Abstract
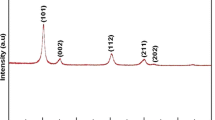
Thermal kinetics of TiO2 NPs at varying time and at different temperatures for anatase–rutile phase transition was investigated by Powder XRD diffraction analysis. Decrease in the diffraction intensities of XRD peaks of anatase phase (101) was observed from 900 °C onwards, which substantiates the evolution of rutile phase. Isothermal curves of transformed rutile mass fraction as a function of varying time were investigated at 925 °C and 950 °C and the data were fitted using Johnson–Mehl–Avrami equation. Thermal behavior of nanostructured TiO2 was characterized using thermogravimetry and differential analyses. In addition, specific heat capacities of the synthesized TiO2 NPs at higher temperature range were reported. By recapitulating the results, we indicate that TiO2 NPs synthesized by controlled hydrolysis technique prolongs the transition state and it can be concluded that interfacial nucleation and growth site improvements are accomplished by activation energy. Activation energy calculated for varying time derivatives was found to be 39 kcal/mol, 64 kcal/mol and 175 kcal/mol.







Similar content being viewed by others
References
S.R. Forrest, Nature 428, 911–918 (2004)
H.H. Park, Y. Choi, D.J. Park, S.Y. Cho, Y.S. Yun, H.J. Jin, Fibers Polym. 14, 1521–1525 (2013)
J. Park, J. Kim, S. Lee, J. Bang, B.J. Kim, Y.S. Kim, J. Cho, J. Mater. Chem. 19, 4488–4490 (2009)
G. Ramis, G. Busca, F. Bregani, Catal. Lett. 18, 299–303 (1993)
M. Gratzel, Nature 414, 338–344 (2001)
A. Hagfeldt, M. Gratzel, Chem. Rev. 95, 49–68 (1995)
A. Mills, S.K. Lee, J. Photochem. Photobiol. A 152, 233–247 (2002)
H. Zhang, J.F. Banfield, J. Phys. Chem. B 104, 3481–3487 (2003)
H. Cheng, J. Ma, Z. Zhao, L. Qi, Chem. Mater. 7, 663–671 (1995)
J. Yang, S. Mei, J.M.F. Ferreira, J. Am. Ceram. Soc. 83, 1361–1368 (2000)
S. Valencia, X. Vargas, L. Rios, G. Restrepo, J.M. Mar´ın, J. Photochem. Photobiol. A Chem. 251, 175–181 (2013)
N.M. Julkapli, S. Bagheri, S.B. Abd, Hamid, Sci. World J. 2014, 1–25 (2014)
V. Ghorbani, M. Ghanipour, D. Dorranian, Opt. Quantum Electron. 48, 1–14 (2016)
I.K. Konstantinou, T.A. Albanis, Appl. Catal. B 49, 1–14 (2004)
M. Ni, M.K.H. Leung, D.Y.C. Leung, K. Sumathy, Renew. Sustain. Energy Rev. 11, 401–425 (2007)
A. Millis, S. Le Hunte, J. Photochem. Photobiol. A 108, 1–35 (1997)
G. Madras, J. Am. Ceram. Soc. 90, 250–255 (2007)
A. Dabler, A. Feltz, J. Jung, W. Ludwig, E. Kaiserberg, J. Therm. Anal. Calorim. 33, 803–809 (1988)
H. Ichinose, M. Terasaki, H. Katsuki, J. Ceram. Soc. Jpn. 104, 715–718 (1996)
C.N. Rao, S.R. Yoganarasimha, P.A. Faeth, Trans. Faraday Soc. 57, 504–510 (1961)
N.T. Nolan, M.K. Seery, S.C. Pillai, J. Phys. Chem. C 113, 16151–16157 (2009)
M. Kakihana, M. Kobayashi, K. Tomita, V. Petrykin, Bull. Chem. Soc. Jpn. 83, 1285–1308 (2010)
N. Murakami, Y. Kurihara, T. Tsubota, T. Ohno, J. Phys. Chem. C 113, 3062–3069 (2009)
W. Li, Y. Bai, C. Liu, Z. Yang, X. Feng, X. Lu, N.K. Van der Laakand, K.Y. Chan, Environ. Sci. Technol. 43, 5423–5428 (2009)
J.Y. Piquemal, E. Briot, J.M. Bregeault, Dalton Trans. 42, 29–45 (2013)
M. Palkovaska, V. Slovak, J. Subrt, J. Bohacek, Z. Barbierikova, V. Brezova, R. Fajgar, J. Therm. Anal. Calorim. 125, 1071–1078 (2016)
F.C. Gennari, D.M. Pasquevich, J. Mater. Sci. 33, 1571–1578 (1998)
B.-K. Yoo, W. Oh-Hoon Kwon, H. Liu, J. Tang, A.H. Zewail, Nat. Commun. 6, 86391–86396 (2015)
H. Mehranpour, M. Askari, M. Sasani, Ghamsari, H. Farzalibeik, J. Nanomater. 2010, 5 (2010)
J. Pagáčová, A. Plško, K. Michalková, V. Zemanová, I. Papučová, Procedia Eng. 136, 280–286 (2016)
G. Li, L. Li, J. Boerio-Goates, B.F. Woodfield, J. Am. Chem. Soc. 127, 8659–8666 (2005)
J. Malek, T. Mitsuhashi, J. Am. Ceram. Soc. 83, 2103–2105 (2000)
N. Nakayama, T. Hayashi, Colloids Surf. A Physicochem. Eng. Asp. 317, 543–550 (2008)
M. Mazhar, M.A. Ehsan, H. Khaledi, A. Pandikumar, P. Rameshkumar, H.N. Ming, Z. Arifin, New J. Chem. 39, 7442–7452 (2015)
P. Piątkowski Jarosław, R.J. Magda, Solid State Phenomen. 203, 431–434 (2013)
C. Marinescu, A. Sofronia, C. Rust, J. Therm. Anal. Calorim. 103, 49–57 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalaiarasi, S., Jose, M. Kinetics of anatase phase transformation of TiO2 NPs synthesized using controlled hydrolysis technique. Appl. Phys. A 124, 589 (2018). https://doi.org/10.1007/s00339-018-2021-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-018-2021-7