Abstract
Purpose
To characterize patient outcomes following visually directed high-intensity focused ultrasound (HIFU) for focal treatment of localized prostate cancer.
Methods
We performed a systematic review of cancer-control outcomes and complication rates among men with localized prostate cancer treated with visually directed focal HIFU. Study outcomes were calculated using a random-effects meta-analysis model.
Results
A total of 8 observational studies with 1,819 patients (median age 67 years; prostate-specific antigen 7.1 mg/ml; prostate volume 36 ml) followed over a median of 24 months were included. The mean prostate-specific antigen nadir following visually directed focal HIFU was 2.2 ng/ml (95% CI 0.9–3.5 ng/ml), achieved after a median of 6 months post-treatment. A clinically significant positive biopsy was identified in 19.8% (95% CI 12.4–28.3%) of cases. Salvage treatment rates were 16.2% (95% CI 9.7–23.8%) for focal- or whole-gland treatment, and 8.6% (95% CI 6.1–11.5%) for whole-gland treatment. Complication rates were 16.7% (95% CI 9.9–24.6%) for de novo erectile dysfunction, 6.2% (95% CI 0.0–19.0%) for urinary retention, 3.0% (95% CI 2.1–3.9%) for urinary tract infection, 1.9% (95% CI 0.1–5.3%) for urinary incontinence, and 0.1% (95% CI 0.0–1.4%) for bowel injury.
Conclusion
Limited evidence from eight observational studies demonstrated that visually directed HIFU for focal treatment of localized prostate cancer was associated with a relatively low risk of complications and acceptable cancer control over medium-term follow-up. Comparative, long-term safety and effectiveness results with visually directed focal HIFU are lacking.
Similar content being viewed by others
Introduction
Over 1.4 million men worldwide receive a prostate cancer (PCa) diagnosis each year [1], and 1 in 8 men receive this diagnosis during their lifetime [2]. Approximately 87% of these cancers are localized to the prostate without the involvement of nearby organs [3]. While whole-gland tumors are typically treated with radical radiotherapy or prostatectomy, localized tumors may be treated with organ-sparing focal therapies intended to minimize side effects while bridging active surveillance and radical treatment in low- and intermediate-risk patients. High-intensity focused ultrasound (HIFU) is a therapy for PCa that targets energy at the index lesion, resulting in coagulating necrosis of malignant tissue by thermal and mechanical effects while sparing the surrounding non-cancerous prostatic tissue. HIFU is an attractive option for focal therapy of localized tumors, since the lesion with the largest focus of cancer largely determines patient prognosis and metastases risk [4].
HIFU can be classified into algorithm-directed or visually directed treatment protocols. Algorithm-directed HIFU assumes specific tissue-related properties, tissue homogeneity, and fixed ultrasound absorption coefficients that produce thermal ablation using pre-defined power/time combinations at given tissue depths. In contrast, visually directed HIFU allows the user to view prostate tissue changes in real time and make power adjustments to account for natural tissue variability. While several systematic reviews have summarized safety and effectiveness outcomes with HIFU for PCa [5,6,7,8], none have reported outcomes of focal therapy with visually directed HIFU. Therefore, the purpose of this systematic review with meta-analysis was to characterize cancer-control outcomes and complications following visually directed HIFU for focal treatment of PCa.
Methods
The systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [9]. The review protocol was prospectively registered at http://www.researchregistry.com (reviewregistry1564).
Study eligibility criteria
Randomized trials and observational studies of visually directed HIFU for focal treatment of PCa were eligible for inclusion in the systematic review. We excluded studies of algorithm-directed HIFU, studies reporting combined results of algorithm- and visually directed HIFU, studies of whole-gland HIFU, studies of salvage HIFU, studies of combination therapy, studies published in abstract form only, review articles or commentaries, studies with insufficient sample size (< 10 patients), studies published in non-English journals, and studies that did not report any outcomes specified in this review.
Search strategy and study selection process
Two researchers (LM, DF) with experience in systematic review methodology independently searched Medline, Embase, and the Cochrane Central Register of Controlled Trials for potentially eligible studies. The pre-defined search strategies included combinations of diagnosis- and procedure-specific keywords. The Medline search strategy is provided in Supplement Table 1; search strategies for other databases were adapted as necessary. We also manually searched the Directory of Open Access Journals, Google Scholar, and the reference lists of eligible papers and relevant review articles. To account for multiple papers derived from the same primary study or subsamples of the primary study, we preferentially extracted data from the paper with the largest sample size and supplemented missing data using secondary sources as needed. This was an essential element of the review, since previous reviews of HIFU for PCa have included duplicate publications in the analysis. Disagreements related to study eligibility were resolved by discussion. The last search was performed in December 2022.
Data extraction and outcomes
Data were independently extracted from eligible studies using standardized data collection forms, which included study characteristics, patient characteristics, treatment data, study methodological quality, and main outcomes. Data extraction discrepancies between researchers were resolved by discussion. The methodological quality of eligible studies was evaluated with The National Institute of Health assessment tool applied to before-after studies [10]. Outcomes of this review included prostate-specific antigen (PSA) nadir, the proportion of patients with clinically significant positive biopsy, the proportion of patients receiving whole-gland or focal salvage treatment, the proportion of patients receiving whole-gland salvage treatment, and the prevalence of complications including de novo erectile dysfunction (ED), urinary retention, urinary tract infection, urinary incontinence, and bowel injury.
Data analysis
We used a random-effects meta-analysis model to calculate a weighted estimate and 95% confidence interval (CI) for each outcome. We estimated heterogeneity among studies with the I2 statistic where a value of 0% represented no heterogeneity and larger values represented increasing heterogeneity. We evaluated the robustness of the meta-analysis conclusions with a one-study removed sensitivity analysis where the analysis was recalculated following iterative one-at-a-time removal of each study. We performed meta-regressions to identify potential prognostic factors for outcomes reported in at least six studies and with substantial heterogeneity (I2 > 50%) [11, 12]. The variables of interest included in the meta-regression were patient age, baseline PSA, prostate volume, percentage of patients with extra-prostatic tumor (cT3), percentage of patients receiving neoadjuvant androgen deprivation therapy, median year of treatment, and duration of post-treatment follow-up. Potential publication bias was assessed by visually examining funnel plot symmetry.
Results
Study selection
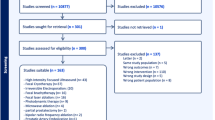
Among 312 papers identified in the literature search, 8 observational studies [13,14,15,16,17,18,19,20] with supplemental data derived from 9 duplicate publications [21,22,23,24,25,26,27,28,29] were included in the systematic review (Supplement Fig. 1).
Study characteristics and risk of bias
The review included 1819 unique patients from 5 countries treated with visually directed focal HIFU from 2003 to 2021. The treatment plans for focal HIFU varied widely among studies, ranging from no more than quadrant ablation [20] to urethra-sparing subtotal ablation [18]. The percentage of treated prostate volume was rarely reported. Follow-up duration after visually directed focal HIFU ranged from 6 to 36 months (median 24 months) (Table 1). Among the included studies, the mean patient age ranged from 64 to 72 years (median 67 years), baseline PSA ranged from 5.4 to 8.7 mg/ml (median 7.1 ng/ml), prostate volume ranged from 24 to 46 ml (median 36 ml), the percentage of patients receiving neoadjuvant androgen deprivation therapy ranged from 0 to 27% (median 13%), and most patients were staged as cT1 or cT2 (Table 2). Study quality was rated good for seven studies, fair for one study, and poor for none (Supplement Table 2).
Meta-analysis results
The mean PSA nadir following visually directed focal HIFU varied considerably among studies, ranging from 0.1 to 3.5 ng/ml. The weighted PSA nadir was 2.2 ng/ml (95% CI 0.9–3.5; I2 = 98%), which was achieved after a median of 6 months post-treatment (Fig. 1). Over a median of 9 month follow-up, a clinically significant positive biopsy was identified in 19.8% (95% CI 12.4–28.3%; I2 = 83%) of cases (Fig. 2). The rates of salvage treatment were 16.2% (95% CI 9.7–23.8%; I2 = 84%) for focal- or whole-gland treatment (Fig. 3) and 8.6% (95% CI 6.1–11.5%; I2 = 35%) for whole-gland treatment (Fig. 4). Complications with visually directed focal HIFU are summarized in Fig. 5. The weighted rates of specific complications were 16.7% (95% CI 9.9–24.6%; I2 = 63%) for de novo ED (Supplement Fig. 2), 6.2% (95% CI 0.0–19.0%; I2 = 95%) for urinary retention (Supplement Fig. 3), 3.0% (95% CI 2.1–3.9%; I2 = 0%) for urinary tract infection (Supplement Fig. 4), 1.9% (95% CI 0.1–5.3%; I2 = 71%) for urinary incontinence (Supplement Fig. 6), and 0.1% (95% CI 0.0–1.4%; I2 = 66%) for bowel injury [all rectourethral fistulae] (Supplement Fig. 6).
The meta-analysis results were largely unchanged in the one-study removed sensitivity analyses, suggesting minimal single-study influences on overall outcomes (Supplement Table 3). The meta-regression findings are reported in Supplement Table 4. Larger prostate volume was associated (p = 0.002) with a higher clinically significant positive biopsy rate (Supplement Fig. 7), and longer follow-up duration was associated (p = 0.007) with lower rates of de novo ED (Supplement Fig. 8). No patient or study characteristic was associated with PSA nadir or the risk of salvage treatment. Funnel plot asymmetry was not evident for any outcome; a formal assessment of publication bias was not performed due to the small number of studies in the review.
Discussion
Patients with small-volume prostate tumors may be unnecessarily overtreated with whole-gland PCa treatments, which are associated with considerable morbidity [30,31,32]. Focal treatment approaches are ideal for men with small-volume, single-lobe prostatic tumors who wish to preserve erectile function and continence. We performed the first known meta-analysis of visually directed HIFU for focal treatment of localized PCa. There were several major findings in this meta-analysis of 8 studies comprising 1819 patients treated with visually directed focal HIFU. First, the risks of urinary complications and de novo ED following visually directed focal HIFU were low. Second, visually directed focal HIFU conferred promising cancer-control outcomes with acceptable recurrence rates and 91.4% freedom from whole-gland salvage treatment over mid-term follow-up. Finally, the variability in results among studies included in this meta-analysis was high and only partly explained using meta-regression techniques.
Although there is no consensus for biochemical failure definition after focal therapy [33], a PSA decrease of 70% or greater indicates proper ablation of the index cancer [34,35,36]. PSA levels after visually directed focal HIFU decreased by approximately 70% among the studies in this review. However, considerable variability was observed in the PSA nadirs in this review (I2 = 98%). Although we did not identify patient- or study-related factors that influenced the PSA nadir, it is plausible that unmeasured factors, such as the extent and location of malignancy or the volume of ablated tissue, may have contributed to the inconsistency among studies. For example, Yee et al. [20] treated the smallest prostate volume and reported the highest PSA nadir. Conversely, Shoji et al. [18] treated the largest prostate volume and reported the lowest PSA nadir. While objective analysis of this association was not possible due to inadequate reporting of treatment details among studies, a negative association between treated prostate volume and PSA nadir was apparent. Since PSA has poor sensitivity to predict positive biopsies after focal HIFU [37], the clinical importance of these results is unclear.
The weighted rate of clinically significant positive biopsy after visually directed focal HIFU was 19.8%, ranging from 8.9% [18, 19] to 42.5% [13]. This heterogeneity was partially explained in meta-regression where larger prostate volume was associated with higher rates of a clinically significant positive biopsy. While posterior tumors are easily accessible even in larger prostates, HIFU effectiveness in anterior tumors may be limited in larger prostates where energy penetration may be insufficient [26]. Prostate downsizing with neoadjuvant TURP may be considered in patients with larger prostates (> 50 cc) to remove prostatic calcification or abscesses that could attenuate HIFU energy. Unfortunately, the relationship between tumor location and prostate volume was unclear in this review. A second possible reason for the variability in clinically significant positive biopsy rate was that repeat biopsy was performed routinely in some studies, while others reserved re-biopsy only for suspected recurrence or high-risk patients.
An advantage of visually directed HIFU is the ability to make real-time power adjustments based on hyperechoic changes visualized on B-mode ultrasound images. However, only one known study has directly compared the outcomes of visually directed HIFU with algorithm-directed HIFU. In the observational study of Illing et al. [38], men treated with visually directed HIFU for localized PCa achieved statistically lower PSA nadirs than those receiving algorithm-directed HIFU, while rates of urinary complications were numerically lower. Additional support for the potential clinical advantages of visually directed focal HIFU comes from comparing the results of the current review with visually directed focal HIFU to a previous review of focal HIFU in which 67% of studies used algorithm-based HIFU [7]. In that review, the mean PSA nadir ranged from 1.9 to 2.7 ng/ml (vs. 2.2 ng/ml in the current review), the rate of positive biopsy ranged from 14 to 38% (vs. 19.8%), and the incidence of complications was 21% for ED (vs. 16.7%), 11% for urinary tract infection (vs. 3.0%), 9% for retention (vs. 6.2%), and 2% for incontinence (vs. 1.9%). Due to a lack of comparative studies, whether cancer-control outcomes differ between visually directed and algorithm-directed HIFU remains to be determined and warrants further study. Further, no known studies have directly compared the safety or effectiveness of visually directed focal HIFU to active surveillance, radiotherapy, or surgery; thus, any treatment comparisons with visually directed focal HIFU should be considered hypothesis-generating only.
We observed a lower risk of de novo ED in studies with longer follow-up duration. However, only some studies in this review reported temporal trends in ED. In Lovegrove et al. [28], the percentage of men with ED was 10% pre-treatment, increasing to 21% at 1–2 years, and declining to 18% at 2–3 years. In Shoji et al. [18], de novo ED rates decreased during follow-up, from 55% at 3 months, 45% at 6 months, 40% at 1 year, and 37% at 2 years. In Shoji et al. [19], de novo ED rates were 33% at 1 month, 19% at 3 months, 12% at 6 months, 9% at 9 months, and 14% at 1 year. In contrast to these studies, Yee et al. [20] reported increasing ED rates over time, with 0% at baseline, 15% at 3 months, and 30% at 6 months. Overall, most evidence suggests that de novo ED after focal HIFU may be temporary in some men, a finding reported in other reviews [5].
Several limitations pertaining to the quality of the studies included in this review warrant discussion. First, while the high observed heterogeneity in cancer-control outcomes and complications after visually directed focal HIFU afforded the opportunity to explore factors associated with these outcomes, the results of the meta-analysis should be interpreted cautiously. Meta-analysis results are prone to ecological fallacy risks, since inference about individuals is attempted using only study-level information [39]. Additionally, meta-regression is inherently an exploratory analysis considered hypothesis-generating only, and the number of studies available for meta-regression was limited. Consequently, readers are cautioned against drawing causal inferences from the results of this study. Second, the evidence from this review was derived exclusively from observational studies, which have limited internal validity, since they are prone to bias and confounding risks. No clear evidence exists that focal HIFU improves cancer control, quality of life, or comorbidities relative to radiation, surgery, or other focal treatments. Finally, this meta-analysis included results obtained during short- and medium-term follow-up. Although a minimum of 5 years of follow-up was recommended in a Delphi consensus of focal therapies for PCa [33], none of the studies in this review followed patients for this duration. Overall, long-term cancer-control results following visually directed focal HIFU are lacking.
Conclusion
Limited evidence from eight observational studies demonstrated that visually directed HIFU for focal treatment of localized PCa was associated with a relatively low risk of complications and acceptable cancer control over medium-term follow-up. Future comparative studies with longer term follow-up are warranted to further elucidate the safety and effectiveness of visually directed HIFU for focal treatment of localized PCa.
Data availability
The data from this review will be made available upon reasonable request.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Siegel RL, Miller KD, Fuchs HE et al (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33
Jemal A, Culp MB, Ma J et al (2021) Prostate cancer incidence 5 years after US Preventive Services Task Force recommendations against screening. J Natl Cancer Inst 113:64–71
Ahmed HU (2009) The index lesion and the origin of prostate cancer. N Engl J Med 361:1704–1706
Bakavicius A, Marra G, Macek P et al (2022) Available evidence on HIFU for focal treatment of prostate cancer: a systematic review. Int Braz J Urol 48:263–274
Guo RQ, Guo XX, Li YM et al (2021) Cryoablation, high-intensity focused ultrasound, irreversible electroporation, and vascular-targeted photodynamic therapy for prostate cancer: a systemic review and meta-analysis. Int J Clin Oncol 26:461–484
He Y, Tan P, He M et al (2020) The primary treatment of prostate cancer with high-intensity focused ultrasound: a systematic review and meta-analysis. Medicine (Baltimore) 99:e22610
Hopstaken JS, Bomers JGR, Sedelaar MJP et al (2022) An updated systematic review on focal therapy in localized prostate cancer: what has changed over the past 5 years? Eur Urol 81:5–33
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65-94
National Institute of Health Quality Assessment Tool for before-after (pre-post) studies with no control group. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 19 Sept 2023
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Fu R, Gartlehner G, Grant M et al (2010) Conducting quantitative synthesis when comparing medical interventions: AHRQ and the effective health care program. Methods guide for effectiveness and comparative effectiveness reviews. Agency for Healthcare Research and Quality, Rockville
Bass R, Fleshner N, Finelli A et al (2019) Oncologic and functional outcomes of partial gland ablation with high intensity focused ultrasound for localized prostate cancer. J Urol 201:113–119
Collins K, Brocken E, Bahler CD et al (2022) High-intensity focused ultrasound for the treatment of prostate cancer: assessing location of failure after focal therapy in prostate cancer and review of histological characteristics and clinicopathologic correlates after treatment-a 5-year experience. Hum Pathol 119:79–84
Khandwala YS, Morisetty S, Ghanouni P et al (2022) Evaluation of post-ablation mpMRI as a predictor of residual prostate cancer after focal high intensity focused ultrasound (HIFU) ablation. Urol Oncol 40:489.e9-489.e17
Muto S, Yoshii T, Saito K et al (2008) Focal therapy with high-intensity-focused ultrasound in the treatment of localized prostate cancer. Jpn J Clin Oncol 38:192–199
Reddy D, Peters M, Shah TT et al (2022) Cancer control outcomes following focal therapy using high-intensity focused ultrasound in 1379 men with nonmetastatic prostate cancer: a multi-institute 15-year experience. Eur Urol 81:407–413
Shoji S, Nakano M, Fujikawa H et al (2015) Urethra-sparing high-intensity focused ultrasound for localized prostate cancer: functional and oncological outcomes. Int J Urol 22:1043–1049
Shoji S, Hiraiwa S, Uemura K et al (2020) Focal therapy with high-intensity focused ultrasound for the localized prostate cancer for Asian based on the localization with MRI-TRUS fusion image-guided transperineal biopsy and 12-cores transperineal systematic biopsy: prospective analysis of oncological and functional outcomes. Int J Clin Oncol 25:1844–1853
Yee CH, Chiu PK, Teoh JY et al (2021) High-intensity focused ultrasound (HIFU) focal therapy for localized prostate cancer with MRI-US fusion platform. Adv Urol 2021:7157973
Ahmed HU, Hindley RG, Dickinson L et al (2012) Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol 13:622–632
Ahmed HU, Dickinson L, Charman S et al (2015) Focal ablation targeted to the index lesion in multifocal localised prostate cancer: a prospective development study. Eur Urol 68:927–936
Dickinson L, Ahmed HU, Hindley RG et al (2017) Prostate-specific antigen vs. magnetic resonance imaging parameters for assessing oncological outcomes after high intensity-focused ultrasound focal therapy for localized prostate cancer. Urol Oncol 35:30.e39-30.e15
Guillaumier S, Peters M, Arya M et al (2018) A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol 74:422–429
Huber PM, Afzal N, Arya M et al (2020) An exploratory study of dose escalation vs standard focal high-intensity focused ultrasound for treating nonmetastatic prostate cancer. J Endourol 34:641–646
Huber PM, Afzal N, Arya M et al (2021) Focal HIFU therapy for anterior compared to posterior prostate cancer lesions. World J Urol 39:1115–1119
Johnston MJ, Emara A, Noureldin M et al (2019) Focal high-intensity focussed ultrasound partial gland ablation for the treatment of localised prostate cancer: a report of medium-term outcomes from a single-center in the United Kingdom. Urology 133:175–181
Lovegrove CE, Peters M, Guillaumier S et al (2020) Evaluation of functional outcomes after a second focal high-intensity focused ultrasonography (HIFU) procedure in men with primary localized, non-metastatic prostate cancer: results from the HIFU Evaluation and Assessment of Treatment (HEAT) registry. BJU Int 125:853–860
Stabile A, Orczyk C, Hosking-Jervis F et al (2019) Medium-term oncological outcomes in a large cohort of men treated with either focal or hemi-ablation using high-intensity focused ultrasonography for primary localized prostate cancer. BJU Int 124:431–440
Giordano SH, Lee A, Kuo YF et al (2006) Late gastrointestinal toxicity after radiation for prostate cancer. Cancer 107:423–432
Thompson IM, Middleton RG, Optenberg SA et al (1999) Have complication rates decreased after treatment for localized prostate cancer? J Urol 162:107–112
Meraney AM, Haese A, Palisaar J et al (2005) Surgical management of prostate cancer: advances based on a rational approach to the data. Eur J Cancer 41:888–907
Muller BG, van den Bos W, Brausi M et al (2015) Follow-up modalities in focal therapy for prostate cancer: results from a Delphi consensus project. World J Urol 33:1503–1509
Oishi M, Gill IS, Tafuri A et al (2019) Hemigland cryoablation of localized low, intermediate and high risk prostate cancer: oncologic and functional outcomes at 5 years. J Urol 202:1188–1198
Bahn D, de Castro Abreu AL, Gill IS et al (2012) Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur Urol 62:55–63
Kongnyuy M, Islam S, Mbah AK et al (2018) PSA kinetics following primary focal cryotherapy (hemiablation) in organ-confined prostate cancer patients. World J Urol 36:209–213
Mortezavi A, Krauter J, Gu A et al (2019) Extensive histological sampling following focal therapy of clinically significant prostate cancer with high intensity focused ultrasound. J Urol 202:717–724
Illing RO, Leslie TA, Kennedy JE et al (2006) Visually directed high-intensity focused ultrasound for organ-confined prostate cancer: a proposed standard for the conduct of therapy. BJU Int 98:1187–1192
Geissbuhler M, Hincapie CA, Aghlmandi S et al (2021) Most published meta-regression analyses based on aggregate data suffer from methodological pitfalls: a meta-epidemiological study. BMC Med Res Methodol 21:123
Acknowledgements
The authors would like to thank David Fay, PhD for assistance with the literature review and data extraction.
Funding
This work was supported by Sonablate. The funding source was not involved in the conduct of the systematic review or meta-analysis.
Author information
Authors and Affiliations
Contributions
Conceptualization: SJ Peretsman and LE Miller. Methodology: SJ Peretsman and LE Miller. Software: LE Miller. Formal analysis: LE Miller. Investigation: All authors. Writing—original draft: LE Miller. Writing—review and editing: all authors. Visualization: LE Miller. Supervision: SJ Peretsman. Project administration: SJ Peretsman and LE Miller. Funding acquisition: SJ Peretsman.
Corresponding author
Ethics declarations
Conflict of interest
S. Peretsman reports employment with Sonablate. M Emberton serves as a consultant, investigator, and proctor/educator for Sonacare Inc and Angiodynamics Inc. Neil Fleshner reports no conflicts of interest. Sunao Shoji reports no conflicts of interest. C. Bahler reports consultancy with Sonablate, Telix Pharma, and Early is Good. L. Miller received research support from Sonablate.
Research involving human participants and/or animals
This research did not involve human participants or animals.
Informed consent
Informed consent was not applicable, since this was a review of published studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peretsman, S.J., Emberton, M., Fleshner, N. et al. High-intensity focused ultrasound with visually directed power adjustment for focal treatment of localized prostate cancer: systematic review and meta-analysis. World J Urol 42, 175 (2024). https://doi.org/10.1007/s00345-024-04840-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-04840-6