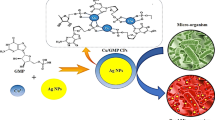
Abstract
Size of the nanoparticles plays a very crucial role in their bactericidal activity as smaller size render better perforation via bacterial cell wall and are thus found to be more effective against resistant bacterial infections also. In this regard, fluorescent silver nanoclusters (Ag NCs) having a size less than 2 nm ought to be a potent bactericidal agent against both Gram positive and Gram negative bacterial strains. However, stabilization of such small sized NPs in aqueous medium that is amenable for biological applications is very challenging. Herein, a simple and fast method of synthesis of Ag NCs was proposed using metal–organic framework (MOFs) as scaffold, which was found to be very stable in the ambient conditions. As-synthesized Ag NCs-in-MOF nanocomposite was characterized by UV–Vis spectroscopy, transmission electron microscopy (TEM), and X-ray photoelectron spectroscopy (XPS). The nanocomposite was highly bactericidal against both Gram positive and Gram negative bacterial strains and caused cell wall perforation that was evident from flow cytometry analysis. Results also showed that DNA damage had occurred due to treatment of Ag NCs-in-MOF, ultimately leading to bacterial cell death with considerably lower dose of silver.






Similar content being viewed by others
References
A. Singh, P.K. Gautam, A. Verma et al., Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 25, e00427 (2020). https://doi.org/10.1016/j.btre.2020.e00427
T. Sadeghi Rad, A. Khataee, F. Vafaei, S. Rahim Pouran, Chromium and cerium co-doped magnetite/reduced graphene oxide nanocomposite as a potent antibacterial agent against S. aureus. Chemosphere 274, 129988 (2021). https://doi.org/10.1016/j.chemosphere.2021.129988
A. Bayrami, S. Alioghli, S. Rahim Pouran et al., A facile ultrasonic-aided biosynthesis of ZnO nanoparticles using Vaccinium arctostaphylos L. leaf extract and its antidiabetic, antibacterial, and oxidative activity evaluation. Ultrason. Sonochem. 55, 57–66 (2019). https://doi.org/10.1016/j.ultsonch.2019.03.010
T. Sadeghi Rad, A. Khataee, S. Arefi-Oskoui et al., Graphene-based ZnCr layered double hydroxide nanocomposites as bactericidal agents with high sonophotocatalytic performances for degradation of rifampicin. Chemosphere 286, 131740 (2022). https://doi.org/10.1016/j.chemosphere.2021.131740
M. Mansoorianfar, A. Khataee, Z. Riahi et al., Scalable fabrication of tunable titanium nanotubes via sonoelectrochemical process for biomedical applications. Ultrason. Sonochem. 64, 104783 (2020). https://doi.org/10.1016/j.ultsonch.2019.104783
Y. Su, T. Xue, Y. Liu et al., Luminescent metal nanoclusters for biomedical applications. Nano Res. 12, 1251–1265 (2019). https://doi.org/10.1007/s12274-019-2314-y
M. Zhu, E. Lanni, N. Garg et al., Kinetically controlled, high-yield synthesis of Au 25 clusters. J. Am. Chem. Soc. 130, 1138–1139 (2008). https://doi.org/10.1021/ja0782448
K. Zheng, M.I. Setyawati, D.T. Leong, J. Xie, Antimicrobial gold nanoclusters. ACS Nano 11, 6904–6910 (2017). https://doi.org/10.1021/acsnano.7b02035
G. Chu, C. Zhang, Y. Liu et al., A gold nanocluster constructed mixed-metal metal-organic network film for combating implant-associated infections. ACS Nano 14, 15633–15645 (2020). https://doi.org/10.1021/acsnano.0c06446
A. Verma, S. Shivalkar, M.P. Sk et al., Nanocomposite of Ag nanoparticles and catalytic fluorescent carbon dots for synergistic bactericidal activity through enhanced reactive oxygen species generation. Nanotechnology 31, 405704 (2020). https://doi.org/10.1088/1361-6528/ab996f
M. Akter, Md.T. Sikder, Md.M. Rahman et al., A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 9, 1–16 (2018). https://doi.org/10.1016/j.jare.2017.10.008
C. Liao, Y. Li, S. Tjong, Bactericidal and cytotoxic properties of silver nanoparticles. IJMS 20, 449 (2019). https://doi.org/10.3390/ijms20020449
A.W. Simonson, M.R. Aronson, S.H. Medina, Supramolecular peptide assemblies as antimicrobial scaffolds. Molecules 25, 2751 (2020). https://doi.org/10.3390/molecules25122751
A. Singh, A. Verma, R. Singh et al., Combination therapy of biogenic C-dots and lysozyme for enhanced antibacterial and antibiofilm activity. Nanotechnology 32, 085104 (2021). https://doi.org/10.1088/1361-6528/abc2ed
S. Tang, J. Zheng, Antibacterial activity of silver nanoparticles: Structural effects. Adv. Healthc. Mater. 7, e1701503 (2018). https://doi.org/10.1002/adhm.201701503
S. Prabhu, E.K. Poulose, Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2, 32 (2012). https://doi.org/10.1186/2228-5326-2-32
B. Han, X. Hu, M. Yu et al., One-pot synthesis of enhanced fluorescent copper nanoclusters encapsulated in metal–organic frameworks. RSC Adv. 8, 22748–22754 (2018). https://doi.org/10.1039/C8RA03632B
Y.-P. Xie, Y.-L. Shen, G.-X. Duan et al., Silver nanoclusters: synthesis, structures and photoluminescence. Mater. Chem. Front. 4, 2205–2222 (2020). https://doi.org/10.1039/D0QM00117A
I. Díez, R.H.A. Ras, Fluorescent silver nanoclusters. Nanoscale 3, 1963 (2011). https://doi.org/10.1039/c1nr00006c
J.M.J. Santillán, D. Muñetón Arboleda, D. Muraca et al., Highly fluorescent few atoms silver nanoclusters with strong photocatalytic activity synthesized by ultrashort light pulses. Sci. Rep. 10, 8217 (2020). https://doi.org/10.1038/s41598-020-64773-z
N. Cathcart, P. Mistry, C. Makra et al., Chiral thiol-stabilized silver nanoclusters with well-resolved optical transitions synthesized by a facile etching procedure in aqueous solutions. Langmuir 25, 5840–5846 (2009). https://doi.org/10.1021/la9005967
X. Yuan, M.I. Setyawati, A.S. Tan et al., Highly luminescent silver nanoclusters with tunable emissions: cyclic reduction–decomposition synthesis and antimicrobial properties. NPG Asia Mater. 5, e39–e39 (2013). https://doi.org/10.1038/am.2013.3
L. Fan, D. Zhao, H. Zhang et al., A hydrolytically stable amino-functionalized Zinc(II) metal-organic framework containing nanocages for selective gas adsorption and luminescent sensing. Microporous Mesoporous Mater. 326, 111396 (2021). https://doi.org/10.1016/j.micromeso.2021.111396
B. Li, D. Zhao, F. Wang et al., Recent advances in molecular logic gate chemosensors based on luminescent metal organic frameworks. Dalton Trans. 50, 14967–14977 (2021). https://doi.org/10.1039/D1DT02841C
J. King, L. Zhang, S. Doszczeczko et al., How to functionalise metal–organic frameworks to enable guest nanocluster embedment. J. Mater. Chem. A 8, 4889–4897 (2020). https://doi.org/10.1039/C9TA12837A
A. Verma, F. Arshad, K. Ahmad et al., Role of surface charge in enhancing antibacterial activity of fluorescent carbon dots. Nanotechnology 31, 095101 (2020). https://doi.org/10.1088/1361-6528/ab55b8
P. Shah, P.W. Thulstrup, S.K. Cho et al., In-solution multiplex miRNA detection using DNA-templated silver nanocluster probes. Analyst 139, 2158–2166 (2014). https://doi.org/10.1039/C3AN02150E
S. Mallick, S. Sharma, M. Banerjee et al., Iodine-stabilized cu nanoparticle chitosan composite for antibacterial applications. ACS Appl. Mater. Interfaces 4, 1313–1323 (2012). https://doi.org/10.1021/am201586w
S. Mallick, P. Sanpui, S.S. Ghosh et al., Synthesis, characterization and enhanced bactericidal action of a chitosan supported core–shell copper–silver nanoparticle composite. RSC Adv. 5, 12268–12276 (2015). https://doi.org/10.1039/C4RA12770F
R. Jin, Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2, 343–362 (2010). https://doi.org/10.1039/B9NR00160C
R.J.T. Houk, B.W. Jacobs, F.E. Gabaly et al., Silver cluster formation, dynamics, and chemistry in metal−organic frameworks. Nano Lett. 9, 3413–3418 (2009). https://doi.org/10.1021/nl901397k
H. Xu, K.S. Suslick, Sonochemical synthesis of highly fluorescent Ag nanoclusters. ACS Nano 4, 3209–3214 (2010). https://doi.org/10.1021/nn100987k
L. Chen, R. Luque, Y. Li, Controllable design of tunable nanostructures inside metal–organic frameworks. Chem. Soc. Rev. 46, 4614–4630 (2017). https://doi.org/10.1039/C6CS00537C
J. Pizzorno, Glutathione! Integr. Med. (Encinitas) 13, 8–12 (2014)
K.H. Lee, B.M. Lee, Study of mutagenicities of phthalic acid and terephthalic acid using in vitro and in vivo genotoxicity tests. J. Toxicol. Environ. Health Part A 70, 1329–1335 (2007). https://doi.org/10.1080/15287390701432277
E. Ruel-Gariépy, J.-C. Leroux, Chitosan: A Natural Polycation with Multiple Applications (American Chemical Society, Washington, 2006). https://doi.org/10.1021/bk-2006-0934.ch012
S. Marpu, E. Benton, Shining light on chitosan: A review on the usage of chitosan for photonics and nanomaterials research. IJMS 19, 1795 (2018). https://doi.org/10.3390/ijms19061795
M. Farrag, R.A. Mohamed, Ecotoxicity of ∼1 nm silver and palladium nanoclusters protected by l -glutathione on the microbial growth under light and dark conditions. J. Photochem. Photobiol. A 330, 117–125 (2016). https://doi.org/10.1016/j.jphotochem.2016.07.027
G.S. Das, J.P. Shim, A. Bhatnagar et al., Biomass-derived carbon quantum dots for visible-light-induced photocatalysis and label-free detection of Fe(III) and ascorbic acid. Sci. Rep. 9, 15084 (2019). https://doi.org/10.1038/s41598-019-49266-y
R. Qiang, S. Yang, K. Hou, J. Wang, Synthesis of carbon quantum dots with green luminescence from potato starch. New J. Chem. 43, 10826–10833 (2019). https://doi.org/10.1039/C9NJ02291K
R. Sharma, A. Dhillon, D. Kumar, Mentha-stabilized silver nanoparticles for high-performance colorimetric detection of Al(III) in aqueous systems. Sci. Rep. 8, 5189 (2018). https://doi.org/10.1038/s41598-018-23469-1
Z. Han, X.-Y. Dong, P. Luo et al., Ultrastable atomically precise chiral silver clusters with more than 95% quantum efficiency. Sci. Adv. 6, eaay0107 (2020). https://doi.org/10.1126/sciadv.aay0107
L. Wang, C. Hu, L. Shao, The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed. 12, 1227–1249 (2017). https://doi.org/10.2147/IJN.S121956
P.V. AshaRani, G. Low Kah Mun, M.P. Hande, S. Valiyaveettil, Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3, 279–290 (2009). https://doi.org/10.1021/nn800596w
N.P. Panpaliya, P.T. Dahake, Y.J. Kale et al., In vitro evaluation of antimicrobial property of silver nanoparticles and chlorhexidine against five different oral pathogenic bacteria. Saudi Dent. J. 31, 76–83 (2019). https://doi.org/10.1016/j.sdentj.2018.10.004
K. Zheng, M.I. Setyawati, T.-P. Lim et al., Antimicrobial cluster bombs: Silver nanoclusters packed with daptomycin. ACS Nano 10, 7934–7942 (2016). https://doi.org/10.1021/acsnano.6b03862
A.K. Sahoo, M.P. Sk, S.S. Ghosh, A. Chattopadhyay, Plasmid DNA linearization in the antibacterial action of a new fluorescent Ag nanoparticle–paracetamol dimer composite. Nanoscale 3, 4226 (2011). https://doi.org/10.1039/c1nr10389j
M. Köping-Höggård, K. Vårum, M. Issa et al., Improved chitosan-mediated gene delivery based on easily dissociated chitosan polyplexes of highly defined chitosan oligomers. Gene Ther. 11, 1441–1452 (2004). https://doi.org/10.1038/sj.gt.3302312
K.E. Siters, M.A. Fountain, J.R. Morrow, Selective binding of Zn 2+ Complexes To Human Telomeric G-quadruplex DNA. Inorg. Chem. 53, 11540–11551 (2014). https://doi.org/10.1021/ic501484p
A.D. Miroshnikova, A.A. Kuznetsova, Y.N. Vorobjev et al., Effects of mono- and divalent metal ions on DNA binding and catalysis of human apurinic/apyrimidinic endonuclease 1. Mol. BioSyst. 12, 1527–1539 (2016). https://doi.org/10.1039/C6MB00128A
S. Lu, Zn2+ blocks annealing of complementary single-stranded DNA in a sequence-selective manner. Sci. Rep. 4, 5464 (2015). https://doi.org/10.1038/srep05464
Acknowledgements
Authors acknowledge department of Chemistry, AMU, Central research facility, Indian Institute of Information Technology Delhi, XPS facility, Indian Institute of Information Technology Kanpur and University Sophisticated Instrument Facility (USIF), Aligarh Muslim University, Aligarh for the instrumental support. Authors are thankful to Mr. Somnath Sengupta for his help in conducting Flow cytometry in BD-Jamia Hamdard FACS Academy New Delhi. This work also received financial support from Seed grant of Indian Institute of Information Technology Allahabad and DBT, Gov. of India (BT/IN/Indo-US/Foldscope/39/2015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare they have no financial interests or conflicts.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verma, A., Singh, A., Shukla, N. et al. Synthesis of highly stable luminescent silver nanoclusters in metal–organic framework for heightened antibacterial activity. Appl. Phys. A 128, 292 (2022). https://doi.org/10.1007/s00339-022-05431-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-05431-1