Abstract
Melatonin (N-acetyl-5-methoxytriptamine) is a novel plant regulatory molecule currently under study. Its role as a biostimulator and protector against abiotic and biotic stressors, through the regulation of the redox network and change in the expression of many elements of primary and secondary metabolism, is of great interest. The possible protective effect of melatonin in mungbean seedlings, previously primed seed treated with the fungicide copper oxychloride, was studied. The effect of melatonin and fungicide in growth of seedlings and photosynthetic pigments, leakage membranes, lipid peroxidation, antioxidant activity, and phytomelatonin content was studied. Also, the effect of exogenous melatonin on endogenous plant hormones indoleacetic acid, gibberellins, cytokinins, abscisic acid, salicylic acid, and jasmonic acid levels, in the absence and presence of fungicide, was analyzed. Melatonin improved growth of roots and aerial parts in the presence of fungicide; chlorophyll and carotenoid contents were protected by melatonin in the presence of melatonin and in melatonin-fungicide co-treatments. Membrane damage due to fungicide was lessened by melatonin. The hormonal profile (auxin, gibberellins, cytokinins, abscisic acid, ethylene precursor, salicylic acid, and jasmonic acid) in roots and leaves was greatly affected by copper fungicide and melatonin treatments. In general, an increasing in plant tolerance response has been detected, proposing melatonin as a natural safener molecule of plants in the presence of copper fungicide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melatonin (MEL, N-acetyl-5-methoxytryptamine), an important hormone that regulates day/night rhythms and acts as a chronological signal giving information to the brain and peripheral organs, was discovered in 1958 in the pineal gland of the cow and also in humans (Lerner et al. 1958, 1959a, b). In plants, it was discovered in 1995 almost simultaneously by three research groups (Dubbels et al. 1995; Hattori et al. 1995; Kolar et al. 1995). Among the physiological processes, phytomelatonin (name given to melatonin in plants) plays a role related to the germination, growth and rooting of plants, and also foliar senescence (Hernández-Ruiz et al. 2004, 2005; Arnao and Hernández-Ruiz 2007, 2009a). Additionally, phytomelatonin is presented with a leading role as a protective molecule and activator of tolerance and resistance responses to many stressors (Lei et al. 2004; Afreen et al. 2006; Arnao and Hernández-Ruiz 2009b; Posmyk et al. 2009). Currently, it has been shown that MEL acts as a phytohormone with biostimulation capacity of plants against biotic stress caused by plant pathogens such as bacteria, fungi, and viruses (Sharif et al. 2018; Moustafa-Farag et al. 2020a; Tiwari et al. 2021; Zhao et al. 2021a), and also against abiotic stresses such as drought, waterlogging, salinity, cold/heat, toxic agents and heavy metals, UV radiation, etc. (Arnao and Hernández-Ruiz 2014, 2019c, 2021b; Moustafa-Farag et al. 2020b, c; Altaf et al. 2021a; Arnao et al. 2022).
This role as a protective agent has been widely studied in many plant species and in multiple stressful situations. Several recent reviews on this topic can be consulted (Moustafa-Farag et al. 2020a, c; Pardo-Hernández et al. 2020; Sharma et al. 2020; Nawaz et al. 2020; Ahn et al. 2021; Arnao and Hernández-Ruiz 2021a, 2022; Hoque et al. 2021; Tripathi et al. 2021; Zhao et al. 2021a, 2022; Giraldo-Acosta et al. 2022). Generally, phytomelatonin actions through phytomelatonin receptor (PMTR1), and it appears to activate metabolic, hormonal, and defense responses, providing the plant with greater tolerance against biotic/abiotic stressor (Wei et al. 2018a; Wang et al. 2021; Yang et al. 2021; Li et al. 2022).
The properties of copper as a fungicide and bactericide product were discovered to control mildew on the vine by Dr. Millardet and Gayon in France, elaborating the “Bordeaux formula” in the 1880s (https://fr.wikipedia.org/wiki/Bouillie_bordelaise). Although copper pesticides are one of the oldest classes of fungicides, they are still used for the management of many different diseases today. The active ingredient in all copper-based formulations is the positively charged copper ion (Cu+2). Cu-based products have broad-spectrum activity against microorganisms due to copper’s interaction with nucleic acids, interference with energy transport and disruption of enzyme activity and integrity of cell membranes. Copper at moderate to high doses can become toxic for plants. Diverse copper forms are used for plant disease management, such as copper hydroxide, copper oxide, copper oxychloride, and copper octanoate, which were formulated to produce low doses of free Cu ions, reducing toxicity to plants (Burkhead et al. 2009; Elalfy et al. 2021).
Copper excess is highly toxic in plants because it generates hydroxyl radicals through Fenton reactions, causing several damage to lipids, proteins, and nucleic acids, and important changes in antioxidative enzymes (Mocquot et al. 1996; Dra̧zkiewicz et al. 2004). Generally, Cu excess symptoms are the reduction of plant biomass, the inhibition of root growth, chlorosis, bronzing, and necrosis (Maksymiec 1997; Pätsikkä et al. 2002; Zhao et al. 2009). Also, tolerance mechanisms to decrease the accumulation of Cu ions in cells are activated by plants (Burkhead et al. 2009; Lequeux et al. 2010).
One of the most interesting aspects is the possible role of MEL as a protective agent (safener) in treatments with pesticides, such as herbicides, fungicides, and others (Hoffman 1969). The use of melatonin (MEL) as a natural safener against herbicides was first studied in 2013 in rice. That safener effect of MEL has also been described by paraquat and bentazone, two widely used herbicides (Szafranska et al. 2017; Wei et al. 2018b; Ding et al. 2018a; Caputo et al. 2020). This safener action against herbicides was proposed in sweetpotato (Ipomoea batatas L.) (Caputo et al. 2020) treated with the herbicide bentazone (a post-emergence contact diazinone herbicide) used to control annual weeds in a variety of crops (Motsenbocker and Monaco 1991). Bentazone-treated sweet potato seedlings caused severe losses (41–75%), but applied together with MEL, 30% fewer injuries and twice the biomass yield compared to treatments with the herbicide alone was observed. The authors suggested using MEL as a possible safener in weed control. Also, the application of MEL in co-treatments with butafenacil (a protoporphyrinogen IX oxidase inhibitor) prevented damage by the herbicide on the photosynthetic apparatus, improving yields (Park et al. 2013). About 20–30 herbicide safeners have been developed and applied so far in pre- and post-emergence. In recent years, no new safeners have appeared due to legal restrictions on the use of synthetic substances in crops.
On the other hand, in some studies combining fungicides and MEL, the protective action of MEL has been proven, along with a synergistic action against fungal infection. Thus, due to the mode of action of MEL as a plant master regulator in the redox network (Arnao and Hernández-Ruiz 2019a, c), regulating reactive oxygen species (ROS) and reactive nitrogen species (RNS) levels, joint fungicide, and MEL treatments appear to decrease ROS production and lipid peroxidation levels, improving fungicidal efficacy. In addition, an optimal modulation of the ascorbic acid-glutathione (ASC-GSH) cycle by MEL was verified, increasing the levels of ASC, GSH, and antioxidant enzymes, improving the detoxification capacity of plant cells and being able to metabolize the fungicide with minimal collateral damage. Thus, according to some authors, MEL makes plants "smarter" to withstand stressful phytotoxic conditions (Yan et al. 2019).
In this paper, we present a study of the protective effect of MEL in mungbean seedlings primed with the fungicide copper oxychloride (F). The action of MEL on root and aerial growth; also the chlorophyll and carotenoid content, the membrane damage, and the antioxidant activity in roots and leaves have been measured. Also, the effect of MEL and/or F on the hormonal profile in roots and leaves was presented. A proposal of MEL as a safener molecule for plants in F treatments was proposed.
Materials and Methods
Chemicals
The chemicals, solvents (methanol, ethanol, acetone, acetonitrile, and ethyl acetate) and reagents used were from Sigma-Aldrich Co. (Madrid, Spain). Milli-Q system (Milli-Q Corp, Merck KGaA, Darmstadt, Germany) ultra-pure water was used. The fungicide (F) used was copper oxychloride (H6Cl2Cu4O6) from Sigma-Aldrich Co. (Madrid, Spain).
Plant Material
Seeds of mungbean (Vigna radiata L., Fabaceae) were sterilized with 10% sodium hypochlorite, for 5 min with gentle agitation. Then, the seeds were washed 3 times with distilled water. Fifty seeds were placed in Petri dishes containing four filter paper disks. There were at least three biological replicates per treatment. The following hydropriming treatments with fungicide (F) and melatonin (MEL) were applied in seeds: distilled water (C), 0.3 mM fungicide (F0.3), 3 mM fungicide (F3), 30 mM fungicide (F30), combined with 20 μM melatonin (MEL20), or 100 μM melatonin (MEL100). The petri dishes were placed in a controlled chamber in darkness at 22 ℃, allowed to germinate for 4 days.
Then, 45 seedlings per treatment were transferred to trays (15 plants per tray) containing vermiculite as an inert substrate. Trays were initially irrigated with 500 mL of water and 3 mL of an NPK universal liquid fertilizer and the third and fifth day of growth with two water irrigations of 200 mL. Trays were placed in a controlled chamber with a photoperiod light/darkness (18/6 h) equipped with 18-Watt fluorescent white light lamps (PAR: 35.6 μE/m2·s), at 24 ℃. Then, three samples (root, stem, and leaf) of 10-day-old seedlings from each treatment were frozen in liquid nitrogen and stored at −80 ℃ until analysis. There were at least three biological replicates per treatment.
Mungbean and copper oxychloride have been selected because they are a model plant and a widely used fungicide, respectively. The concentrations of MEL and F selected for the study were chosen after a previous study of a wider range.
Measurement of Morphological Parameters
Seed germination was recorded after 4 days of treatment application. A seed was considered to be germinated if its radicle was emerged. The germination percentage was calculated from the number of total seeds (50) and germinated seeds in a petri dish.
The length of the roots of 45 seedlings of 4 and 10 day old were measured using a graph paper. Also, the number of secondary roots and length of stem in 45 seedling of 10 day old were determinate.
Twenty seedlings of 10 day old were selected to measure the fresh and dry weight. The fresh weight of the plants was measured on a precision scale. Then, seedlings were dried in an oven for 48 h at a temperature of 55 ℃ and its dry weight was recorded.
The first two leaves of 15 seedlings per treatment were selected and scanned to determine the leaf area, using the image processing software ImageJ.
Determination of Chlorophyll and Carotenoid Content
Chlorophyll and carotenoid contents were determined according to (Lichtenthaler and Wellburn 1983). Briefly, frozen leaf disks (0.3 g) and 5 mL acetone (80%) were ground in a mortar to extract pigments. Three consecutive extractions were made, and the supernatants were collected and centrifuged at 10000xg for 10 min at 4 ℃. The absorbance of the supernatant was measured at 470, 645, 652, and 663 nm to determine the chlorophylls a and b, and carotenoid content. The determinations were made by triplicate. Results were expressed in mg of chlorophyll (a or b) or carotenoid/g FW.
Determination of Malondialdehyde
Malondialdehyde (MDA) content was estimated using thiobarbituric acid (TBA) assay, which is related to cell membrane damage (Niehaus Jr. and Samuelsson 1968). Briefly, frozen plant material (0.2 g) was homogenized in 5 mL of 5% trichloroacetic acid (TCA) and centrifuged at 10000xg for 10 min at 4 ℃. The supernatant was collected, and 1 mL was added to 4 mL of TCA (20%) and TBA (0.5%) solution. The resultant solution was heated at 90 ℃ for 30 min, and then the solution was cooled in ice and centrifuged at 10000xg for 10 min at 4 ℃. The changes in absorbance at 450, 532, and 600 nm were monitored in a Perkin-Elmer Lambda-2 UV–visible spectrophotometer (Überlingen, Germany). A standard curve with MDA was made, and results were expressed in µg MDA/g FW. The determinations were made by triplicate.
Determination of Electrolyte Leakage
Electrolyte leakage (EL) was determined according to (Hatsugai and Katagiri 2018), obtaining information on the integrity of cell membranes. Measurements were obtained using a conductivity meter (Bante Instruments, Shanghai, China) and expressed in % of EL (EL1/EL2·100%). Frozen plant material (0.2 g) was immersed in 8 mL of distilled water. The initial EL measurement was performed after leaving the samples for 1 h at room temperature (EL1). The final EL measurement of the samples was recorded after an hour in a boiling water bath (EL2). The determinations were made by triplicate.
Determination of Hydrophilic Antioxidant Activity
Hydrophilic antioxidant activity (HAA) was determined according to (Arnao et al. 1999). This method is based on the ability of the antioxidants of a sample to reduce the radical cation of 2,2′-azino-bis-3-(ethylbenzothiazoline-6-sulfonic acid) (ABTS· +), determined by the discoloration of ABTS· + and measuring the quenching of the absorbance at 730 nm. This activity was calculated by comparing the values of the sample with a standard curve of ascorbic acid and is expressed as mg of ascorbic acid equivalents/g FW (Cano and Arnao 2018). The determinations were made by triplicate.
Determination of Melatonin and Phytohormones
MEL content was measured by liquid chromatography with fluorescence detection (LC-FLUO) according to (Arnao and Hernández-Ruiz 2009c). Briefly, 0.2 g of plant material (leaves or roots) was placed in vials with ethyl acetate (4 mL) and left overnight (15 h) in darkness with shaking. The extract of each sample was evaporated to dryness under vacuum using a SpeedVac (ThermoSavant SPD11V, Thermo-Fisher Sci, Waltham, MA, USA) coupled to a refrigerated RCT400 vapor trap. The dry residue was redissolved in methanol (1 mL), filtered (0.2 µm), and analyzed. A Jasco liquid chromatograph Serie-2000 (Tokyo, Japan) equipped with an online degasser, binary pump, auto sampler, thermo-stated column, and a Jasco FP-2020-Plus fluorescence detector were used to analyze the endogenous MEL. A Waters Spherisorb-S5 ODS2 column (250 × 4.6 mm) was used. The mobile phase consisted of water:acetonitrile (80:20) at a flow rate of 0.5 mL/min. The fluorescence detector was programmed with an excitation value of 280 and 350 nm of emission. The data were analyzed using the Jasco ChromNAV v.1.09.03 Data System Software (Tokyo, Japan). MEL identification was carried out by comparing the excitation and emission spectra of standard MEL with the corresponding peak of MEL in the samples. MEL quantification was determined using a standard curve, and data were expressed as mg MEL/g FW. The determinations were made by triplicate.
The determination of phytohormones was carried out by Q-Exactive LC–MS/MS according to (Villanova et al. 2017). Briefly, 0.1 g of fresh plant material (leaf or root) was extracted twice with 1 mL of cold (4 ℃) extraction mixture of methanol:water (80:20) and separated by centrifugation (20000xg, 15 min). Pooled supernatants were passed through a Sep-Pak Plus-C18 cartridge (SepPak Plus, Waters, USA) and evaporated to dryness. The residue was resuspended in 1 mL of methanol:water (20:80). Filtered extracts (10 µL) were injected into a U-HPLC–MS system consisting of an Accela Series U-HPLC (ThermoFisher Scientific Waltham, MA, USA) coupled to a Q-ExactiveMass Orbitrap Spectrometer (ThermoFisher Scientific) using heated electrospray ionization (HESI) interface. Mass spectra were obtained using the Xcalibur software version 2.2 (ThermoFisher Scientific). For quantification of the plant hormones, calibration curves were constructed for each analyzed component (1, 10, 50, and 100 µg L−1) and corrected for 10 µg L−1 deuterated internal standards. The determinations were made by triplicate.
Statistical Analysis
All the data were graphically illustrated using SigmaPlot program version 14 (SYSTAT Software Inc., California, USA). Analysis of variance was performed using IBM SPSS Statistics 22.0 (IBM, New York, USA). All data are represented as mean ± standard error (SE) values. The statistical significance was considered for p values less than 0.05 in ANOVA and a post-hoc with Duncan Test.
Results
Melatonin Alleviated the Harmful Effects of Fungicide on Morphological Parameters
An extensive set of germination and growth assays in mungbean to determine the possible safener properties of MEL against chemical stress induced by the fungicide copper oxychloride (F) were conducted. In the seed germination test, no significant differences in germination in the different treatments were observed, presenting a germination percentage greater than 96.6% (see Table S1 in supplementary material).
The root length was measured in 4- and 10-day-old seedlings (Fig. 1). In the absence of F, MEL exerts a beneficial effect on root length, clearly observed at 4 days in 20 μM MEL, increasing the root length by 15–20%, compared to control (C) (Fig. 1A). In the presence of F, root length was severely affected at 3 and 30 mM F, but not at lower F treatment (F0.3). In co-treatments (F + MEL), MEL improved root length at 20 and 100 µM in lower F treatment, but a significant effect of MEL at 3 mM F and 100 µM MEL at 4 days was observed (Fig. 1A). In addition, significant differences in root length in 10-day-old seedlings were also obtained in the 30 mM F and 100 µM MEL co-treatments when compared to the F alone treatment. In 10-day old seedlings, the positive effect of MEL in 0.3 mM F treatment was observed (Fig. 1B).
Root length of 4-day-old (A) and 10-day-old (B) seedlings of Vigna radiata L. treated with distilled water (C, white bars), melatonin (MEL, 20 or 100 µM, yellow bars), fungicide (F, 0.3, 3, or 30 mM, blue bars), and yellow-blue bars represent the co-treatments. Data are represented as means ± SE (n = 45). Different superscript letters indicate statistically significant differences at p < 0.05 (Color figure online)
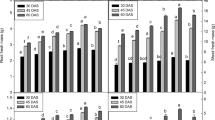
Fresh and dry weights were determined in 10-day-old seedlings (Fig. 2). MEL treatments improved both parameters (FW and DW), both in the absence and in the presence of the F. It is observed that the increasing concentrations of F significantly decreased the values of FW and DW, MEL significantly increased both parameter in a concentration depending on way except for F30ME20 in FW, being these values in FW around 7% at 0.3 and 30 mM F and 14% at 3 mM F (Fig. 2A), and DW up to 23% in treatments with F30 (Fig. 2B).
Fresh (A) and dry weight (B) of 10-day-old seedlings of Vigna radiata L. treated with distilled water (C, white bars), MEL (MEL, 20 or 100 µM, yellow bars), fungicide (F, 0.3, 3, or 30 mM, blue bars), and yellow-blue bars represent the co-treatments. Data are represented as means ± SE (n = 20). Different superscript letters indicate statistically significant differences at p < 0.05 (Color figure online)
Figure 3 shows the effect of MEL and F on parameters such as leaf area, stem length, and secondary root number in 10-day-old seedlings. It can be observed how the presence of F negatively affects all of them, having MEL co-treatments a clear safener effect, so in the leaf area at 0.3 mM F only, compared to its counterpart (Fig. 3A), respect to stem length both treatments with MEL improved significantly to F 0.3 y 30 (Fig. 3B), and in the number secondary roots both MEL treatments improved them (Fig. 3C).
Leaf area (A), stem length (B), and number of secondary roots (C) of 10-day-old seedlings of Vigna radiata L. treated with distilled water (C, white bars), MEL (MEL, 20 or 100 µM, yellow bars), fungicide (F, 0.3, 3, or 30 mM, blue bars), and yellow-blue bars represent the co-treatments. Data are represented as means ± SE (n = 15 to Fig. 3A, n = 45 to Figs. 3B and C). Different superscript letters indicate statistically significant differences at p < 0.05 (Color figure online)
Melatonin Protects Photosynthetic Pigments at Low Fungicide Concentrations
A set of biochemical tests were carried out on roots and leaves of mungbean seedlings subjected to stress by F, and the effect of MEL was analyzed. The effects of co-treatment (F + MEL) were studied in photosynthetic pigments (Fig. 4). In chlorophyll-a (Chl-a) only the combination 0.3 mM F + 100 µM MEL showed significant results compared to only F hydroprimed seedlings, while the chlorophyll-b content increased not only in the 0.3 mM F + 100 µM MEL combination but also in both MEL only treatments (Fig. 4A and B). The F had a decreasing effect on the contents of Chl-a and Chl-b, but not in a concentration-dependent manner, decreasing total Chls levels by a maximum approximately of 17% with 30 mM F compared to control without treatments (Fig. 4C). Only in the case of 0.3 mM F, a significant protective effect of MEL100 was observed on Chl-a, Chl-b, and total Chl contents, but also in the latter case, a slight effect was also observed with MEL20. These protective effects, only at low F, are possibly due to the fact that, at high concentrations of F, the protective system is overloaded and does not work properly. Respect to carotenoids, the presence of MEL alone increases their contents, but no significant effect is showed in co-treatment with F (Fig. 4D).
Chlorophyll a (A), chlorophyll b (B), total chlorophyll (C), and carotenoid (D) contents of 10-day-old seedlings of Vigna radiata L. treated with distilled water (C, white bars), MEL (MEL, 20 or 100 µM, yellow bars), fungicide (F, 0.3, 3 or 30 mM, blue bars), and yellow-blue bars represent the co-treatments. Data are represented as means ± SE (n = 3). Different superscript letters indicate statistically significant differences at p < 0.05 (Color figure online)
Membrane Damage is Reduced by Melatonin
Malondialdehyde (MDA) is a final product of lipid peroxidation that can be determined by TBA assay. MDA content in leaves and roots of 10-day-old seedlings by each treatment is shown in Fig. 5. Figure 5A shows that when the higher concentration of F is applied, the highest value of MDA is obtained, being 42% higher than control without treatments, reflecting lipid peroxidation damage caused by the F in leaves. Only in the combination F30MEL20, a protective effect of MEL is observed against the F. In the roots (Fig. 5B), compared to only F-primed plants, MEL priming significantly decreased the MDA contents, a maximum value of 16% for F30MEL20 treatment. The protective effect of MEL against the F followed this order F30MEL20 > F3MEL20 = F0.3MEL20 = F30MEL100 > F0.3MEL100 > F3MEL100.
MDA content in leaves (A) and roots (B) of 10-day-old seedlings of Vigna radiata L. treated with distilled water (C, white bars), MEL (MEL, 20 or 100 µM, yellow bars), fungicide (F, 0.3, 3, or 30 mM, blue bars), and yellow-blue bars represent the co-treatments. Data are represented as means ± SE (n = 3). Different superscript letters indicate statistically significant differences at p < 0.05 (Color figure online)
Protection of Membrane Integrity by Melatonin in Fungicide-Stressed Seedlings
Electrolyte leakage (EL) is a useful parameter to determine membrane operativity. In both leaves and roots, EL was clearly increased by F due to its damage in membranes, and significantly decreased by the protective action of MEL, in a concentration-dependent manner (Fig. 6).
Electrolyte leakage (EL) percentage in leaves (A) and roots (B) of 10-day-old seedlings of Vigna radiata L. treated with distilled water (C, white bars), MEL (MEL, 20 or 100 µM, yellow bars), fungicide (F, 0.3, 3, or 30 mM, blue bars), and yellow-blue bars represent the co-treatments. Data are represented as means ± SE (n = 3). Different superscript letters indicate statistically significant differences at p < 0.05 (Color figure online)
Antioxidant Activity is Compromised by Fungicide but Restored by Melatonin
Hydroprimed seeds with MEL significantly change the antioxidant activity of roots and leaves of 10-day old seedlings (Fig. 7). In leaves, in the absence of F, MEL at both concentrations (MEL20 and MEL100) showed an increase in the antioxidant capacity (Fig. 7A). Only the F30 treatment caused a significant decrease in antioxidant activity. The co-treatments with MEL increased the values of antioxidant activity, especially at 100 μM MEL. A similar behavior appeared in roots, with a gradual response in the co-treatments at 0.3, 3, and 30 mM F, in both MEL treatments (Fig. 7B).
Antioxidant activity in leaves (A) and roots (B) of 10-day-old seedlings of Vigna radiata L. treated with distilled water (C, white bars), MEL (MEL, 20 or 100 µM, yellow bars), fungicide (F, 0.3, 3, or 30 mM, blue bars), and yellow-blue bars represent the co-treatments. Data are represented as means ± SE (n = 3). Different superscript letters indicate statistically significant differences at p < 0.05 (Color figure online)
Phytohormone and Melatonin Content are Differently Affected in Roots and Leaves
MEL content in leaves and roots of 10-day-old seedlings grown from hydroprimed seeds in the different treatments was determined (Fig. 8). In leaves (Fig. 8A), the endogenous MEL content was 0.3 µg/g FW approximately (C). The presence of F (F0.3, F3, and F30) produces an increase in endogenous melatonin compared with the control (C). In roots (Fig. 8B), increased amounts of MEL in the absence of fungicide were measured due to exogenous MEL treatments (MEL20 and MEL100), and natural endogenous MEL content was around 0.7 µg/g FW (C). The presence of F at 3 and 30 mM decreased MEL contents in roots, but its content was recovered in exogenous MEL treatments, especially at 100 μM. A higher MEL content in 0.3 mM F + 100 μM MEL (F0.3MEL100) than in its respective treatment without F (MEL100) was found, which points to a possible stimulating effect of MEL biosynthesis due to F. In the roots, co-treatments with 100 μM MEL, an increase in MEL content was observed, being the highest value for the 0.3 mM F (Fig. 8B).
Melatonin content in leaves (A) and roots (B) of 10-day-old seedlings of Vigna radiata L. treated with distilled water (C, white bars), MEL (MEL, 20 or 100 µM, yellow bars), fungicide (F, 0.3, 3, or 30 mM, blue bars), and yellow-blue bars represent the co-treatments. Data are represented as means ± SE (n = 3). Different superscript letters indicate statistically significant differences at p < 0.05 (Color figure online)
Table S2 shows the analysis of several phytohormones in the roots and leaves of 10-day-old seedlings grown from hydroprimed seeds. In this case, for simplicity, only one treatment (3 mM F and 100 μM MEL) was analyzed. As a first assessment, note that the contents of the different phytohormones differed significantly between the roots and leaves in many cases. Antagonist responses were observed between both organs for some treatments regarding phytohormone contents. Thus, for a simpler visualization, some of the data in Table S2 have been shown graphically (Fig. 9).
Phytohormone content of 10-day-old seedlings of Vigna radiata L. treated with distilled water (C, white bars), MEL (MEL, 100 µM, yellow bars), fungicide (F, 3 mM, blue bars), and yellow-blue bars represent the co-treatments. Total cytokinin (CK) content in leaves (A) and roots (B), total gibberellin (GA) content in leaves (C) and roots (D), indoleacetic acid (IAA) content in leaves (E) and roots (F), 1-aminocyclopropane-1-carboxylic acid (ACC) content in leaves (G) and roots (H), abscisic acid (ABA) content in leaves (I) and roots (J), jasmonic acid (JA) content in leaves (K) and roots (L), and salicylic acid (SA) content in leaves (M) and roots (N). Data are represented as means ± SE (n = 3). Different superscript letters indicate statistically significant differences at p < 0.05 (Color figure online)
MEL alone increased the content of abscisic acid (ABA), 1-aminocyclopropane-1-carboxylic acid (ACC), and total cytokinins (CKs) in the leaves, as well as total gibberellins (GAs) in the roots. Fungicide alone increased the content of CKs, GAs, and jasmonic acid (JA) in the leaves, and salicylic acid (SA) and indoleacetic acid (IAA) contents were increased in the roots. In the leaves, all the phytohormones except IAA increased their values in the co-treatment (F + MEL) compared to control, while in the roots, only IAA and SA showed an increase.
In particular, total CKs were increased by MEL and higher in co-treatment in the leaves (Fig. 9A). Total GAs were decreased by MEL and increased by F and co-treatment in the leaves but decreased by fungicide in the roots (Fig. 9C and D). IAA content was not changed by MEL, induced by F and, in a minor extension, in co-treatment in the roots; but in the leaves, the decrease in IAA content by F was reduced in co-treatment with MEL (Fig. 9E and F). As for ACC, the precursor of ethylene, F and MEL acted synergically to minimize ACC content in roots (Fig. 9H). ABA was accumulated in MEL treatment in the leaves, being reduced its content by F, but in a minor extension in co-treatment. The effects of F and co-treatment were similar in the roots than in the leaves (Fig. 9I and J). JA content was decreased by MEL but increased by F and more by co-treatment in the leaves. On the other hand, in the roots, the opposite effect was observed, a decrease in the JA content induced by F and in the co-treatment (Fig. 9K and L). Regarding SA, co-treatment induced higher SA content, in both leaves and roots, with a higher effect in the roots (Fig. 9M and N).
Discussion
Effect of Cu-Fungicide on Plants
In most studies with toxic agents such as Cu-fungicides, plants are continuously exposed to specific concentrations of the toxic agent, assessing the plant’s physiological response. In our case, we developed an experimental model in which the toxic agent (copper oxychloride) was in contact with plant tissues from the beginning of its development. Thus, the seeds were exposed to Cu-fungicide solutions by hydropriming, being able to integrate Cu ions in tissues, to subsequently monitor its effects on plant development during the initial days.
The applied F does not show any negative effect on the germination rate (Table S1), in a similar way that mungbean seeds treated with copper sulfate (Verma et al. 2011), but inhibition in the development of seedlings was measured. Thus, roots showed a noticeable decreasing growth concerning control seedlings without F, being higher for higher concentration of F, at 4- and 10-day-old seedlings (Fig. 1). This inhibition action of F can be seen also in other organs such as leaf, stem, and secondary roots (Fig. 3). By observing the fresh and dry weight of the seedlings, we could also see this inhibitory effect respecting control (Fig. 2). However, it should be noted that, of the three concentrations of F applied, the lowest (0.3 mM) hardly shows inhibition or appears slightly in almost all the parameters studied.
Effect of Melatonin and Cu-Fungicide Co-Treatments on Plants
Co-treatments of F and MEL (F + MEL) showed a positive effect in most parameters, showing the effect in a concentration-dependent manner. Practically in all the parameters studied, the presence of MEL together with the F induced growth in the roots, especially in 0.3 mM F (Fig. 1), in fresh weight and dry weight at the three concentrations of fungicide tested (Fig. 2), and in the leaf area, stem length, and number of secondary roots (Fig. 3).
When the mungbean seeds were primed with MEL alone, a growth-promoting action was observed on roots, in fresh and dry weight, the leaf area, in the number of secondary roots (Figs. 1, 2, 3A and B, respectively), but not in stem length (Fig. 3B). Thus, the inhibitory action of Cu-fungicide, widely described in many plant species (Mocquot et al. 1996; Maksymiec 1998; Yruela and Yruela 2009; Lequeux et al. 2010), was also observed in our assay in mungbean seedlings, where the inhibitory action of F was reversed or diminished by the presence of MEL as observed in the co-treatments for all growing parameters, in a widely described action of MEL as a biostimulator compound. Many reviews on the biostimulator role of MEL in stress conditions in plants can be consulted (Arnao and Hernández-Ruiz 2019b, c, d; Menhas et al. 2022; Altaf et al. 2021a; Arnao et al. 2022; Moustafa-Farag et al. 2020b, c). Similar results were obtained in red cabbage seedlings where the toxic effect of Cu was not observed in seedlings grown with seeds primed with MEL (Posmyk et al. 2008). Also, in Cu-treated cucumber seedlings, several growth and morphological parameters were improved in the presence of MEL (Cao et al. 2018).
A common response of plants to excess Cu-fungicide is the appearance of chlorosis (Mocquot et al. 1996; Adrees et al. 2015; Ambrosini et al. 2018). In our study, the effect of F on chlorophyll contents (a, b and total, Fig. 4A, B and C) was very noticeable, decreasing up to 20% in its total chlorophyll content at high fungicide concentrations (Fig. 4C). Melatonin had a positive effect on Chl-b and total Chls contents without the presence of F. In the co-treatments (F + MEL), at low F concentration (F0.3 + MEL), a positive effect of MEL was observed on the contents of Chl-a, b and total, but not at high concentrations of F. Concerning carotenoids, MEL alone slightly increased its content in the seedling leaves, having a slightly positive effect on co-treatments (Fig. 4D). Our results fit well with existing knowledge about melatonin's role in photosynthesis and photosynthetic pigments (Arnao et al. 2022). MEL protected the chlorophyll and carotenoid contents in the leaves through two mechanisms: (i) MEL protected from dark-induced senescence in barley leaves, preserving chlorophyll contents (Arnao and Hernández-Ruiz 2009a, b, c). Later, several studies demonstrated that MEL promoted a higher level of chlorophylls and carotenoids in treated plants compared with control plants (Li et al. 2012; Sarropoulou et al. 2012; Szafranska et al. 2017), and in some algae such as Ulva sp. (Tal et al. 2011) and Chara australis (Lazar et al. 2013). MEL downregulates key senescence leaf genes, such as senescence associated genes (SAG12 and SEN4), and chlorophyll degradation-related genes such as pheophorbide a oxygenase (PAO), stay-green (SGR) and red chlorophyll catabolite reductases (RCCR), preserving high chlorophyll contents (Wang et al. 2012, 2013; Arnao and Hernández-Ruiz 2019c; Arnao et al. 2022), and (ii) MEL increased α-, β-carotene, lutein, and zeaxanthin levels in kiwifruit leaves (Liang et al. 2019). Also, several carotenogenesis transcripts, such as 1-deoxy-D-xylulose-5-phosphate synthase (DXS), 1-deoxy-D-xylulose-5-phosphate reducto-isomerase (DXR), geranylgeranyl diphosphate synthase (GGPPS), phytoene synthase (PSY), phytoene desaturase (PDS), ζ-carotene desaturase (ZDS), carotene isomerase (CRTISO), and chromoplast-specific lycopene β-cyclase (CYCB) were upregulated by MEL (Xia et al. 2020, 2021). Similar results were shown in other plants and microalgae (Chen et al. 2018; Ding et al. 2018b; Zhao et al. 2021b, c). Also in tomato fruits, carotenogenesis was induced by MEL in an ethylene-mediated mechanism (Arnao and Hernández-Ruiz 2020; Sun et al. 2020).
Copper ions provoke oxidative stress and cellular damage in membranes as can be seen in Figs. 5 and 6. Malondialdehyde (MDA), a final product of lipid peroxidation, and electrolyte leakage (EL) values were significantly affected by F. Both parameters were increased, in roots and leaves, depending on the concentration of F. In contrast, MEL decreased the values of MDA and EL, showing a protective effect of oxidative damage caused by F. Oxidative stress caused by F could be determined through the antioxidant activity measurements in tissues. Thus, in both roots and leaves, MEL increased antioxidant activity values, more significantly at higher concentrations of F and more strongly at higher concentrations of MEL (Fig. 7). Antioxidant activity data correlate quite well with endogenous MEL levels in 10-day-old leaves and roots. Endogenous MEL contents were increased in seed-primed seedlings with MEL (MEL20 and MEL100) (Fig. 8). In leaves, and to a lesser extent in roots, the F showed a stimulating effect on MEL contents which is explained by the promoting effect of MEL biosynthesis due to oxidative stress. In multiple stress conditions, MEL self-regulates its biosynthesis, increasing the levels of biosynthesis enzyme transcripts to cope with oxidative stress (Arnao and Hernández-Ruiz 2009b, 2013, 2014, 2019a). Also, MEL regulates the homeostasis of the redox network, regulating ROS and RNS levels and related key enzyme expressions such as nitric oxide synthase-like (NOS-like), nitrate reductase (NR), respiratory burst oxidase homologues (RBOHs), ASC-GSH cycle, and antioxidant enzymes (superoxide dismutases, catalases, peroxidases, glutathione transferases, etc.) (Wang et al. 2012; Wei et al. 2015; Siddiqui et al. 2019; Arnao and Hernández-Ruiz 2019c; Yan et al. 2020; Altaf et al. 2021b).
Effect of Co-Treatments on Plant Hormone Contents
In 10-day-old seedlings previously treated through primed seeds, new hormonal homeostasis was established. In general, MEL-alone treatment (MEL100) induced leaf growth, which could be a response to the increase in total CKs, and the number of secondary roots was also increased, possibly due to their own MEL action (Figs. 3C and 9B). Although the action of F was usually inhibitory in all aspects, it was especially in root growth, perhaps for the increase in the levels of IAA in the roots by F (Fig. 9F). This inhibitory effect was also proven in Arabidopsis thaliana where the accumulated auxin in roots by copper excess could explain the reduced primary root growth and the increased density of short lateral roots (Lequeux et al. 2010). MEL usually increases the levels of GAs in roots, but this stimulating effect was significantly inhibited by F (Fig. 9D).
Contrary to previous reports (Potters et al. 2009), but according to (Lequeux et al. 2010), higher ACC content in roots (precursor of ethylene) was not observed in mungbean seedlings in the presence of F, which provoked a high reduction in ACC contents (Fig. 9H); but in leaves, an ACC increased by F and MEL co-treatment was induced (Fig. 9G) which could be responsible for the high inhibition in stem growth and leaf area (Fig. 3A and B). ABA contents were increased by MEL, and curiously decreased by F in leaves (Fig. 9I), and in minor extension in roots (Fig. 9J), possibly due to the homeostasis accommodation of tissues. Regarding SA and JA, plant hormones are usually involved in biotic stress, but they can also have prominence in abiotic stress, several changes in their contents can be pointed out. JA contents in leaves were also increased (Fig. 9K) but decreased in roots by F (Fig. 9L). SA contents were increased importantly in roots by F and co-treatments, and only in co-treatments in leaves (Fig. 9M and N). All these data on the plant hormone contents help us to explain some physiological responses but suffer from the lack of a temporal dynamic study since surely this still photo of 10-day-old seedlings shows us just a status quo of hormonal homeostasis due to previous treatments on the seeds.
The interpretation of the results of the hormonal analysis is not easy, but we have some previous data regarding phytohormones and MEL relationship (Arnao and Hernández-Ruiz 2018). MEL co-participates in the actions of auxin; it is not clear whether it alters endogenous levels of IAA (Arnao and Hernández-Ruiz 2021a). MEL activates or inhibits growth in primary roots depending on their concentration, and promotes rooting, both lateral and adventitious roots, which is in accordance with the data of the present work. MEL not only acts through changes in auxin-signaling elements (ARFs and SAUR) and IAA transport genes (AUX1 and PINs), but also modulates root development transcription factors such as WUSCHEL-related homeobox11 (WOX11) and, in some cases, YUC flavin monooxygenase (YUCCA) genes (Mao et al. 2020). The promoting effect of MEL in rooting has been extensively studied and widely applied (Arnao and Hernández-Ruiz 2017).
MEL upregulated GA biosynthesis genes in cucumber germinated seeds under saline stress (Zhang et al. 2014), in cotton-germinated seeds (Xiao et al. 2019), and in other species, increasing GA levels through the upregulation of gibberellins (GA20ox, GA3ox and GA2ox) genes; also, GID GA-receptor genes were upregulated, which promoted root growth, as can also be seen in our data (Figs. 1 and 9D).
Concerning CKs, exogenous MEL treatments increased CK levels in leaves, and roots of F + MEL co-treatments in roots (Fig. 9A and B). MEL up-regulates CK signaling genes such as ARR- (type A and B) transcription factors, and, reciprocally, CK seems to upregulate some MEL biosynthesis genes, improving physiological responses against stressors (Arnao and Hernández-Ruiz 2021a). The MEL–ABA relationship is controversial. While in studies in cucumber, apple, and cabbage, MEL induced a decrease in ABA level through the upregulation of ABA catabolism genes and the down-regulation of 9-cis-epoxycarotenoid dioxygenase (NCED) gen; in barley, radish, and Elymus mutants, an ABA increased by MEL has been described (Arnao and Hernández-Ruiz 2021a). Our data in mungbean showed an increase in ABA level by MEL in leaves, which was diminished by F, and a negligible response in roots (Fig. 9I and J).
In the case of the relationship between MEL and ethylene, there are many studies but almost all of them are about ripening and post-harvest fruits. During ripening, MEL activated ethylene biosynthesis and several ripening factors, improving shelf life and quality parameters of fruits (Arnao and Hernández-Ruiz 2020). In many fruits, the ACC oxidase (ACO), ACC synthase (ACS) genes, and several ethylene signaling elements (EILs and ERFs) were upregulated by MEL (Arnao and Hernández-Ruiz 2021a). In our study on mungbean seedlings, ACC content increased in leaves and roots, with a presumed promotion of ethylene, which in roots was diminished by the F (Fig. 9G and H).
Regarding JA and SA, MEL treatments alter its content in plant tissues. In Arabidopsis roots, MEL at high concentration inhibited root growth, downregulating JA, CK, and brassinosteroid biosynthesis genes, while GAs, ethylene, and strigolactone biosynthesis genes were upregulated, similarly to our mungbean model (Fig. 9). SA is a plant hormone generally involved in biotic pathogen responses. In Arabidopsis, Pseudomonas syringae DC3000 infection provoked an increase in MEL and SA contents (Lee et al. 2015). In a study in Nicotiana glutinosa and Solanum lycopersicum, MEL increased plant resistance to tobacco mosaic virus increasing antiviral response by increasing SA and NO (nitrogen monoxide) levels (Zhao et al. 2019). Furthermore, a synergistic response between SA and MEL was recently suggested (Haydari et al. 2019; Abd El-Naby et al. 2020). In our study, although MEL alone did not cause a higher SA content, the co-treatment with F marked a clear increase in SA, in leaves and roots (Fig. 9M and N).
Melatonin as a Natural Safener
Based on the above data, MEL improves growth both in isolation and in co-treatments with F, slowing down the inhibitory processes caused by F, with less inhibition being observed at higher concentration of MEL in the different treatments with F. In contrast, MEL reduced membrane damage and increased antioxidant activity in leaves and roots, after 10 days of growth in primed mungbean seeds. For all these reasons, we propose a protective (safener) action for MEL such as that produced with synthetic protectors and herbicides (Giraldo-Acosta et al. 2022).
MEL was also studied as a safener of fungicides in plants. Thus, the application of MEL in co-treatments with the fungicide induced less damage to the plant, and a synergistic effect that increased the effectiveness in pathogen protection, being able to use lower doses of the fungicide ensuring the plant protection (Giraldo-Acosta et al. 2022). The present study describes for the first time the safener effect of MEL from a copper-based fungicide and copper oxychloride. The data show the beneficial effect of MEL on morphological and biochemical parameters in mungbean seedlings, thanks to its biostimulant effect on growth in response to oxidative stress generated by F. As previously proposed, in the co-treatments of MEL and pesticides, the activation of the redox network and the specific response of MEL against toxic substances results in greater tolerance to the stressor, rearranging the homeostasis of the plant, through its hormonal and osmoregulatory response. Figure 10 shows an outline of the possible synergistic action of MEL and F, and their safener function on plants, activating the redox network, antioxidative detoxification pathway, and pathogen response (Moustafa-Farag et al. 2020a), which translates into less damage to the plants (Giraldo-Acosta et al. 2022).
General model of MEL action as a safener in abiotic stress responses induced by a Cu-fungicide adapted from Giraldo-Acosta et al. (2022). The different elements integrated in the response of MEL as a safener in stress situations are represented in green boxes. Red boxes show the elements involved in the damage produced by pathogen (fungi) in plants. Blue box represents the effect of Cu-fungicide which is capable to protect the plant against pathogen disease, but also, if it is not correctly detoxified, to produce plant damage (Color figure online)
Conclusion
MEL is a natural compound with wide possibilities in agronomy and post-harvest. Its action as a biostimulating agent and as a regulator of plant hormonal and redox networks has suggested interesting possibilities as a protection and improvement tool in crops. In this work, we have demonstrated, in mungbean seedlings, the safener effect of MEL against F (copper oxychloride), a widely used fungicide. MEL improved growth and hormonal responses to F excess, increasing plant tolerance. Its application in crops as a natural safener together with pesticides (herbicides, fungicides, insecticides, etc.) opens up a range of possible uses focused on obtaining better resistance and tolerance responses in plants. So far, studies of MEL as a protector are very scarce. It has been tested in co-treatments with 2–3 herbicides and 2–3 fungicides, with very interesting results. The data from our study and other previous data indicate that MEL exerts a biostimulating, detoxifying, and synergistic effect when used together with fungicides and others and can be used in eco-friendly applications that could reduce pesticide doses.
References
Abd El-Naby S, Mohamed AAA, Baiea M (2020) Mitigation of heat stress effects on Whasington navel orange by using melatonin, gibberellin and salicylic treatments. Plant Arch 20:3523–3534
Adrees M, Ali S, Rizwan M et al (2015) The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res 22:8148–8162. https://doi.org/10.1007/s11356-015-4496-5
Afreen F, Zobayed SMA, Kozai T (2006) Melatonin in Glycyrrhiza uralensis : response of plant roots to spectral quality of light and UV-B radiation. J Pineal Res 41:108–115
Ahn HR, Kim YJ, Lim YJ et al (2021) Key genes in the melatonin biosynthesis pathway with circadian rhythm are associated with various abiotic stresses. Plants 10:129
Altaf MA, Shahid R, Ren MX et al (2021a) Phytomelatonin: an overview of the importance and mediating functions of melatonin against environmental stresses. Physiol Plantarum 172:820–846. https://doi.org/10.1111/ppl.13262
Altaf MA, Shahid R, Ren MX et al (2021b) Melatonin alleviates salt damage in tomato seedling: a root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Sci Hort 285:110145
Ambrosini VG, Rosa DJ, Bastos de Melo GW et al (2018) High copper content in vineyard soils promotes modifications in photosynthetic parameters and morphological changes in the root system of ‘Red Niagara’ plantlets. Plant Physiol Biochem 128:89–98. https://doi.org/10.1016/j.plaphy.2018.05.011
Arnao MB, Hernández-Ruiz J (2007) Melatonin promotes adventitious- and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J Pineal Res 42:147–152
Arnao MB, Hernández-Ruiz J (2009a) Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J Pineal Res 46:58–63
Arnao MB, Hernández-Ruiz J (2009b) Chemical stress by different agents affects the melatonin content of barley roots. J Pineal Res 46:295–299
Arnao MB, Hernández-Ruiz J (2009c) Assessment of different sample processing procedures applied to the determination of melatonin in plants. Phytochem Anal 20:14–18
Arnao MB, Hernández-Ruiz J (2013) Growth conditions determine different melatonin levels in Lupinus albus L. J Pineal Res 55:149–155
Arnao MB, Hernández-Ruiz J (2014) Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci 19:789–797. https://doi.org/10.1016/j.tplants.2014.07.006
Arnao MB, Hernández-Ruiz J (2017) Growth activity, rooting capacity, and tropism: three auxinic precepts fulfilled by melatonin. Acta Physiol Plant 39:127
Arnao MB, Hernández-Ruiz J (2018) Melatonin in its relationship to plant hormones. Ann Bot 121:195–207
Arnao M, Hernández-Ruiz J (2019) Melatonin and reactive oxygen and nitrogen species: a model for the plant redox network. Melatonin Res 2:152–168. https://doi.org/10.32794/11250036
Arnao MB, Hernández-Ruiz J (2019b) Role of melatonin to enhance phytoremediation capacity. Appl Sci 9:5293. https://doi.org/10.3390/app9245293
Arnao MB, Hernández-Ruiz J (2019c) Melatonin: a new plant hormone and/or a plant master regulator? Trends Plant Sci 24:38–48. https://doi.org/10.1016/j.tplants.2018.10.010
Arnao MB, Hernández-Ruiz J (2019d) Melatonin as a chemical substance or as phytomelatonin rich-extracts for use as plant protector and/or biostimulant in accordance with EC legislation. Agronomy 9:570. https://doi.org/10.3390/agronomy9100570
Arnao MB, Hernández-Ruiz J (2020) Melatonin in flowering, fruit set and fruit ripening. Plant Reprod 33:77–87. https://doi.org/10.1007/s00497-020-00388-8
Arnao MB, Hernández-Ruiz J (2021a) Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol 23:7–19. https://doi.org/10.1111/plb.13202
Arnao MB, Hernández-Ruiz J (2021b) Melatonin as a plant biostimulant in crops and during post-harvest: a new approach is needed. J Sci Food Agric 101:5297–5304. https://doi.org/10.1002/jsfa.11318
Arnao MB, Hernández-Ruiz J (2022) Melatonin against environmental plant stressors: a review. Curr Protein Pept Sci 22:413–429. https://doi.org/10.2174/1389203721999210101235422
Arnao MB, Cano A, Acosta M (1999) Methods to measure the antioxidant activity in plant material. A comparative discussion. Free Rad Res 31:89–96
Arnao MB, Cano A, Hernández-Ruiz J (2022) Phytomelatonin: an unexpected molecule with amazing performances in plants. J Exp Bot. https://doi.org/10.1093/jxb/erac009
Burkhead JL, Gogolin Reynolds KA, Abdel-Ghany SE et al (2009) Copper homeostasis. New Phytol 182:799–816. https://doi.org/10.1111/j.1469-8137.2009.02846.x
Cano A, Arnao MB (2018) ABTS/TEAC (2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)/trolox-equivalent antioxidant capacity) radical scavenging mixed-mode assay. In: Apak R, Capanoglu E, Shahidi F (eds) Measurement of antioxidant activity & capacity. Recent trends and applications. John Wiley & Sons, Oxford, pp 117–139
Cao YY, Qi CD, Li S et al (2018) Melatonin alleviates copper toxicity via improving copper sequestration and ROS scavenging in cucumber. Plant Cell Physiol 60:562–574. https://doi.org/10.1093/pcp/pcy226
Caputo G, Wadl P, McCarty L et al (2020) In vitro safening of bentazon by melatonin in sweetpotato (Ipomoea batatas). HortScience 55:1406–1410
Chen YE, Mao J-J, Sun L-Q et al (2018) Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol Plant 164:349–363. https://doi.org/10.1111/ppl.12737
Ding F, Wang G, Zhang S (2018a) Exogenous melatonin mitigates methyl viologen-triggered oxidative stress in poplar leaf. Molecules 23:2852. https://doi.org/10.3390/molecules23112852
Ding W, Zhao P, Peng J et al (2018b) Melatonin enhances astaxanthin accumulation in the green microalga Haematococcus pluvialis by mechanisms possibly related to abiotic stress tolerance. Algal Res 33:256–265
Dra̧zkiewicz M, Skórzyńska-Polit E, Krupa Z, (2004) Copper-induced oxidative stress and antioxidant defence in Arabidopsis thaliana. Biometals 17:379–387. https://doi.org/10.1023/B:BIOM.0000029417.18154.22
Dubbels R, Reiter RJ, Klenke E et al (1995) Melatonin in edible plants identified by radioimmunoassay and by HPLC-MS. J Pineal Res 18:28–31
Elalfy M, Abomosallam M, Elhadidy M, Sleem F (2021) Copper and copper containing pesticide as copper oxychloride toxicity and its adverse effects on animal and human health. Med Res Chronicles 8:2021. https://doi.org/10.26838/MEDRECH.2021.8.2.486
Giraldo-Acosta M, Cano A, Hernández-Ruiz J, Arnao MB (2022) Melatonin as a possible natural safener in crops. Plants 11:890. https://doi.org/10.3390/plants11070890
Hatsugai N, Katagiri F (2018) Quantification of plant cell death by electrolyte leakage assay. Bio-Protoc 8:e2758–e2758
Hattori A, Migitaka H, Iigo M et al (1995) Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int 35:627–634
Haydari M, Rigano D et al (2019) Salicylic acid and melatonin alleviate the effects of heat stress on essential oil composition and antioxidant enzyme activity in Mentha x Piperita and Mentha Arvensis L. Antioxidants 8:547
Hernández-Ruiz J, Cano A, Arnao MB (2004) Melatonin: a growth-stimulating compound present in lupin tissues. Planta 220:140–144. https://doi.org/10.1007/s00425-004-1317-3
Hernández-Ruiz J, Cano A, Arnao MB (2005) Melatonin acts as a growth-stimulating compound in some monocot species. J Pineal Res 39:137–142
Hoffman OL (1969) Chemical antidotes for EPTC on corn. Abstracts Weed Sci Soc Am 9:12
Hoque M, Tahjib-Ul-Arif M, Hannan A et al (2021) Melatonin modulates plant tolerance to heavy metal stress: morphological responses to molecular mechanisms. Int J Mol Sci 22:11445
Kolar J, Machackova I, Illnerova H et al (1995) Melatonin in higher plant determined by radioimmunoassay and liquid chromatography-mass spectrometry. Biol Rhythm Res 26:406–409
Lazar D, Murch SJ, Beilby MJ, Al Khazaaly S (2013) Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant Sign Behav 8:e23279
Lee HY, Byeon Y, Tan DX et al (2015) Arabidopsis serotonin N-acetyltransferase knockout mutant plants exhibit decreased melatonin and salicylic acid levels resulting in susceptibility to an avirulent pathogen. J Pineal Res 58:291–299
Lei XY, Zhu RY, Zhang GY, Dai YR (2004) Attenuation of cold-induced apoptosis by exogenous melatonin in carrot suspension cells: the possible involvement of polyamines. J Pineal Res 36:126–131
Lequeux H, Hermans C, Lutts S, Verbruggen N (2010) Response to copper excess in Arabidopsis thaliana: Impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol Biochem 48:673–682. https://doi.org/10.1016/j.plaphy.2010.05.005
Lerner AB, Case JD, Takahashi Y et al (1958) Isolation of melatonin, a pineal factor that lightens melanocytes. J Am Chem Soc 80:2587
Lerner AB, Case JD, Heinzelmann RV (1959a) Structure of melatonin. J Am Chem Soc 81:6084–6085
Lerner AB, Case JD, Mori W, Wright MR (1959b) Melatonin in peripheral nerve. Nature 183:1821
Li C, Wang P, Wei Z et al (2012) The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J Pineal Res 53:298–306
Li X, Rengel Z, Chen Q (2022) Phytomelatonin prevents bacterial invasion during nighttime. Trends Plant Sci 27:331–334. https://doi.org/10.1016/j.tplants.2021.12.008
Liang D, Ni Z, Xia H et al (2019) Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci Hort 246:34–43
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592. https://doi.org/10.1042/bst0110591
Maksymiec W (1997) Effect of copper in higher plants. Photosynthetica 34:321–342
Maksymiec W (1998) Effect of copper on cellular processes in higher plants. Photosynthetica 34:321–342. https://doi.org/10.1023/A:1006818815528
Mao J, Chundong N, Li K et al (2020) Melatonin activates adventitious root formation by promoting the function of MdWOX11 in apple. BMC Plant Biol. https://doi.org/10.21203/rs.3.rs-29239/v1
Menhas S, Yang X, Hayat K et al (2022) Exogenous melatonin enhances Cd tolerance and phytoremediation efficiency by ameliorating Cd-induced stress in oilseed crops: a review. J Plant Growth Regul 41:922–935. https://doi.org/10.1007/s00344-021-10349-8
Mocquot B, Vangronsveld J, Clijsters H, Mench M (1996) Copper toxicity in young maize (Zea mays L.) plants: effects on growth, mineral and chlorophyll contents, and enzyme activities. Plant Soil 182:287–300. https://doi.org/10.1007/BF00029060
Motsenbocker CE, Monaco TJ (1991) Sweet potatoes (Ipomoea batatas) differ in response to bentazon. Weed Technol 5:345–350
Moustafa-Farag M, Almoneafy A, Mahmoud A et al (2020a) Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 10:54
Moustafa-Farag M, Elkelish A, Dafea M et al (2020b) Role of melatonin in plant tolerance to soil stressors: salinity, pH and heavy metals. Molecules 25:5359. https://doi.org/10.3390/molecules25225359
Moustafa-Farag M, Mahmoud A, Arnao MB et al (2020c) Melatonin-induced water stress tolerance in plants: recent advances. Antioxidants 9:809
Nawaz K, Chaudhary R, Sarwar A et al (2020) Melatonin as master regulator in plant growth, development and stress alleviator for sustainable agricultural production: current status and future perspectives. Sustainability 13:294
Niehaus WG Jr, Samuelsson B (1968) Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:126–130. https://doi.org/10.1111/j.1432-1033.1968.tb00428.x
Pardo-Hernández M, López-Delacalle M, Rivero RM (2020) ROS and NO regulation by melatonin under abiotic stress in plants. Antioxidants 9:1078
Park S, Lee DE, Jang H et al (2013) Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J Pineal Res 54:258–263
Pätsikkä E, Kairavuo M, Šeršen F et al (2002) Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol 129:1359–1367. https://doi.org/10.1104/pp.004788
Posmyk MM, Kuran H, Marciniak K, Janas KM (2008) Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J Pineal Res 45:24–31
Posmyk MM, Balabusta M, Wieczorek M et al (2009) Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J Pineal Res 46:214–223
Potters G, Pasternak TP, Guisez Y, Jansen MAK (2009) Different stresses, similar morphogenic responses: Integrating a plethora of pathways. Plant Cell Environ 32:158–169. https://doi.org/10.1111/j.1365-3040.2008.01908.x
Sarropoulou VN, Dimassi-Theriou KN, Therios IN, Koukourikou-Petridou M (2012) Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium x Prunus cerasus). Plant Physiol Biochem 61:162–168
Sharif R, Xie C, Zhang H et al (2018) Melatonin and its effects on plant systems. Molecules 23:2352
Sharma A, Kumar V, Shahzad B et al (2020) Photosynthetic response of plants under different abiotic stresses: a review. J Plant Growth Regul 39:509–531
Siddiqui HM, Alamri S, Al-Khaishany YM et al (2019) Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int J Mol Sci 20:353
Sun Q, Liu L, Zhang L et al (2020) Melatonin promotes carotenoid biosynthesis in an ethylene-dependent manner in tomato fruits. Plant Sci 298:110580
Szafranska K, Reiter RJ, Posmyk MM (2017) Melatonin improves the photosynthetic apparatus in pea leaves stressed by paraquat via chlorophyll breakdown regulation and its accelerated de novo synthesis. Front Plant Sci 8:878. https://doi.org/10.3389/fpls.2017.00878
Tal O, Haim A, Harel O, Gerchman Y (2011) Melatonin as an antioxidant and its semi-lunar rhythm in green macroalga Ulva sp. J Exp Bot 62:1903–1910
Tiwari RK, Lal MK, Kumar R et al (2021) Insight into melatonin-mediated response and signaling in the regulation of plant defense under biotic stress. Plant Mol Biol. https://doi.org/10.1007/s11103-021-01202-3
Tripathi GD, Javed Z, Mishra M et al (2021) Phytomelatonin in stress management in agriculture. Heliyon 7:e06150
Verma JP, Singh V, Yadav J (2011) Effect of copper sulphate on seed germination, plant growth and peroxidase activity of mung bean (Vigna radiata). Int J Bot 7:200–204
Villanova J, Cano A, Albacete A et al (2017) Multiple factors influence adventitious rooting in carnation (Dianthus caryophyllus L.) stem cuttings. Plant Growth Regul 81:511–521. https://doi.org/10.1007/s10725-016-0228-1
Wang P, Yin L, Liang D et al (2012) Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate-glutathione cycle. J Pineal Res 53:11–20
Wang P, Sun X, Chang C et al (2013) Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J Pineal Res 55:424–434
Wang LF, Li TT, Zhang Y et al (2021) CAND2/PMTR1 is required for melatonin-conferred osmotic stress tolerance in arabidopsis. Int J Mol Sci 22:4014
Wei W, Li Q, Chu Y-N et al (2015) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot 66:695–707
Wei J, Li D, Zhang J et al (2018a) Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J Pineal Res 65:e12500. https://doi.org/10.1111/jpi.12500
Wei Z, Gao T, Liang B et al (2018b) Effects of exogenous melatonin on methyl viologen-mediated oxidative stress in apple leaf. Int J Mol Sci 19:316
Xia H, Ni Z, Hu R et al (2020) Melatonin alleviates drought stress by a non-enzymatic and enzymatic antioxidative system in kiwifruit seedlings. Int J Mol Sci 21:852
Xia H, Zhou Y, Deng H et al (2021) Melatonin improves heat tolerance in Actinidia deliciosa via carotenoid biosynthesis and heat shock proteins expression. Physiol Plantarum 172:1582–1593. https://doi.org/10.1111/ppl.13350
Xiao S, Liu L, Wang H et al (2019) Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L.). PLoS ONE 14:e0216575. https://doi.org/10.1371/journal.pone.0216575
Yan Y, Sun S, Zhao N et al (2019) COMT1 overexpression resulting in increased melatonin biosynthesis contributes to the alleviation of carbendazim phytotoxicity and residues in tomato plants. Environ Pollut 252:51–61
Yan H, Jia S, Mao P (2020) Melatonin priming alleviates aging-induced germination inhibition by regulating B-oxidation, protein translation, and antioxidant metabolism in oat ( Avena sativa L.) seeds. Int J Mol Sci 21:1898
Yang Q, Peng Z, Ma W et al (2021) Melatonin functions in priming of stomatal immunity in Panax notoginseng and Arabidopsis thaliana. Plant Physiol 187:2837–2851. https://doi.org/10.1093/plphys/kiab419
Yruela I, Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36:409–430. https://doi.org/10.1071/FP08288
Zhang HJ, Zhang N, Yang RC et al (2014) Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J Pineal Res 57:269–279
Zhao C-R, Ikka T, Sawaki Y et al (2009) Comparative transcriptomic characterization of aluminum, sodium chloride, cadmium and copper rhizotoxicities in Arabidopsis thaliana. BMC Plant Biol 9:32. https://doi.org/10.1186/1471-2229-9-32
Zhao L, Chen L, Gu P et al (2019) Exogenous application of melatonin improves plant resistance to virus infection. Plant Pathol 68:1287–1295. https://doi.org/10.1111/ppa.13057
Zhao D, Wang H, Chen S et al (2021a) Phytomelatonin: an emerging regulator of plant biotic stress resistance. Trends Plant Sci 26:70–82. https://doi.org/10.1016/j.tplants.2020.08.009
Zhao Y, Cui J, Li Q, et al (2021b) A joint strategy comprising melatonin and 3-methyladenine to concurrently stimulate biomass and astaxanthin hyperaccumulation by Haematococcus pluvialis. Bioresource Technology 341:125784. https://www.sciencedirect.com/science/article/pii/S0960852421011251
Zhao Y, Song X, Zhao P et al (2021) Role of melatonin in regulation of lipid accumulation, autophagy and salinity-induced oxidative stress in microalga Monoraphidium sp. QLY-1. Algal Res 54:102196
Zhao C, Nawaz G, Cao Q, Xu T (2022) Melatonin is a potential target for improving horticultural crop resistance to abiotic stress. Sci Hort 291:110560
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work has been funded through the project of the Ministry of Science and Innovation "R + D + I Projects," State Program for the Generation of Knowledge and Scientific and Technological Strengthening of the R + D + I System and R + D + I Oriented to the Challenges of Society of the State Plan for Scientific and Technical Research and Innovation 2017–2020, Grant PID2020-113029RB-I00 funded by MCIN/AEI/1013039/501100011033. More information in: https://www.um.es/en/web/phytohormones/, accessed on september 24, 2022 (Phytohormones & Plant Development Lab).
Author information
Authors and Affiliations
Contributions
MBA and JHR: conceptualization. AC and MGA: methodology. AC: software. MBA: validation. MGA, CMA, and PAMM: investigation. MBA: resources. AC and JHR: data curation. MGA and MBA: writing—original draft preparation. MGA, AC, JHR, and MBA: writing—review and editing. MGA: visualization. MBA: supervision. JHR: project administration. JHR and MBA: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Handling Editor: M. Naeem.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giraldo-Acosta, M., Martínez-Andújar, C., Martínez-Melgarejo, P.A. et al. Protective Effect (Safener) of Melatonin on Vigna Radiata L. Seedlings in the Presence of the Fungicide Copper Oxychloride. J Plant Growth Regul 42, 4918–4934 (2023). https://doi.org/10.1007/s00344-022-10886-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10886-w