Abstract
Purpose
Unclear lesions on multiparametric magnetic resonance tomography (mpMRI) are challenging for the indication of biopsy in patients with clinical suspicion of prostate cancer (PCa). The aim of this study is the validation of the detection rate of clinically significant PCa (csPCa) in patients with PI-RADS 3 findings and to determine the appropriate follow-up strategy.
Methods
In this retrospective single-center study, patients with maximum PI-RADS 3 lesions underwent targeted MRI/ultrasound-fusion biopsy (tPbx) combined with systematic 12-core biopsy (sPbx) and follow-up mpMRI with further control biopsy. We assessed the evolution of MRI findings (PI-RADS, volume of the lesion), clinical parameters and histopathology in follow-up MRI and biopsies. The primary objective is the detection rate of csPCa, defined as ISUP ≥ 2 findings.
Results
A total of 126 patients (median PSA 6.65 ng/ml; median PSA-density (PSAD) 0.13 ng/ml2) were included. The initial biopsy identified low-risk PCa in 24 cases (19%). During follow-up biopsy, 22.2% of patients showed PI-RADS upgrading (PI-RADS > 3), and 29 patients (23%) exhibited a tumor upgrading. Patients with PI-RADS upgrading had a higher risk of csPCa compared to those without PI-RADS upgrading (42.9% vs. 9.18%, p < 0.05). PI-RADS upgrading was identified as an independent predictor for csPCa in follow-up biopsy (OR 16.20; 95% CI 1.17–224.60; p = 0.038).
Conclusion
Patients with stable PI-RADS 3 findings may not require a follow-up biopsy. Instead, it is advisable to schedule an MRI, considering that PI-RADS upgrading serves as an independent predictor for csPCa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiparametric magnetic resonance imaging (mpMRI) in the biopsy pathway has become a standard method for the detection of prostate cancer (PCa) and has shown high accuracy in detecting clinically significant PCa (csPCa) [1, 2]. National and international guidelines recommend performing mpMRI for all patients requiring prostate biopsy, regardless of whether they are biopsy-naïve or have undergone a previous biopsy [3, 4]. The EAU guideline recommends a systematic (sPbx) combined with MRI/ultrasound-fusion targeted biopsy (tPbx) in the case of tumor-suspicious lesion in mpMRI for biopsy-naïve patients and a sole tPbx for patients with prior negative biopsy [3]. Nevertheless, the management of PI-RADS 3 lesions, which are considered as equivocal, remains controversial, with conflicting findings regarding their significance [5,6,7,8,9,10]. Currently, there are no established recommendations for managing and following up on these lesions. Some studies suggest incorporating PSA-density (PSAD) values with PI-RADS category to guide biopsy decisions of whether to proceed with or postpone a biopsy [8, 11,12,13]. Several clinical parameters, biomarkers, and nomograms were described to assist in the biopsy decision in patients with lesions of PI-RADS ≥ 3 [14, 15]. In this study, we analyzed the follow-up mpMRI findings and histological results of patients who initially presented PI-RADS 3 lesions in initial biopsy. The analysis included monitoring the development of clinical and imaging parameters over time. The objectives of this study were to confirm the presence of csPCa through imaging and biopsy during subsequent follow-up and to identify clinical and imaging parameters that can predict csPCa during the follow-up.
Patients and methods
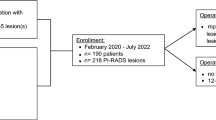
This retrospective single-center study included patients with initial PI-RADS 3 lesions only, who underwent tPbx and sPbx and a further mpMRI combined with a follow-up biopsy. This study was approved by the Ethic committee of the Technische Universität Dresden (EK53022014).
Both biopsy naïve and previously biopsied patients were included in the study. Patients without tumor and patients with ISUP 1 tumors detected in initial biopsy were investigated. PI-RADS, number of lesions, histopathological results, clinical parameters and the risk calculator (RC) based on European Randomized study of Screening for Prostate Cancer (ERSPC) between initial and follow-up mpMRI and biopsy were compared [16]. The RC used for this study are the RC2 and RC3 from ERSPC. The RC2 is a PSA-based RC that examines the PSA value to predict the need for further testing. The RC3 was developed to calculate the risk of PCa detectable by biopsy. The result is expressed as a percentage in any PCa risk (anyERSPC3) and csPCa risk (signERSPC3). The anyERSPC3 assists in prostate biopsy decision making. A prostate biopsy is recommended if the result is 20% or more and no prostate biopsy if the result is less than 12.5%. The signERSPC3 calculates the probability of csPCa (http://www.prostatecancer-riskcalculator.com/). In our study, a PCa with Gleason Score ≥ 7 (3 + 4) or International Society of Urological Pathology (ISUP) ≥ 2 was defined as csPCa.
Primary objective was the detection rate of csPCa in patients with PI-RADS 3 lesions in the initial and follow-up biopsy. Further objective was to evaluate, which clinical and imaging parameters are eligible as predictors for csPCa. The development of the PI-RADS finding and clinical parameters between initial and follow-up biopsy in the context of histopathologic results were analyzed.
The mpMRI was performed at the Institute for Diagnostic and Interventional Radiology at the Technische Universität Dresden using a 3T MRI system (Siemens Medical Solutions, Erlangen, Germany) without an endorectal coil or external radiological practices. The mpMRI followed the ACR guidelines, including T1-weighted, T2-weighted, diffusion-weighted, and perfusion-weighted sequences, evaluated according to PI-RADS criteria. Transperineal tPbx and sPbx were conducted in the Department of Urology using the BioJet system (dk Technologies, Barum, Germany) and bkfusion systems (bk, Herlev, Denmark). In tPbx, at least 2 cores were taken per lesion based on size. Statistical analysis employed SPSS v26.0 (IBM Corp, Armonk, NY, USA), reporting continuous variables using mean, standard deviation, median, interquartile range (IQR), and minimum or maximum values. The McNemar test assessed csPCa detection rate between biopsy methods (p ≤ 0.05). Receiver operating characteristic (ROC) analysis, calculating the area under the curve (AUC), compared clinical parameters' significance in detecting csPCa. Uni- and multivariate binary logistic regression analysis identified predictors for PCa or csPCa presence based on clinical and imaging parameters. Therefore, continuous parameters were dichotomized according the median or the clinical impact. Using a Sankey diagram, the course between the initial and follow-up PI-RADS is presented depending on the biopsy result.
Results
Between January 2016 and October 2022, 126 patients with a maximum PI-RADS 3 lesion underwent initial Pbx (30 patients with primary biopsy, 96 patients with repeat biopsy). 19% (24/126) presented PCa with ISUP 1. sPbx detected more PCa compared to tPbx (83% vs. 54%; p < 0.001). All patients underwent follow-up biopsy at our department (Table 1). The interval between initial and follow-up biopsy was 23 months. During this time 28 patients showed a PI-RADS-upgrading, 26 with PI-RADS 4 and 2 with PI-RADS 5 (Fig. 1). In the follow-up biopsy 35 patients had PCa, whereas 21 patients (60%) had a csPCa (ISUP 2:n = 19, ISUP ≥ 4: n = 2). 23% of patients (29/126) had a downgrading of PI-RADS and a stable MRI-finding with a PI-RADS 3 was found in 54.8% of patients (69/126). CsPCa was detected in 3.4% of men with PI-RADS < 3, 11.6% of men with PI-RADS 3, 42.3% of men with PI-RADS 4, and 50% of men with PI-RADS 5 findings (p < 0.001). In the follow-up biopsy, sPbx detected more csPCa compared to the tPbx for patients with PI-RADS 3 and 4 (PI-RADS 3: 63.6% (7/11) vs. 45% (5/11); p < 0.001, PI-RADS 4: 90.9% (10/11) vs. 63.6% (7/11); p = 0.002). For PI-RADS 5, both sPbx and tPbx detected an equal amount of csPCa with 100% (1/1; p = 0.16).
In the ROC analysis, the PSAD showed the highest accuracy comparing to other parameters in detecting csPCa with an AUC of 0.546 (p = 0.511; 95% CI 0.410–0.681). ERSPC3 for the detection of any PCa discriminated csPCa with an AUC of 0.540 (p = 0.561; 95% CI 0.406–0.648). PSA and ERSPC2 showed a lower AUC of 0.512 (p = 0.861; 95% CI 0.376–0.648). ERSPC3 for the detection of csPCa came last with an AUC of 0.455 (p = 0.515; 95% CI 0.321–0.589). In the uni- and multivariate binary logistic regression analysis, the upgrading of PI-RADS to 4 was the only independent predictor for the detection of csPCa (Table 2).
Discussion
In the present study, the value of mpMRI and tPbx compared to sPbx in PCa detection was examined and compared in a total of 126 men with an initial maximum PI-RADS of 3. The majority of patients (76.2%) had already received at least one pre-biopsy. In the initial biopsy, PCa with ISUP 1 was found in 19% patients. Several studies have shown a variety of the detection rate of PCa for the PI-RADS 3 between 29.7% and 43.9% and for csPCa between 4 and 24.9% [13, 17,18,19,20]. The likelihood of PI-RADS 3 lesions associated with csPCa is considered rather low [5, 6, 20]. Many authors interpret PI-RADS 3 lesions as a gray zone for the detection of csPCa [7], in which the presence of a csPCA is not clearly described [2, 5]. There is still a considerable detection rate of csPCa at 14% [21], as we also demonstrated in our cohort (11.6%). Additionally, it’s important to acknowledge that in a re-biopsy cohort, the overall PCa-detection rate might be lower when compared to a primary biopsy cohort. Within our study population, patients with PI-RADS 3 lesions in the initial biopsy showed the presence of PCa with ISUP 1 in 19% of cases, which decreased to 14.5% in the follow-up biopsy. Moreover, 40% of patients exhibited signs of inflammation consistent with chronic prostatitis.
Thus, the importance of PI-RADS 3 lesion, seems to be contradictory in some works [17, 19, 20]. Considering the frequency of occurrence of PI-RADS 3 lesions, depending on the patient cohort, studies have shown that 18–46% of all mpMRI show PI-RADS 3 lesions [22, 23], as can be calculated from this work with 54.7% in the follow-up mpMRI. This relatively high number poses a significant challenge for clinical management [2, 23].
Current guidelines recommend combined biopsy for biopsy-naïve patients with PI-RADS 3 and solely tPbx for patients with previous negative biopsy [3, 4]. In our follow-up MRI after approximately 2 years, 22.2% of patients showed PI-RADS upgrading, while 23% had PI-RADS downgrading. We found an association between PCa or csPCA presence and PI-RADS progression. The detection rate was 42.3% for PI-RADS 4 and 50% for PI-RADS 5 lesions, respectively. Boschheidgen et al. showed similar results. Of 89 patients with initially maxPI-RADS 3, 19 had PCa, of which 4 cases were csPCa. After the time interval of 12–24 months, PI-RADS upgrading was evident in the follow-up MRI, especially in the patients with PCa at initial biopsy. In patients with a negative initial biopsy, there was even a significant PI-RADS downgrading. Thus, Boschheidgen et al. recommended that patients with PI-RADS 3 primarily receive a follow-up MRI within 12–24 months after the initial MRI instead of a prompt biopsy [20]. Limitation of the study by Boschheidgen et al. was that there was no follow-up of the biopsy, so that an upgrading or downgrading of the histological findings cannot be retraced. In our study, ISUP upgrading was observed in 23% of patients, with ISUP 1 in 27.6%, ISUP 2 in 65.5%, and ISUP ≥ 3 in 6.9% of cases. ISUP downgrading occurred in 7.1% of patients. In this case, the majority of patients with tumor upgrading had an ISUP ≤ 2. Our results align with other studies advocating for follow-up mpMRI to avoid unnecessary biopsies [8, 9, 17, 20, 24]. In case of a repeat biopsy, we recommend performing a combined biopsy. In this study, both sPbx and tPbx were evaluated. In the follow-up biopsy, the detection rate of sPbx was significantly higher for csPCa compared to tPbx for PI-RADS 3 and 4 (PI-RADS 3: 63.6% vs. 45%, p < 0.001; PI-RADS 4: 90.9% vs. 63.6%, p = 0.002). This may be due to the fact that the total number of patients with each associated PI-RADS lesion is relatively low. Nevertheless, this result stands in line with other studies [18, 25]. If diagnostics relied solely on tPbx, 17% of csPCa would be missed [25]. Thus, it can again be concluded that in case of a repeat biopsy the need for additional sPbx to tPbx continues to exist in the cohort with PI-RADS 3 lesions [4, 18, 25, 26].
Several studies have shown, that the additional use of the PSAD with a cut-off of 0.15 ng/ml2 and the risk calculator to the mpMRI contributes to a better risk discrimination for csPCa and can thus be used as a helpful decision index for the biopsy indication. In this study, the PSAD was the strongest predictor for the detection of csPCa, followed by ERSPC3. However, both PSAD and ERSPC were not independent predictors. In the uni- and multivariate analysis, the upgrading of PI-RADS to 4 was the only independent predictor for the detection of csPCa. The relatively low p value of PI-RADS upgrading to 5 is most likely due to the limited patient sample size, particularly with PI-RADS 5. Nevertheless, some studies could demonstrate, that the higher the PSAD, the more likely the probability of csPCa [27, 28]. In patients with negative MRI and PSAD > 0.15–0.20 ng/ml2 the risk of csPCA is up to 27–40% [8, 29, 30]. Alternatively, a biopsy can be omitted for patients with PI-RADS ≤ 3 and PSA density < 0.15 ng/ml2 [8, 30].
Our study has several limitations. First, it is a retrospective, single-center study that includes a heterogeneous group of patients, many of whom had undergone prior biopsies. Additionally, there was variability in the timing of follow-up MRI and biopsy among the patients. However, it is worth noting that our study achieved a realistic median time interval between the initial and follow-up MRI. Other studies have reported time frames of 12–24 months for a follow-up MRI, which reveal PI-RADS upgrading in cases of csPCa [9, 20]. It is important to acknowledge that different time intervals may lead to different outcomes, emphasizing the need for further research to determine the optimal interval. Another limitation to consider is the diversity in the MRI scans, which were performed by different physicians, both internally and externally. Furthermore, there was no secondary evaluation of the external MRI scans, which might have the potential to introduce variability in result interpretation. Moreover, for the minor group of patients with ISUP 1 PCa in the initial biopsy, we did not use the PRECISE Score to have homogenized MRI findings.
Conclusion
Based on our findings, we suggest that patients with PI-RADS 3 findings may primarily undergo a follow-up MRI within 24 months of the initial MRI. The overall detection rate of csPCa in PI-RADS 3 lesions was relatively low. However, if there is a PI-RADS upgrade observed in the follow-up MRI, we strongly recommend a repeat biopsy, as there is a significant association between PI-RADS upgrading and PCa upgrading. Clinical parameters such as the PSAD or ERSPC3 risk calculator may provide valuable support for clinical decision-making.
Data availability
It was performed during the submission process.
Abbreviations
- CI:
-
Confidence interval
- csPCa:
-
Clinically significant prostate cancer (ISUP grade ≥ 2)
- IQR:
-
Interquartile range
- ISUP:
-
International Society of Urological Pathology
- mpMRI:
-
Multiparametric prostate magnetic resonance imaging
- OR:
-
Odds ratio
- PCa:
-
Prostate cancer
- PI-RADS:
-
Prostate Imaging Reporting and Data System
- PSA:
-
Prostate-specific antigen
- PSAD:
-
Prostate-specific antigen density
- sPbx:
-
Systematic 12-core biopsy
- tPbx:
-
targeted biopsy by MRI/ultrasound-fusion biopsy
- US:
-
Ultrasound
References
Barentsz JO, Weinreb JC, Verma S et al (2016) Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol 69:41–49. https://doi.org/10.1016/j.eururo.2015.08.038
Maggi M, Panebianco V, Mosca A et al (2020) Prostate imaging reporting and data system 3 category cases at multiparametric magnetic resonance for prostate cancer: a systematic review and meta-analysis. Eur Urol Focus 6:463–478. https://doi.org/10.1016/j.euf.2019.06.014
Mottet N, Van Den Bergh RCN, Briers E et al (2021) EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 79:243–262. https://doi.org/10.1016/j.eururo.2020.09.042
Deutschland, Deutschland, Bundesärztekammer, Deutsche Gesellschaft für Psychiatrie, Psychotherapie und Nervenheilkunde (2016) S3-Leitlinie Methamphetamin-bezogene Störungen. Springer, Berlin
Liddell H, Jyoti R, Haxhimolla HZ (2015) mp-MRI prostate characterised PIRADS 3 lesions are associated with a low risk of clinically significant prostate cancer—a retrospective review of 92 biopsied PIRADS 3 lesions. Curr Urol 8:96–100. https://doi.org/10.1159/000365697
Bastian-Jordan M (2018) Magnetic resonance imaging of the prostate and targeted biopsy, comparison of PIRADS and Gleason grading. J Med Imaging Radiat Oncol 62:183–187. https://doi.org/10.1111/1754-9485.12678
Scialpi M, Martorana E, Aisa MC et al (2017) Score 3 prostate lesions: a gray zone for PI-RADS v2. Turk J Urol 43:237–240. https://doi.org/10.5152/tud.2017.01058
Washino S, Okochi T, Saito K et al (2017) Combination of prostate imaging reporting and data system (PI-RADS) score and prostate-specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naïve patients. BJU Int 119:225–233. https://doi.org/10.1111/bju.13465
Steinkohl F, Gruber L, Bektic J et al (2018) Retrospective analysis of the development of PIRADS 3 lesions over time: when is a follow-up MRI reasonable? World J Urol 36:367–373. https://doi.org/10.1007/s00345-017-2135-0
Thompson J, Lawrentschuk N, Frydenberg M et al (2013) The role of magnetic resonance imaging in the diagnosis and management of prostate cancer: The role of magnetic resonance imaging in the diagnosis and management of prostate cancer. BJU Int 112:6–20. https://doi.org/10.1111/bju.12381
Distler FA, Radtke JP, Bonekamp D et al (2017) The value of PSA density in combination with PI-RADS™ for the accuracy of prostate cancer prediction. J Urol 198:575–582. https://doi.org/10.1016/j.juro.2017.03.130
Schoots IG, Padhani AR (2021) Risk-adapted biopsy decision based on prostate magnetic resonance imaging and prostate-specific antigen density for enhanced biopsy avoidance in first prostate cancer diagnostic evaluation. BJU Int 127:175–178. https://doi.org/10.1111/bju.15277
Oerther B, Engel H, Bamberg F et al (2022) Cancer detection rates of the PI-RADSv2.1 assessment categories: systematic review and meta-analysis on lesion level and patient level. Prostate Cancer Prostatic Dis 25:256–263. https://doi.org/10.1038/s41391-021-00417-1
Schoots IG, Roobol MJ (2020) Multivariate risk prediction tools including MRI for individualized biopsy decision in prostate cancer diagnosis: current status and future directions. World J Urol 38:517–529. https://doi.org/10.1007/s00345-019-02707-9
Grönberg H, Eklund M, Picker W et al (2018) Prostate cancer diagnostics using a combination of the Stockholm3 blood test and multiparametric magnetic resonance imaging. Eur Urol 74:722–728. https://doi.org/10.1016/j.eururo.2018.06.022
Steyerberg EW, Roobol MJ, Kattan MW et al (2007) Prediction of indolent prostate cancer: validation and updating of a prognostic nomogram. J Urol 177:107–112. https://doi.org/10.1016/j.juro.2006.08.068
Schlenker B, Apfelbeck M, Armbruster M et al (2019) Comparison of PIRADS 3 lesions with histopathological findings after MRI-fusion targeted biopsy of the prostate in a real world-setting. CH 71:165–170. https://doi.org/10.3233/CH-189407
Sathianathen NJ, Konety BR, Soubra A et al (2018) Which scores need a core? An evaluation of MR-targeted biopsy yield by PIRADS score across different biopsy indications. Prostate Cancer Prostatic Dis 21:573–578. https://doi.org/10.1038/s41391-018-0065-6
Wadera A, Alabousi M, Pozdnyakov A et al (2021) Impact of PI-RADS Category 3 lesions on the diagnostic accuracy of MRI for detecting prostate cancer and the prevalence of prostate cancer within each PI-RADS category: a systematic review and meta-analysis. BJR 94:20191050. https://doi.org/10.1259/bjr.20191050
Boschheidgen M, Schimmöller L, Doerfler S et al (2022) Single center analysis of an advisable control interval for follow-up of patients with PI-RADS category 3 in multiparametric MRI of the prostate. Sci Rep 12:6746. https://doi.org/10.1038/s41598-022-10859-9
Yilmaz EC, Shih JH, Belue MJ et al (2023) Prospective evaluation of PI-RADS version 2.1 for prostate cancer detection and investigation of multiparametric MRI–derived markers. Radiology 307:e221309. https://doi.org/10.1148/radiol.221309
Filson CP, Natarajan S, Margolis DJA et al (2016) Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: the role of systematic and targeted biopsies: CaP Detection With MR-US Fusion Biopsy. Cancer 122:884–892. https://doi.org/10.1002/cncr.29874
Schoots IG (2018) MRI in early prostate cancer detection: how to manage indeterminate or equivocal PI-RADS 3 lesions? Transl Androl Urol 7:70–82. https://doi.org/10.21037/tau.2017.12.31
Ullrich T, Quentin M, Arsov C et al (2018) Risk stratification of equivocal lesions on multiparametric magnetic resonance imaging of the prostate. J Urol 199:691–698. https://doi.org/10.1016/j.juro.2017.09.074
Recabal P, Assel M, Sjoberg DD et al (2016) the efficacy of multiparametric magnetic resonance imaging and magnetic resonance imaging targeted biopsy in risk classification for patients with prostate cancer on active surveillance. J Urol 196:374–381. https://doi.org/10.1016/j.juro.2016.02.084
Rosenkrantz AB, Verma S, Choyke P et al (2016) Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with a prior negative biopsy: a consensus statement by AUA and SAR. J Urol 196:1613–1618. https://doi.org/10.1016/j.juro.2016.06.079
Omri N, Kamil M, Alexander K et al (2020) Association between PSA density and pathologically significant prostate cancer: the impact of prostate volume. Prostate 80:1444–1449. https://doi.org/10.1002/pros.24078
Nordström T, Akre O, Aly M et al (2018) Prostate-specific antigen (PSA) density in the diagnostic algorithm of prostate cancer. Prostate Cancer Prostatic Dis 21:57–63. https://doi.org/10.1038/s41391-017-0024-7
Boesen L, Nørgaard N, Løgager V et al (2019) Prebiopsy biparametric magnetic resonance imaging combined with prostate-specific antigen density in detecting and ruling out gleason 7–10 prostate cancer in Biopsy-naïve Men. European Urology Oncology 2:311–319. https://doi.org/10.1016/j.euo.2018.09.001
Oishi M, Shin T, Ohe C et al (2019) Which PATIENTS WITH NEGATIVE MAGNETIC RESONANCE IMAGING CAN SAFELY AVOID BIOPSY FOR PROSTATE CANCER? J Urol 201:268–277. https://doi.org/10.1016/j.juro.2018.08.046
Acknowledgements
We thank Andre Sonnenfeld for helping to collect the data of the study cohort.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JK—data collection, data interpretation, writing of manuscript. MB—revision of manuscript. KB—revision of manuscript. IP—revision of manuscript. CT—supervision, revision of manuscript. AB—supervision, study concept, data collection, data interpretation, revision of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. The authors declare have no financial or non-financial interests that are directly or indirectly related to that work.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable standards. Ethical approval was obtained by the Ethical Committee of the Technische Universität Dresden. This study does not contain any studies with animals.
Informed consent
All patients provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kwe, J., Baunacke, M., Boehm, K. et al. PI-RADS upgrading as the strongest predictor for the presence of clinically significant prostate cancer in patients with initial PI-RADS-3 lesions. World J Urol 42, 84 (2024). https://doi.org/10.1007/s00345-024-04776-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-04776-x