-021-01583-z
Zhang X, Guo J, Vogt RD et al (2020) Soil acidification as an additional driver to organic carbon accumulation in major Chinese croplands. Geoderma 366:114234. https://doi.org/10.1016/j.geoderma.2020.114234
Acknowledgements
We are grateful to the colleagues from the University of Agricultural Science Bangalore, especially to Prof. Dr. Subbarayappa, Dr. Hanumanthappa, and Dr. Mudalagiriyappa for the management of the Acrisol and Nitisol fields. We are grateful to Gabriele Dormann for her technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. This project was funded by the Graduate Program BangaDyn from the University of Kassel. V.E.M-R. received additional funding from the STIBET program from DAAD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moran-Rodas, V.E., Joergensen, R.G. & Wachendorf, C. Does liming improve microbial carbon use efficiency after maize litter addition in a tropical acidic soil?. Biol Fertil Soils 59, 619–627 (2023). https://doi.org/10.1007/s00374-023-01722-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-023-01722-8