Abstract
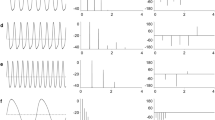
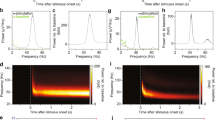
An apteronotid weakly electric fish, Adontosternarchus, emits high-frequency electric organ discharges (700–1500 Hz) which are stable in frequency if no other fish or artificial signals are present. When encountered with an artificial signal of higher frequency than the fish’s discharge, the fish raised its discharge frequency and eventually matched its own frequency to that of the artificial signal. At this moment, phase locking was observed, where the timing of the fish’s discharge was precisely stabilized at a particular phase of the artificial signal over a long period of time (up to minutes) with microsecond precision. Analyses of the phase-locking behaviors revealed that the phase values of the artificial stimulus at which the fish stabilizes the phase of its own discharge (called lock-in phases) have three populations between −180° and +180°. During the frequency rise and the phase-locking behavior, the electrosensory system is exposed to the mixture of feedback signals from its electric organ discharges and the artificial signal. Since the signal mixture modulates in both amplitude and phase, we explored whether amplitude or phase information participated in driving the phase-locking behavior, using a numerical model. The model which incorporates only amplitude information well predicted the three populations of lock-in phases. When phase information was removed from the electrosensory stimulus, phase-locking behavior was still observed. These results suggest that phase-locking behavior of Adontosternarchus requires amplitude information but not phase information available in the electrosensory stimulus.







Similar content being viewed by others
Abbreviations
- AM:
-
Amplitude modulation
- Df :
-
Frequency difference: f st − f eod
- EOD:
-
Electric organ discharge
- f eod :
-
Frequency of electric organ discharge
- f st :
-
Frequency of electrosensory stimulus
- f rest :
-
Resting frequency of electric organ discharge
- LIP:
-
Lock-in phase: the value of ψ at which fish maintains phase locking
- PLB:
-
Phase-locking behavior
- ψ :
-
Phase difference defined as the phase of the stimulus in reference to the Phase of the EOD: ψ = [stimulus phase] − [EOD phase]
- ST:
-
Electrosensory stimulus
References
Baker CLJ (1980) Jamming avoidance behavior in gymnotoid electric fish with pulse-type discharges: sensory encoding for a temporal pattern discrimination. J Comp Physiol 136:165–181
Bastian J, Chacron MJ, Maler L (2002) Receptive field organization determines pyramidal cell stimulus-encoding capability and spatial stimulus selectivity. J Neurosci 22(11):4577–4590
Bennett MVL (1971) Electric organs. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 5. Academic Press, New York, pp 493–574
Bullock T, Hamstra R, Scheich H (1972) The jamming avoidance response of high frequency electric fish. II. Quantitative aspects. J Comp Physiol 77:23–48
Bullock TH, Behrend K, Heiligenberg W (1975) Comparison of the jamming avoidance responses in Gymnotoid and Gymnarchid electric fish: a case of convergent evolution of behavior and its sensory basis. J Comp Physiol 103:97–121
Carlson BA, Kawasaki M (2006) Ambiguous encoding of stimuli by primary sensory afferents causes a lack of independence in the perception of multiple stimulus attributes. J Neurosci 26(36):9173–9183
Carlson BA, Kawasaki M (2007) Behavioral responses to jamming and ‘phantom’ jamming stimuli in the weakly electric fish Eigenmannia. J Comp Physiol A 193(9):927–941
Chacron M, Doiron B, Maler L, Longtin A, Bastian J (2003) Non-classical receptive field mediated switch in a sensory neuron’s frequency tuning. Nature 423:77–81
Crampton WGR, Albert JS (2006) Evolution of electric signal diversity in gymnotiform fishes. In: Ladich F, Collin SP, Moller P, Kapoor BG (eds) Communication in fishes, vol 2. Science Publishers, Enfield, pp 647–731
Dye J (1987) Dynamics and stimulus-dependence of pacemaker control during behavioral modulations in the weakly electric fish, Apteronotus. J Comp Physiol A 161(2):175–185
Gottschalk B, Scheich H (1979) Phase sensitivity and phase coupling: common mechanisms for communication behaviors in gymnotid wave and pulse species. Behav Ecol Sociobiol 4(4):395–408
Heiligenberg W (1973) Electrolocation of objects in the electric fish Eigenmannia (Rhamphichthydae, Gymotoidei). J Comp Physiol 87:137–164
Heiligenberg W (1980) The evaluation of electroreceptive feedback in a gymnotoid fish with pulse-type electric organ discharges. J Comp Physiol 138:173–185
Heiligenberg W (1991) Neural nets in electric fish. The MIT Press, Cambridge, MA
Heiligenberg W, Bastian J (1980) The control of Eigenmannia’s pacemaker by distributed evaluation of electroreceptive afferences. J Comp Physiol 136:113–133
Heiligenberg W, Metzner W, Wong C, Keller C (1996) Motor control of the jamming avoidance response of Apteronotus leptorhynchus: evolutionary changes of a behavior and its neuronal substrate. J Comp Physiol 179:653–674
Hopkins CD (1986) Behavior of Mormyridae. In: Bullock TH, Heiligenberg W (eds) Electroreception. Wiley, New York, pp 527–576
Huang C, Chacron MJ (2014) Differential neural responses to naturally occurring envelopes in the electrosensory system. BMC Neurosci 15(1):P192. doi:10.1186/1471-2202-15-s1-p192
Kawasaki M (2009) Evolution of time-coding systems in weakly electric fishes. Zoolog Sci 26(9):587–599
Kawasaki M, Prather J, Guo Y-X (1996) Sensory cues for the gradual frequency fall responses of the gymnotiform electric fish, Rhamphichthys rostratus. J Comp Physiol 178:453–462
Konishi M (1996) Neuroethology of orientation and navigation. Biol Bull 191:101–102
Langner G, Scheich H (1978) Active phase coupling in electric fish—behavioral control with microsecond precision. J Comp Physiol 128(3):235–240
Livingstone M, Hubel D (1988) Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science 240:740–749
Mago-Leccia F (1994) Electric fishes of the continental waters of America. Fudeci, Caracas
Matsushita A, Pyon G, Kawasaki M (2012) Time disparity sensitive behavior and its neural substrates of a pulse-type gymnotiform electric fish, Brachyhypopomus gauderio. J Comp Physiol A 199(7):583–599
Quintana L, Sierra F, Silva A, Macadar O (2011) A central pacemaker that underlies the production of seasonal and sexually dimorphic social signals: functional aspects revealed by glutamate stimulation. J Comp Physiol 197(2):211–225. doi:10.1007/s00359-010-0603-8
Saunders J, Bastian J (1984) The physiology and morphology of two types of electrosensory neurons in the weakly electric fish Apteronotus leptorhynchus. J Comp Physiol 154:199–209
Takizawa Y, Rose GJ, Kawasaki M (1999) Resolving competing theories for control of the jamming avoidance response: the role of amplitude modulations in electric organ discharge decelerations. J Exp Biol 202:1377–1386
Zhou MC, Smith GT (2006) Structure and sexual dimorphism of the electrocommunication signals of the weakly electric fish, Adontosternarchus devenanzii. J Exp Biol 209(23):4809–4818. doi:10.1242/jeb.02579
Acknowledgements
We thank Prof. DeForest Mellon and two anonymous referees for critical comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawasaki, M., Leonard, J. Phase-locking behavior in a high-frequency gymnotiform weakly electric fish, Adontosternarchus . J Comp Physiol A 203, 151–162 (2017). https://doi.org/10.1007/s00359-017-1148-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-017-1148-x