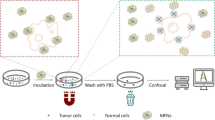
Abstract
Cancer-related deaths are rising every day due to the lack of efficient detection methods in the primary stages, especially before metastasis, which is the main reason for the uncontrollability of cancer. Thus, a novel method is urgently required to efficiently and accurately identify circulating cancer cells scattered in the blood of patients with the primary stages of cancer. Here, in this work, a new fluorescent biosensor based on amine-functionalized graphene quantum dot (af-GQD) was constructed through a hydrothermal method from graphene oxide. Afterward, af-GQD was grafted with HA (GQD-HA) for specific detection through a ligand–receptor concept in which HA recognizes CD44 receptors, which are overexpressed on certain cancer cells such as A549, MDA-MB-231, MCF-7, and HeLa. Then, the intensity of fluorescent signals detected from each cell line media was assessed and quantified. Besides, the designed nanocomposite can be used as a bioimaging agent due to its remarkable properties, such as high fluorescent emission, good compatibility, low cytotoxicity, high resistance to photobleaching, and chemical stability. The results indicated that the designed nanocomposite could act either as an efficient a bioimaging agent or as a biosensor with high specificity and sensitivity with a linear detection range from 500 to 5000 cells/ml for the detection of CTCs. The limit of detection for cell lines was 95, 98, 112, 123 cell/ml for MDA-MB-231, A549, MCF-7, and HeLa, respectively.











Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article
Abbreviations
- CTC:
-
Circulating tumor cell
- DI:
-
Deionized
- DLS:
-
Dynamic light scattering
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMF:
-
Dimethyl formamide
- DMSO:
-
Dimethyl sulfoxide
- EDC:
-
1-Ethyl-3-(3-dimethyl-aminopropyl) carbodiimide
- EDX:
-
Energy-dispersive X-ray spectroscopy
- EMT:
-
Epithelial–mesenchymal transition
- FBS:
-
Fetal bovine serum
- FTIR:
-
Fourier-transform infrared spectroscopy
- GO:
-
Graphene oxide
- GQD:
-
Graphene quantum dot
- af-GQD:
-
Amino-functionalized graphene quantum dots
- GQD-HA:
-
Hyaluronic acid-amino-functionalized graphene quantum dots
- HA:
-
Hyaluronic acid
- rGO:
-
Reduced graphene oxide
- NHS:
-
N-Hydroxysuccinimide
- MES:
-
2-(N-Morpholino) ethanesulfonic acid hemi sodium salt
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS:
-
Phosphate-buffered saline
- PEG:
-
Polyethylene glycol
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscopy
- UV:
-
Ultraviolet
- Vis:
-
Visible
References
H. Sung et al., Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021)
S. Asghari, M. Mahmoudifard, Core-shell nanofibrous membrane of polycaprolactone-hyaluronic acid as a promising platform for the efficient capture and release of circulating tumor cells. Polym. Adv. Technol. 32(3), 1101–1113 (2020)
P. Rodrigues, S. Vanharanta, Circulating tumor cells: come together, right now, over metastasis. Cancer Discov. 9(1), 22–24 (2019)
F. Chemi et al., Early dissemination of circulating tumor cells: biological and clinical insights. Front. Oncol. 11, 1423 (2021)
Y. Yu et al., Design of a biocompatible and ratiometric fluorescent probe for the capture, detection, release, and reculture of rare number CTCs. Anal. Chem. 90(22), 13290–13298 (2018)
D. Naor et al., CD44 in cancer. Crit. Rev. Clin. Lab. Sci. 39(6), 527–579 (2002)
H. Xu et al., CD44 as a tumor biomarker and therapeutic target. Exp. Hematol. Oncol. 9(1), 1–14 (2020)
C. Chen et al., The biology and role of CD44 in cancer progression: therapeutic implications. J. Hematol. Oncol. 11(1), 1–23 (2018)
N. Al-Othman et al., Role of CD44 in breast cancer. Breast Dis. 39(1), 1–13 (2020)
O. Kearns, A. Camisasca, S. Giordani, Hyaluronic acid-conjugated carbon nanomaterials for enhanced tumour targeting ability. Molecules 27(1), 48 (2021)
N.J. Singhai, R. Maheshwari, S. Ramteke, CD44 receptor targeted ‘smart’multi-walled carbon nanotubes for synergistic therapy of triple-negative breast cancer. Colloid Interface Sci. Commun. 35, 100235 (2020)
M. Ma et al., Hyaluronic acid-conjugated mesoporous silica nanoparticles: excellent colloidal dispersity in physiological fluids and targeting efficacy. J. Mater. Chem. 22(12), 5615–5621 (2012)
X. Pan et al., Light-nutrition coupling effect of degradable fluorescent carbon dots on lettuce. Environ. Sci. Nano 10(2), 539–551 (2023)
A. Wang et al., Rare earth-free composites of carbon dots/metal–organic frameworks as white light emitting phosphors. J. Mater. Chem. C 7(8), 2207–2211 (2019)
F. Kang et al., Spectral tuning, stabilities under external exposures, and spontaneous enhancement of emission intensity in grown-into-glass all-inorganic metal halide perovskite nanocrystals. Laser Photonics Rev. 17(1), 2200166 (2022)
S. Asghari, et al., The role of the nanofibers in lateral flow assays enhancement: a critical review. Int. J. Polym. Mater. Polym. Biomater. 1–14 (2022)
M. Mahmoudifard, Thermosensetive Nanofibrous Structure for Exosome Isolation. Google Patents (2021)
E. Ekrami, M. Khodabandeh Shahraky, M. Mahmoudifard, M.S. Mirtaleb, P. Shariati, Biomedical applications of electrospun nanofibers in industrial world: a review. Int. J. Polym. Mater. Polym. Biomater. 72(7), 561–575 (2022)
M. Mahmoudifard, A.M. Shoushtari, A. Mohsenifar, Fabrication and characterization study of electrospun quantum dot—poly vinyl alcohol composite nanofiber for novel engineering applications. Fibers Polym. 13(8), 1031–1036 (2012)
P. Ranjbarvan et al., Natural compounds for skin tissue engineering by electrospinning of nylon-Beta vulgaris. ASAIO J. 64(2), 261–269 (2018)
S. Zamanlui et al., Enhanced chondrogenic differentiation of human bone marrow mesenchymal stem cells on PCL/PLGA electrospun with different alignments and compositions. Int. J. Polym. Mater. Polym. Biomater. 67(1), 50–60 (2018)
N. Borzooee Moghadam et al., Graphene family in cancer therapy: recent progress in cancer gene/drug delivery applications. J. Mater. Chem. B 11(12), 2568–2613 (2023)
N. Vahedi, F. Tabandeh, M. Mahmoudifard, Hyaluronic acid–graphene quantum dot nanocomposite: potential target drug delivery and cancer cell imaging. Biotechnol. Appl. Biochem. 69(3), 1068–1079 (2021)
M. Pouresmaieli et al., A comprehensive review on efficient approaches for combating coronaviruses. Biomed. Pharmacother. 144, 112353 (2021)
E. Ekrami et al., A review on designing biosensors for the detection of trace metals. Appl. Geochem. 127, 104902 (2021)
M. Mahmoudifard, A.M. Shoushtari, M. Shanehsaz, Quantum dot/polyvinyl alcohol composite nanofibers membrane as highly sensitive fluorescence quenching-based sensors. Fibers Polym. 15(9), 1797–1803 (2014)
E. Ekrami et al., Potential diagnostic systems for coronavirus detection: a critical review. Biol. Proced. Online 22(1), 21 (2020)
S. Asghari et al., Nanostructure materials: efficient strategies for circulating tumor cells capture, release, and detection. Biotechnol. Bioprocess. Eng. 26(4), 529–545 (2021)
S. Asghari, M. Mahmoudifard, Core-shell nanofibrous membrane of polycaprolactone-hyaluronic acid as a promising platform for the efficient capture and release of circulating tumor cells. Polym. Adv. Technol. 32(3), 1101–1113 (2021)
F. Cui et al., A novel magnetic fluorescent biosensor based on graphene quantum dots for rapid, efficient, and sensitive separation and detection of circulating tumor cells. Anal. Bioanal. Chem. 411(5), 985–995 (2019)
L. Ruiyi et al., Electrochemical detection of cancer cells in human blood using folic acid and glutamic acid-functionalized graphene quantum dot-palladium@ gold as redox probe with excellent electrocatalytic activity and target recognition. Sens. Actuators B Chem. 309, 127709 (2020)
X. Zhang et al., Shining luminescent graphene quantum dots: synthesis, physicochemical properties, and biomedical applications. Trends Anal. Chem. 116, 109–121 (2019)
J. Zhao et al., Graphene quantum dots as effective probes for label-free fluorescence detection of dopamine. Sens. Actuators B Chem. 223, 246–251 (2016)
J. Shi et al., A fluorescence turn-on biosensor based on graphene quantum dots (GQDs) and molybdenum disulfide (MoS2) nanosheets for epithelial cell adhesion molecule (EpCAM) detection. Biosens. Bioelectron. 93, 182–188 (2017)
F. Du et al., Dual-ligand functionalized carbon nanodots as green fluorescent nanosensors for cellular dual receptor-mediated targeted imaging. Analyst 144(22), 6729–6735 (2019)
Y. Liu et al., Paclitaxel delivered by CD44 receptor-targeting and endosomal pH sensitive dual functionalized hyaluronic acid micelles for multidrug resistance reversion. Colloids Surf. B 170, 330–340 (2018)
J. Yoon et al., CD44 receptor-mediated/reactive oxygen species-sensitive delivery of nanophotosensitizers against cervical cancer cells. Int. J. Mol. Sci. 23(7), 3594 (2022)
M. Mahmoudifard et al., The different fate of satellite cells on conductive composite electrospun nanofibers with graphene and graphene oxide nanosheets. Biomed. Mater. 11(2), 025006 (2016)
Z. Sun, D.K. James, J.M. Tour, Graphene chemistry: synthesis and manipulation. J. Phys. Chem. Lett. 2(19), 2425–2432 (2011)
H. Sun et al., Highly photoluminescent amino-functionalized graphene quantum dots used for sensing copper ions. Chemistry 19(40), 13362–13368 (2013)
M. Soleymani et al., One-pot preparation of hyaluronic acid-coated iron oxide nanoparticles for magnetic hyperthermia therapy and targeting CD44-overexpressing cancer cells. Carbohydr. Polym. 237, 116130 (2020)
J.-D. Xie, G.-W. Lai, M.M. Huq, Hydrothermal route to graphene quantum dots: effects of precursor and temperature. Diam. Relat. Mater. 79, 112–118 (2017)
A. Basu et al., Hyaluronic acid engrafted metformin loaded graphene oxide nanoparticle as CD44 targeted anti-cancer therapy for triple negative breast cancer. Biochimica et Biophysica Acta (BBA) Gen. Subj. 1865(3), 129841 (2021)
A.-A. Nahain et al., Target delivery and cell imaging using hyaluronic acid-functionalized graphene quantum dots. Mol. Pharm. 10(10), 3736–3744 (2013)
J. Tao, S. Feng, B. Liu, J. Pan, C. Li, Y. Zheng, Hyaluronic acid conjugated nitrogen-doped graphene quantum dots for identification of human breast cancer cells. Biomed. Mater. 16(5), 055001 (2021)
S. Asghari, M. Mahmoudifard, The detection of the captured circulating tumor cells on the core-shell nanofibrous membrane using hyaluronic acid-functionalized graphene quantum dots. J. Biomed. Mater. Res. B Appl. Biomater. 111(5), 1121–1132 (2023)
X. Hu et al., Construction and comparison of BSA-stabilized functionalized GQD composite fluorescent probes for selective trypsin detection. New J. Chem. 42(21), 17718–17724 (2018)
P. Mishra, B.R. Bhat, A study on the electro-reductive cycle of amino-functionalized graphene quantum dots immobilized on graphene oxide for amperometric determination of oxalic acid. Microchim. Acta 186(9), 1–10 (2019)
Acknowledgements
This study was made possible by a grant with the number of 750 from the National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Avatefi, M., Mahmoudifard, M. & Moghadam, N.B. Amine-functionalized graphene quantum dots–hyaluronic acid nanocomposite as a high-resolution cancer cell bioimaging and biosensing system. Appl. Phys. A 129, 435 (2023). https://doi.org/10.1007/s00339-023-06700-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06700-3