Abstract
The role of species interactions in setting species range limits is rarely empirically explored. Here, we quantify host and parasite densities in subtropical eastern Australia (26.65°–30.20°S) to examine whether parasitism might contribute to range limitation of Acropora corals at their cold-range boundary. 79% of Acropora corals had endolithic barnacles (family Pyrgomatidae), with higher parasite load in larger corals and up to 141 barnacles per coral. Parasite load increased poleward and closer to the mainland and was greater in cooler and high nutrient environments. Parasite burden was higher at sites with fewer Acropora corals, broadly consistent with the hypothesis that parasites can fragment host populations where host densities are low, and the parasite is a better disperser than the host. Whilst the mechanism is unclear, our findings suggest that at the high densities recorded here, coral-barnacles could influence range dynamics of Acropora corals at their poleward range limit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Whilst the role of environmental stress in setting species range limits has been well documented, the contribution of species interactions, such as parasitism, is less frequently explored (Briers 2003; Sexton et al. 2009). Environmental stress can make hosts more susceptible to parasites (Esch et al. 1975; Lafferty and Kuris 1999), and it is plausible that range edge populations in naturally stressful environments might be particularly prone to parasite infection. In a mathematical study, Hochberg and Ives (1999) found that a specialised parasite can fragment its host population and enforce a range limit when the host density is low and the parasite disperses at higher rates than the host, highlighting density dependence and dispersal as important drivers of these dynamics. In a rare empirical study, Briers (2003) found that trematode parasitism in a freshwater gastropod snail increased sharply at the species’ range limit.
Biogeographic transition zones are ideal study systems in which to investigate host–parasite dynamics along gradients of environmental stress. In subtropical eastern Australia, corals occur in marginal and extreme conditions (Schoepf et al. 2023) at their poleward range limits along a gradient of increasing abiotic stress towards higher latitudes (Sommer et al. 2018). In this region, coral assemblages are characterised by widely distributed, stress-tolerant species with massive and horizontally spreading morphologies and by diminishing poleward and nearshore prevalence of tropical species (Sommer et al. 2014). Acropora species in particular tend to be narrowly distributed and rare (Sommer et al. 2014) and are frequently infested by obligate coral-inhabiting barnacles (Cirripedia: Sessilia: Pyrgomatidae) that bore into the coral skeleton (Fig. 1). Barnacle settlement and metamorphosis are highly invasive and trigger a physical stress response as the coral defends itself (Liu et al. 2016). In these challenging high-latitude settings (Sommer et al. 2018), directing energy towards chemical defence could reduce energy available for growth, reproduction and survival and ultimately reduce coral fitness (Kaposi et al. 2022).
Here, we examine the potential role of parasitism in contributing to range limitation in Acropora corals as they approach their poleward range limit in subtropical eastern Australia (26.65°–30.20°S). This region is characterised by cooler and more eutrophic conditions towards higher latitudes (Sommer et al. 2018), and we test whether parasite burden increases as Acropora corals approach their lower thermal limits, and environmental conditions become more stressful at higher latitudes. We also examine the predictions that the density of macrobioeroders increases in high nutrient settings (Le Grand and Fabricius 2011; van der Schoot and Hoeksema 2022) and that parasite loads are higher, where host abundance is lower (Maher et al. 2018). We further test whether barnacle density scales with coral size, expecting that larger corals host more barnacles, due to larger surface area available for barnacle recruitment and longer time on the reef.
Methods
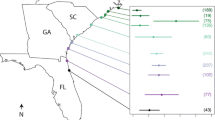
We conducted surveys at nine sites arrayed along a latitudinal environmental gradient (26.65°–30.20°S, Fig. 2) in subtropical eastern Australia in the austral spring of 2011. At each of the sites, we conducted visual census of Acropora corals and their macrobioeroders (family Pyrgomatidae, Fig. 1) along five 10 m long by 1 m wide belt transects that were haphazardly placed in the spatially restricted coral habitat (Sommer et al. 2014). We focused on Acropora corals due to their high infestation with coral-barnacles (pers. obs., Fig. 1), their predominantly tropical distribution, rarity and latitudinal decline compared to other coral taxa in the region (Sommer et al. 2014). Visual differentiation amongst Acropora species was not practical in the field and results are presented at the genus level. For each Acropora coral, we recorded its maximum diameter as a proxy for coral size, whether it had barnacles (parasite incidence), the number of externally visible barnacles (parasite load), and the number of barnacles over the host’s maximum diameter (parasite crowding) to account for host size. To characterise environmental conditions, we compiled monthly sea surface temperate (SST) and chlorophyll a (Chl a) data from the MODIS Aqua Satellite (Parkinson 2003) and calculated the mean annual means for SST and Chl a at the study sites.
Statistical analysis
We used Bayesian generalised linear models (BGLMs) to assess the effect of coral size on whether a coral had barnacles (family = bernoulli), whether larger corals had more barnacles (poisson) and to examine whether parasite incidence (bernoulli), parasite load (poisson) and parasite crowding (gaussian) varied systematically with latitude, distance from the mainland (log transformed), SST and Chl a. Due to multi-collinearity (r > 0.7), separate BGLMs were built for the predictors. To determine which parameters best explained parasite patterns, we calculated Bayes R2 values and used approximate leave-one-out cross-validation with LOO information criterion (LOOIC) to assess model fit. All models were created using the probabilistic framework offered by Bayesian statistics and executed in Stan, accessed with the R package ‘brms’ (Bürkner 2017). We chose the Bayesian approach as it quantifies uncertainty by sampling from the posterior distributions and can account for asymmetrically distributed uncertainty distributions (Bürkner 2017). We summarised model fits using the 95% highest posterior density interval as the credible interval and computed median point estimates for all chains. To improve convergence and guard against overfitting, we specified weakly informative conservative priors and ran each model with four chains of 5,000 iterations (warmup = 1000) and a thinning rate of 5. We examined chain mixing, performed posterior predictive checks to assess model fit, and used the Gelman–Rubin convergence diagnostic (R-hat) to assess model convergence.
Results and discussion
We recorded high parasite burden in Acropora corals at their poleward range limit in eastern Australia, supporting the notion that parasite load increases in areas of elevated abiotic stress. 79% of surveyed Acropora corals had endolithic barnacles, with up to 141 barnacles recorded per coral. Parasite load (i.e. the number of barnacles per coral) and parasite crowding (i.e. parasite load relative to coral size) varied systematically and increased poleward and closer to the mainland (Fig. 3, Table 1). Distance from the mainland better explained spatial patterns than latitude (Table 1), likely due to greater offshore influence of the East Australia Current shaping environmental conditions and larval connectivity in this biogeographic transition zone (Harriott and Banks 2002; Malcolm et al. 2011).
Larger corals had higher probability of barnacle infestation (95% CI, 0.34, 1.24) than smaller corals and had elevated parasite loads (95% CI, 0.91, 1.01; Fig. 4a). As age positively scales with coral size, these patterns are consistent with increased chance of barnacle colonisation over time, more available space for barnacle larvae to settle on, and preferential settlement of barnacle larvae near conspecifics, likely in response to chemical or behavioural cues during host and site selection (Liu et al. 2016; Yap et al. 2023). Species-specific life spans of coral-barnacles (e.g. 2–6 years) could also mediate these dynamics (Brickner et al. 2010). Notably, parasite load was higher at sites with fewer Acropora corals (95% CI, − 0.04, − 0.03; Fig. 4b) suggesting that gradients in host abundance might also be influencing the observed patterns. Indeed, in addition to other factors, the range borders of parasites are further constrained by the geographic ranges and ecology of their host species (Bozick et al. 2015). Pyrgomatids are the most host-specific amongst coral associated fauna, with a high proportion of unique symbiont-host relationships in the Acroporidae family (van der Schoot and Hoeksema 2023). It is thus possible that low density of Acropora corals at their poleward range limits could lead to specialised barnacles crowding onto few available hosts, consistent with the hypothesis that host-barnacle associations are phylogenetically conserved (Tsang et al. 2014). Although information on parasite burden in other potential host genera (e.g. Turbinaria, Pocillopora, Paragoniastrea; Harriott and Banks 2002; Sommer et al. 2024) would be needed to test this, the prevalence of pyrgomatid barnacles in other coral genera appeared comparatively low (Sommer pers. obs.). Host traits likely also mediate these dynamics (van der Schoot and Hoeksema 2023), especially as Acroporidae and Pocilloporidae are the only branching taxa in this region (Sommer et al. 2017), with Pocillopora preferentially occupied by endolithic bivalves (Smith 2011). In combination, our results suggest that increased host susceptibility under abiotic stress, and higher availability of infective stages of barnacles per coral surface area might mediate the observed dynamics, although more data are needed to formally test this hypothesis.
Parasite load and parasite crowding were higher in cooler areas and where Chlorophyll a concentrations were higher (Fig. 3c), with the latter better predicting the observed patterns (Table 1). This corroborates the role of nutrients in driving macroboring detected in other systems, including the Great Barrier Reef (Le Grand and Fabricius 2011), French Polynesia (Rice et al. 2020), and the Caribbean (van der Schoot and Hoeksema 2022). Elevated nutrients, organic matter and plankton biomass tend to favour filter feeding bioeroder populations (Fig. 1) over autotrophic calcifiers, such as corals (Glynn and Manzello 2015).
The high explanatory power of distance from the mainland (Table 1, Fig. 3b) suggests that other factors likely also influence parasite dynamics in the region, possibly in relation to dispersal of barnacles and hosts (Hochberg and Ives 1999; Smith 2011). Smith (2011) hypothesised that differential supply of Lithophagia lessepsiana larvae might contribute to higher offshore densities of endolithic bivalves on Pocillopora corals in this region (Smith 2011). Many barnacles reproduce continuously (Brickner et al. 2010), whilst Acropora corals have a restricted reproductive season, with less synchronous spawning patterns at high-latitude compared to the tropics (Baird et al. 2009). Moreover, typically lower growth rates of Acropora corals in the subtropics compared to the tropics (Harriott 1999) could give barnacles a growth advantage over their hosts and prevent shell-openings from being overgrown by the coral (Brickner et al. 2010).
Although our study only covers one genus, Acropora corals are key framework building taxa on tropical coral reefs (van Woesik and Done 1997), whose absence or rarity has been linked to lack of reef development in high-latitude settings (Harriott and Banks 2002). Moreover, a recent study found limited tropicalisation of coral assemblages in this region despite warming (Mizerek et al. 2021). Our study suggests that in addition to cooler and light-limited conditions (Sommer et al. 2018), high parasite burden could further challenge Acropora populations in this region. As such it is possible that, at the higher densities recorded here, coral parasites could depress coral population growth rates and expansion into adjacent habitats, further supported by high parasite loads where Acropora corals were rare. At high parasite loads, endolithic barnacles can negatively impact host fitness, causing stress or damage (Fig. 1) to nearby coral polyps (Benzoni et al. 2010) and inhibiting coral growth (Barton et al. 2020), fecundity (Thamrin and Nojima 2001) and heterotrophic feeding (Barton et al. 2020), in this region where Acropora corals are often seen tentacle feeding during the day. Endolithic organisms also weaken coral skeletons and make them more susceptible to breakage (Fig. 1) and bioerosion (Rice et al. 2020; Li et al. 2022), which has been linked to lack of reef development at high latitudes (Harriott and Banks 2002). In the eastern tropical Pacific, a region marginal for coral growth, for instance, macroboring contributed 85–95% to total bioerosion pressure (Rodriguez-Ruano et al. 2023). Whilst the mechanisms are unclear, our results are broadly consistent with the hypothesis that parasitism by pyrgomatid barnacles could affect Acropora range dynamics in this region, where corals already exist in environmentally stressful conditions at their poleward range limits.
References
Baird AH, Guest JR, Willis BL (2009) Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol Syst, pp 551–571
Barton JA, Bourne DG, Humphrey C, Hutson KS (2020) Parasites and coral-associated invertebrates that impact coral health. Rev Aquac 12:2284–2303
Benzoni F, Galli P, Pichon M (2010) Pink spots on Porites: Not always a coral disease. Coral Reefs 29:153–153
Bozick BA, Real LA, Associate Editor Paul WE (2015) Integrating parasites and pathogens into the study of geographic range limits. The Quarterly Review of Biology 90:361–380
Brickner I, Loya Y, Achituv Y (2010) Diverse life strategies in two coral-inhabiting barnacles (Pyrgomatidae) occupying the same host (Cyphastrea chalcidicum), in the northern Gulf of Eilat. J Exp Mar Biol Ecol 392:220–227
Briers RA (2003) Range limits and parasite prevalence in a freshwater snail. Proc R Soc Lond B 270:S178–S180
Bürkner P-C (2017) brms: An R package for Bayesian Multilevel Models using Stan. J Stat Softw 80:1–28
Esch GW, Gibbons JW, Bourque JE (1975) An analysis of the relationship between stress and parasitism. Am Midl Nat 93:339–353
Glynn PW, Manzello DP (2015) Bioerosion and coral reef growth: a dynamic balance. In: Birkeland C (ed) Coral Reefs in the Anthropocene. Springer, Netherlands, Dordrecht, pp 67–97
Harriott VJ (1999) Coral growth in subtropical eastern Australia. Coral Reefs 18:281–291
Harriott VJ, Banks SA (2002) Latitudinal variation in coral communities in eastern Australia: a qualitative biophysical model of factors regulating coral reefs. Coral Reefs 21:83–94
Hochberg ME, Ives AR (1999) Can natural enemies enforce geographical range limits? Ecography 22:268–276
Kaposi KL, Courtney RL, Seymour JE (2022) Implications of bleaching on cnidarian venom ecology. Toxicon: X 13:100094
Lafferty KD, Kuris AM (1999) How environmental stress affects the impacts of parasites. Limnol Oceanogr 44:925–931
Le Grand HM, Fabricius KE (2011) Relationship of internal macrobioeroder densities in living massive Porites to turbidity and chlorophyll on the Australian Great Barrier Reef. Coral Reefs 30:97–107
Li Y, Wang F, Liu Z, Jiang J, Han T, Liao X, He C, Lu Z (2022) The formation of biogenic reef stone: from coral skeleton to reef rubble. J Oceanogr 78:135–149
Liu JCW, Høeg JT, Chan BKK (2016) How do coral barnacles start their life in their hosts? Biol Let 12:20160124
Maher RL, Johnston MA, Brandt ME, Smith TB, Correa AMS (2018) Depth and coral cover drive the distribution of a coral macroborer across two reef systems. PLoS ONE 13:e0199462
Malcolm HA, Davies PL, Jordan A, Smith SDA (2011) Variation in sea temperature and the East Australian Current in the Solitary Islands region between 2001–2008. Deep-Sea Research Part II-Topical Studies in Oceanography 58:616–627
Mizerek TL, Madin JS, Benzoni F, Huang D, Luiz OJ, Mera H, Schmidt-Roach S, Smith SDA, Sommer B, Baird AH (2021) No evidence for tropicalization of coral assemblages in a subtropical climate change hot spot. Coral Reefs 40:1451–1461
Parkinson CL (2003) Modis Aqua: an Earth-Observing Satellite mission to examine water and other climate variables. Geoscience and Remote Sensing, IEEE Transactions on 41:173–183
Rice MM, Maher RL, Correa AMS, Moeller HV, Lemoine NP, Shantz AA, Burkepile DE, Silbiger NJ (2020) Macroborer presence on corals increases with nutrient input and promotes parrotfish bioerosion. Coral Reefs 39:409–418
Rodriguez-Ruano V, Toth LT, Enochs IC, Randall CJ, Aronson RB (2023) Upwelling, climate change, and the shifting geography of coral reef development. Sci Rep 13:1770
Schoepf V, Baumann JH, Barshis DJ, Browne NK, Camp EF, Comeau S, Cornwall CE, Guzmán HM, Riegl B, Rodolfo-Metalpa R, Sommer B (2023) Corals at the edge of environmental limits: A new conceptual framework to re-define marginal and extreme coral communities. Sci Total Environ 884:163688
Sexton JP, McIntyre PJ, Angert AL, Rice KJ (2009) Evolution and ecology of species range limits Annu Rev Ecol Evol Syst, pp. 415–436
Smith SDA (2011) Densities of the endolithic bivalve Lithophaga lessepsiana (Vaillant, 1865) in Pocillopora damicornis, Solitary Islands Marine Park, northern NSW, Australia. Molluscan Res 31:42–46
Sommer B, Harrison PL, Beger M, Pandolfi JM (2014) Trait-mediated environmental filtering drives assembly at biogeographic transition zones. Ecology 95:1000–1009
Sommer B, Beger M, Harrison PL, Babcock RC, Pandolfi JM (2018) Differential response to abiotic stress controls species distributions at biogeographic transition zones. Ecography 41:478–490
Sommer B, Sampayo EM, Beger M, Harrison PL, Babcock RC, Pandolfi JM (2017) Local and regional controls of phylogenetic structure at the high-latitude range limits of corals. Proc R Soc Biol Sci Ser B 284
Sommer B, Hodge JM, Lachs L, Cant J, Pandolfi JM, Beger M (2024) Decadal demographic shifts and size‐dependent disturbance responses of corals in a subtropical warming hotspot. Scientific Reports. https://doi.org/10.1038/s41598-024-56890-w
Thamrin TM, Nojima S (2001) Effect of coral-inhabiting barnacle (Cantellius pallidus) on planula production in a scleractinian coral Alveopora japonica. Ophelia 55:93–100
Tsang LM, Chu KH, Nozawa Y, Chan BKK (2014) Morphological and host specificity evolution in coral symbiont barnacles (Balanomorpha: Pyrgomatidae) inferred from a multi-locus phylogeny. Mol Phylogenet Evol 77:11–22
van der Schoot RJ, Hoeksema BW (2023) Host specificity of coral-associated fauna and its relevance for coral reef biodiversity. Int J Parasitol
van der Schoot RJ, Hoeksema BW (2022) Abundance of coral-associated fauna in relation to depth and eutrophication along the leeward side of Curaçao, southern Caribbean. Mar Environ Res 181:105738
van Woesik R, Done TJ (1997) Coral communities and reef growth in the southern Great Barrier Reef. Coral Reefs 16:103–115
Yap F-C, Chen H-N, Chan BKK (2023) Host specificity and adaptive evolution in settlement behaviour of coral-associated barnacle larvae (Cirripedia: Pyrgomatidae). Sci Rep 13:9668
Acknowledgements
Funding was provided by an Australian Research Council Discovery Early Career Research Award (DE230100141) to BS and the Australian Research Council Centre of Excellence for Coral Reef Studies (CE140100020) to JMP.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sommer, B., Chang, Y.Y., Beger, M. et al. Does high parasite load contribute to limitation of the poleward range of Acropora corals?. Coral Reefs (2024). https://doi.org/10.1007/s00338-024-02518-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00338-024-02518-4