Abstract
Function-based studies have opened a new chapter in our understanding of coral reefs. Unfortunately, we are opening this chapter as the world’s reefs rapidly transform. In this context, one of the most important roles of function-based studies is to inform coral reef conservation. At this critical juncture, we have a chance to reflect on where we have come from, and where we are going, in coral reef functional ecology, with specific consideration of what this means for our approaches to conserving reefs. As focal examples, we examine the role of corals on reefs, and the practice of culling crown-of-thorns starfish, from a functional perspective. We also consider how the papers in this special issue build on our current understanding. Ultimately, we highlight how robust scientific investigation, based on an understanding of ecosystem functions, will be key in helping us navigate reefs through the current coral reef crisis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compared to other disciplines in ecology, the study of ecosystem functions harbours a unique suite of challenges. In addition to species-specific abundances, studies of function require detailed information on organismal traits (e.g. size, diet, mobility), as well as the role of species in ecosystem-scale processes (e.g. herbivory, predation). While this need for additional information can impede the pace at which research on function progresses, the rewards for integrating this information are clear. This is especially true for high-diversity coral reefs, where strong biotic interactions can lead to a handful of species disproportionally contributing to ecosystem processes. For example, studies of coral reef functions have revealed that Bolbometopon parrotfish can erode the reef at a rate equal to total calcification, with each individual producing over 5 tonnes of sand per year (Bellwood et al. 2003), and that 60% of all fish flesh consumed on reefs may come from ‘unseen’ cryptobenthic fishes (Brandl et al. 2019b). Such information is critical in our quest to decipher how coral reefs operate and, increasingly, to inform coral reef conservation efforts.
We have now entered a critical period for coral reef conservation, where the actions we take today will dictate the nature of coral reefs tomorrow and for many decades to come. Successful coral reef conservation requires that these actions are grounded in robust science, in which functional studies can play an important role (Kuffner and Toth 2016; Bellwood et al. 2019a). Given that we have reached this critical juncture, it is prudent to take stock and reflect on where we have come from, and where we are going, in terms of function-based studies on coral reefs, with specific consideration of what this means for the conservation of coral reefs in the Anthropocene. We also consider how the papers included in this special issue Functional studies on coral reefs: insights from a changing world have changed our understanding of coral reef ecosystem functioning.
The evolution of functional studies on coral reefs
There is a long history of research studying functions on coral reefs, and observations with functional relevance began centuries ago, including observations by Darwin (1842) who’s quantitative reasoning on links between coral growth and reef production are still relevant today. Nevertheless, although studies of coral reef functions are scattered throughout most of the history of coral reef ecology, systematic and cohesive efforts to understand functions have only recently begun to emerge. From the 1700s until the 1970s, most reef research was centred on taxonomy and basic ecological descriptions of which organisms occurred where (e.g. Randall 1955; Goreau 1959; Talbot 1965). From the 1970s onwards, the increased prevalence of SCUBA diving in science underpinned a bourgeoning interest in the quantification of marine organisms, with increasingly sophisticated analytical approaches facilitating a growing interest in documenting abundance patterns, be they at biogeographic or population levels (e.g. Sheppard 1980; Anderson et al. 1981; Williams and Sale 1981; Done 1982; Alevizon et al. 1985). Documenting spatiotemporal variation was in vogue; indeed, reef scientists were primarily interested in understanding patterns (e.g. community composition), rather than examining processes (i.e. functions). The term ‘function’ was largely left to functional morphologists who, at this time, were exploring the potential for combining morphology and ecology in the rapidly expanding field of eco-morphology (Motta 1988; Wainwright 1988), or in the delineation of ‘functional groups’ of coral reef organisms (Steneck 1983, 1988). At this time, the term functional was most commonly used when examining how organisms worked, not ecosystems.
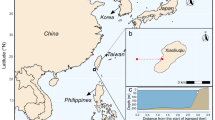
As our understanding of coral reef organisms grew, our approaches to quantifying where they occur on reefs, and why, became increasingly structured. In the 1980s, coral work increasingly focused on distribution and abundance censuses (quadrats, line intercept methods etc.) (Bak and Luckhurst 1980; Done 1982). Moreover, on the Australian Great Barrier Reef (GBR), the Australian Institute of Marine Science was implementing new cross-shelf fish survey approaches that captured the different environmental contexts in which reefs, and their associated communities, existed—from turbid inshore reefs to clear-water high energy offshore reefs (Fig. 1); each with their own characteristic faunas and associated regimes of ecosystem processes such as calcification and erosion (Done 1983; Kleypas et al. 1999). At this time, the best available fish data were based on either mass of fishes per family from explosive samples (Williams and Hatcher 1983) or early underwater visual census (UVC) methods, e.g. using point censuses (Bohnsack and Bannerot 1986) or a Log3 abundance scale (e.g. Russ 1984a, 1984b). Fish numbers (i.e. abundance) and identities were the focus; ecologically informative traits, like body size, were often not recorded. Thus, identification and counting were still relatively crude and, indeed, the first full fish identification book for the GBR was not published until 1990 (Randall et al. 1990).
‘Coral reefs’ come in a variety of forms, all of which are embedded within their own environmental context. a Tropical, high coral cover reef system (Linnett Reef, Great Barrier Reef [GBR], 2021), b low coral cover, high turf cover reef system (Lizard Island, GBR, 2021 post disturbance by cyclones and bleaching events), c high-energy, high crustose coralline algae cover reef system (Yonge Reef, GBR, 2021), d turbid, nearshore reef system (Bowen, GBR, 2019), e low diversity, subtropical reef system (Flinders Reef, South East Queensland, Australia, 2020), and f high macroalgal cover, low coral cover reef system (Turtle Group, GBR, 2021) (Photographs: SB Tebbett)
Our understanding of what reef organisms actually did was even less refined and there was still no united field of ‘coral reef functional ecology’. Nevertheless, the process-focussed foundations for the subdisciplines within this field, that would ultimately become major avenues of research, had already been laid. This included threads of ecological understanding emerging from earlier feeding observations and dietary analyses (e.g. Hiatt and Strasburg 1960; Randall 1967; Jones 1968; Choat 1969; Robertson et al. 1979). Moreover, a basic understanding of system-wide coral reef productivity and trophic energy flows was established (Odum and Odum 1955), as well as the roles of reef fishes in controlling (Randall 1961) and utilizing (Bardach 1959) this productivity. There was an early appreciation of the central role of corals in reef growth (Goreau 1963; Stoddart 1969; Chave et al. 1972; Stearn et al. 1977; Chappell 1980). Ecological aspects of reef growth and carbonate budgets (Smith and Kinsey 1976; Stearn et al. 1977), as well as the role of fishes in these processes (Bardach 1961), was also already underway.
Building on this pioneering work, the study of processes operating on coral reefs rapidly expanded in the late 1980s and into the 1990s. The field of reef fish research shifted from documenting communities to understanding where they came from, through population biology and recruitment-oriented work. A focus on the ‘black-box’ of the pelagic realm (e.g. Doherty and Williams 1988; Sale 2004) developed into work on population connectivity, especially pertaining to marine protected areas (Jones et al. 2009; Harrison et al. 2020), while benthic studies showed an increased interest in settlement, growth, and demography (e.g. Hughes et al. 1999; Pratchett et al. 2015). Other major advances included the direct measurement of reef processes, like herbivory and benthic productivity (e.g. Hatcher and Larkum 1983; Steneck 1983; Hay 1984; Carpenter 1986; Russ 1987; Klumpp and McKinnon 1989). These early findings were so fundamental to our understanding of herbivory that today we are still drawing on the lessons learnt during this period (see Tebbett et al. 2023b in this special issue for an overview). Discoveries also expanded beyond the reef benthos, with increased attention to off-reef habitats such as seagrasses and mangroves and, perhaps most importantly, the pelagic realm. Pioneering work by Hamner et al. (1988) shaped our understanding of planktivory on reefs, with recent studies (e.g. Leray et al. 2019 and Gahan et al. 2023 in this special issue) expanding our insights into the nutritional value of key planktonic groups for fishes. This period also saw a major expansion in our understanding of reef growth, primarily through coring, in both the Indo-Pacific and Western Atlantic (Adey 1978; Scoffin and Dixon 1983), as well as the role of key bioeroding organisms in these processes (Hutchings 1986; Scoffin 1992; Bak 1994; Bellwood 1995). Indeed, researchers were already focusing on understanding the interaction between reef growth and global climate change/sea level rise (Buddemeier and Smith 1988), with this field expanding in the twenty-first century as the effects of climate change intensified (Perry et al. 2018; Kench et al. 2022; Toth et al. 2023).
Consequently, by the late 1980s, for both fishes and corals, many of the methods that would lay the foundations for studies of coral reef functions, as we know them today, were already in place. However, a unified and cohesive understanding of reef functions was still lacking, and it was not until the mid-1990s that the broader concept of reef ‘ecosystem function’, was more formally expressed (see Done et al. 1996). Unfortunately, in many areas, this pioneering research on coral reef ecosystem functions was not immediately followed up. This may be, in part, due to the changes that began rapidly unfolding on the world’s coral reefs. The halcyon days of interest-based science on coral reefs ended in 1998 with the first global coral bleaching event (Fig. 2). The 1998 event triggered a great deal of concern, monitoring, and funding (Goreau et al. 2000; Obura et al. 2019), but not necessarily new ideas of what to measure or how. Indeed, such was the parlous state of reef function studies that it was not until 2019 that the term ‘function’ was defined (Bellwood et al. 2019b) and clarified (Brandl et al. 2019a), and only in 2022 was the related concept of ‘functional traits’ similarly clarified (Streit and Bellwood 2023). In the meantime, we continued to quantify coral reef declines at increasingly large spatial scales, especially in terms of ‘coral cover’ (e.g. Gardner et al. 2003; De’ath et al. 2012; Hughes et al. 2017b; Tebbett et al. 2023c) (Fig. 2). To this day, monitoring is largely based on fish counts (often of a select group of fishes in a select set of reef habitats; Bellwood et al. 2020) and coral cover data (in groups separated by growth morphology); all on relatively short transects (e.g. 30–50 m).
Coral cover dynamics on Anthropocene coral reefs. a Temporal variation in coral cover on the world’s coral reefs (blue line) and atmospheric carbon dioxide concentration (grey line) from the onset of the first global bleaching event (1998) to the end of the most recent global bleaching event (2015–2017). Global coral bleaching events during this period are shown as red bars (Skirving et al. 2019). b Temporal variation in coral cover (blue line) on Australia’s Great Barrier Reef (GBR) and crown-of-thorns starfish (CoTS) densities (orange line) on the GBR. c A corallivorous butterflyfish and two yellow damselfishes, interacting with a bleached Acropora coral at Lizard Island during the 2016 bleaching event (photograph: RP Streit), and d two corallivorous CoTS feeding on an Acropora coral (white areas are feeding scars) (photograph: A Hoggett). These two corallivorous organisms have fundamentally different reputations; one will make it onto postcards (the butterflyfish in c), while the other is the subject of a multi-million dollar culling program (the CoTS in d). Coral cover data in a were sourced from a dataset compiled in Tebbett et al. (2023c) (see this reference for original data sources and relevant citations) and are based on 15,668 mean site level coral cover datapoints (collected using point-intercept or photo-quadrat transect methods) from reef crest and slope habitats at depths < 15 m. The atmospheric CO2 concentration data in a were sourced from Keeling et al. (2001, 2023) and were based on in situ air measurements from the Mauna Loa Observatory, Hawaii. Coral cover data in b were based on photo transects and were sourced from AIMS (2015a), while CoTS density data were based on manta tows and sourced from AIMS (2015b). All lines in a and b were fitted using gam smoothers for visualisation purposes in ggplot2 (Wickham 2016) in the software R (version 4.2.2; R Core Team 2022)
The current special issue explores the growing diversity of methods for quantifying reef functions, including video-based methods (Collins et al. 2023; Magneville et al. 2023), or the integration of remote-sensing (Lutzenkirchen et al. 2024); two areas in which information on reef functions has benefited greatly from technological advances (e.g. DiFiore et al. 2019; Madin et al. 2019; Schiettekatte et al. 2022b). Nevertheless, with ongoing global bleaching events, it has become increasingly clear that reefs are in serious danger (Hughes et al. 2017a; Sully et al. 2019) and that, despite many advances, we still have little idea of: (i) how reefs function and (ii) what the implications of coral bleaching are for critical reef processes. What is missing is a clear, quantitative, understanding of reef processes, i.e. ecosystem functions, and their respective importance across reef types and environmental conditions. Currently, we are left with rapidly transforming ecosystems, largely non-transformed scientific methods, and little idea of which ecosystem functions (processes) are still operating, needed, or manageable (Bellwood et al. 2019a). To focus research and to address these shortcomings, it may pay to re-examine the three key questions: (i) what do we want to protect on coral reefs? (ii) why? and (iii) how can this be achieved?
What do we want to protect on reefs and why?
There is no simple, single answer to the questions: what do we want to protect on coral reefs and why? Answers are invariably context dependent (cf. Bellwood et al. 2019b), with people valuing coral reefs for a plethora of different services in different parts of the world (Lau et al. 2019; Woodhead et al. 2019). However, arguably one of the most ubiquitous features of coral reefs that we want to protect is their physical structure (Hoegh-Guldberg et al. 2019; Perry and Alvarez-Filip 2019), which is tied to nearly every key service that we derive from reefs, including their capacity to (a) protect coastal communities from hydrodynamic activity (Ferrario et al. 2014), (b) enhance fisheries productivity (Rogers et al. 2018), and (c) support tourist/recreational activities (Santavy et al. 2021), including the organismal biodiversity for which reefs are renowned (Siqueira et al. 2023). Indeed, the importance of focusing on this goal was recently expressed by Kuffner and Toth (2016) when they stated: “A better understanding of the processes that control the long-term resilience of reefs as geomorphic structures, not just as ecological communities, may help optimize management activities aimed at increasing both reef longevity and the delivery of critical ecosystem services”.
Importantly, to protect the physical structure of reefs, we know that reef growth via carbonate deposition/production needs to match or exceed erosion. This may not always require sustained vertical accretion (e.g. Toth et al. 2018, 2022), but in the wake of climate change and sea-level rise, sufficient accretion will be needed to accommodate future sea-level changes (Kennedy et al. 2013; Kench et al. 2022). We thus have a near-universal key function of interest—reef growth (accretion). With this clear focus at hand, functional studies offer a rare opportunity to critically evaluate whether our conservation priorities match natural ecological processes and our increasingly prevalent response to climate change: reef restoration.
The vast majority of reef restoration approaches have a single target: to increase coral cover (Hughes et al. 2023). This appears to be a logical goal, the presumed rationale being that: (a) corals have suffered the greatest impact from global warming, and (b) corals provide critical ecosystem functions. Most importantly, the latter includes both the promotion of reef growth/accretion and ancillary functions, such as maintaining fish populations, increasing fisheries productivity, increasing reef complexity for the thousands of associated reef creatures, and, finally, looking attractive to humans (Pratchett et al. 2014b; Rogers et al. 2018). Despite its instinctive appeal, it is important to evaluate whether this focus on corals is based on ecological evidence or our intuition. If it transpires that reef functions (e.g. reef growth or the creation of 3D habitat) are not directly dependent on the corals that are being targeted for restoration, then can current practices of restoring corals be justified in a functional context (at least from the perspective of reef growth)?
It is undoubtedly true that a large proportion of the carbonate in reef cores comes from corals (Montaggioni 2005; Gischler 2014; Webster et al. 2018; Toth et al. 2019) and that corals are one of the dominant calcifiers on coral reefs (Perry et al. 2018; Perry and Alvarez-Filip 2019; Cornwall et al. 2021). It thus appears logical that the loss of corals would result in diminished reef growth and eventually the loss of reef structures. And, indeed, this may well be the case in many regions, including the East Pacific, where, for example, the loss of corals, reduced coral growth, and exceptionally high urchin-based bioerosion have been associated with the disappearance of reef structures (Cortés 1997; Manzello et al. 2017; Glynn et al. 2018; Enochs et al. 2021). However, the relationship between coral abundance, diversity, or growth rates and reef accretion may not be linear and in extreme cases, may not be present at all.
Indeed, recent research suggests that coral growth rates and coral diversity may be largely unrelated to reef growth, at least across geological timescales. For example, reefs in the Atlantic have just 7% of the coral species richness found in the Indo-Pacific (and have just two fast-growing Acropora species) (Roff 2021). They have also had 6000 years less time with high sea-levels in which to grow (Gischler 2010). Thus, in theory, Indo-Pacific reefs should be 500–1000 m wider than their Caribbean counterparts; yet the widths of Caribbean and Indo-Pacific reefs are almost identical (Lutzenkirchen et al. 2023). This suggests that horizontal shallow water reef area and coral diversity are not inherently correlated; instead, reef size appears to be constrained by hydrodynamics, not coral species richness nor sea-level stability (Lutzenkirchen et al. 2023; also see Adey 1978; Montaggioni 2005).
A similar pattern has been documented when looking at reef growth through vertical accretion. In the Caribbean, vertical reef accretion was not found to be affected by significant coral extinction events (i.e. a 50% loss of species; Johnson et al. 2008). Similarly, on Indo-Pacific reefs, vertical accretion appears to be independent of the growth rates of the local dominant framework-building coral species (Roff 2020). Indeed, it is widely accepted in geological contexts that coral growth rates and diversity are not directly correlated with the onset or extent of reef development or growth (Pandolfi and Kiessling 2014; Pomar et al. 2017). The lack of a clear relationship between coral diversity and reef growth also means that, if the goal of restoration activities is to enhance reef growth, then selecting the key coral species is likely to be extraordinarily difficult on high-diversity Indo-Pacific reefs (cf. Madin et al. 2023).
If coral diversity and coral growth rates are poorly correlated with reef accretion, perhaps coral abundances are a better indicator? Today, and for the last few thousand years, Caribbean reef accretion has depended largely on just two Acropora species; with recent losses raising concerns over the functional persistence of these structures (Toth et al. 2018, 2022). However, the disconnects between coral growth and reef growth may also manifest over shorter timescales and incorporate local coral abundances. Today, global mean coral cover is only approximately 25% (Tebbett et al. 2023c), yet even then some Indo-Pacific corals, especially branching Acropora, may contribute little to the consolidated reef matrix and net accretion processes in critical habitats, such as on the high-energy reef crest. For example, Morais et al. (2022) found that the vast majority of Acropora colonies disappeared completely within five years, with no detectable contribution to net in-situ accretion. Presumably, these corals contribute primarily to reef growth in lower energy habitats via production of sediment and rubble that contribute to infilling (with corals invariably constituting the dominant component of cores, and being the major source of carbonate, in such habitats). Indeed, detailed quantification of reef growth over ecological timescales has revealed that accretion can continue despite coral loss, with the contribution of crustose coralline algae to this process being particularly notable (Kench et al. 2022).
Unfortunately, to date, virtually all contemporary reef growth/reef budget studies have focused on the generally high coral cover reef slope (especially ecological studies) or the down-current reef flat habitat (especially coring studies) where dead coral skeletons often accumulate, with far less attention given to reef growth in the reef crest habitat; the primary place where the reef breaks the waves (see Kench et al. 2022 for an overview). Indeed, comparisons between reef habitats raise the possibility that there may be two functionally distinct types of reef growth: ‘reef growth’ version 1 sensu lato, i.e. growth of the total reef structure, especially in terms of backreef progradation—as detected by most cores and which is indeed coral dominated versus ‘reef growth’ version 2 sensu stricto, i.e. in terms of the formation of the structure that breaks waves and allows the backreef to exist. The latter type of growth may depend to a substantial extent on crustose coralline algae and encrusting corals, and results in the actual presence of a reef based on the definition provided by Hopley and Smithers (2008): “Coral reefs are biogenic limestone structures built by corals and other carbonate-producing organisms in shallow tropical and subtropical marine settings, where they grow upwards or towards sea level as landforms able to resist wave action” (for other definitions see Kleypas et al. 2001). Beyond the reef structure that breaks the waves, reef growth sensu lato as defined above (version 1), is largely infilling and widening (notably the substantial amount of carbonate produced by corals appears to be key in these reef-building processes). The study by Kench et al. (2022) appears to be one of the first ecological studies to comprehensively quantify growth on the critical wave-breaking reef crest, though this study is restricted to one location at small spatial and temporal scales (1 m2 over 2 years). Additional studies of reef growth in the most exposed reef habitats are clearly needed.
This evidence inevitably raises the question: are corals always necessary for reef growth (especially vertical growth at the reef crest)? The answer is: probably not. However, reefs built by non-coral organisms are likely to be functionally different, even though they can deliver the key function of reef construction. In this respect, there is growing recognition within coral reef geology and ecology that crustose coralline algae may be critical for reef growth and carbonate production (e.g. Rasser and Riegl 2002; Nash et al. 2013; Vargas-Ángel et al. 2015; Weiss and Martindale 2017; Teichert et al. 2020; Cornwall et al. 2023; Raja et al. 2023), following recognition of this important association decades earlier (e.g. Goreau 1963; Womersley and Bailey 1969; Adey and Macintyre 1973; Littler and Littler 1984). Indeed, some physically complex biogenic tropical reefs function with virtually no corals, such as the Atol das Rocas in Brazil (Gherardi and Bosence 2001). Similarly, coralline algae also dominate framework construction in various other scenarios, including in the formation of the critical algal ridge/rim habitat, which breaks the waves in high-energy settings (Adey 1975; Littler and Doty 1975; Bosence 1983; Rees et al. 2005). These reef structures constructed by coralline algae may offer a glimpse into potential benthic configurations in the Anthropocene, if non-coral calcifiers are not as sensitive as corals to climate change (note the response of CCA to future climate change conditions have been varied [see Cornwall et al. 2019, 2022 for reviews on this topic]). Therefore, it could be that, under some circumstances, some of the functions of corals on coral reefs can be sustained independent of coral diversity (e.g. long-term reef crest development) and perhaps also independent of coral composition or abundance (e.g. short-term carbonate production). From a functional perspective, this opens the possibility of a conceptual shift from ‘coral reefs’ to ‘reefs with corals’ (also see Womersley and Bailey 1969; Kleypas et al. 1999). As continued anthropogenic impacts decimate coral populations on reefs this conceptual shift may become more apparent.
Ultimately, this also means, that if the goal of coral restoration is to maintain reef growth, then our method of doing this (increasing the cover of ‘weedy’ corals in the Indo-Pacific; i.e. fast growing, highly fecund, rapidly recruiting, and highly competitive Acropora) may not be the most appropriate strategy (cf. Madin et al. 2023); especially if we take this action before we stop the main driver of coral declines (i.e. climate change [Hughes et al. 2023]). Even in the Caribbean/Western Atlantic, where benthic systems operate in fundamentally different ways to the Indo-Pacific (Roff and Mumby 2012; Tebbett et al. 2023c), and where two Acropora species can be easily identified as the main contributors to the carbonate framework, care is needed. Even if A. palmata and A. cervicornis can be reared, transplanted, and kept alive through successive bleaching events and disease outbreaks, they may not adequately perform the processes required for consolidation and reef accretion in monocultures. Indeed, an inherently coral-centric perspective (perhaps guided by their high vulnerability) may have left us with only a partial understanding of the impacts of climate change and its implications for coral reefs.
The key problem is that a reef with corals is likely to be functional, but adding corals may not always guarantee reef health or growth. Accepting that the coral contributions to reef growth may be negligible is challenging, as is the fact that many reefs are not dominated by corals (Vroom 2011; Tebbett et al. 2023d). But this changing mindset may be essential if we are to accurately and responsibly evaluate our actions to preserve reefs into the future; simply adding corals, while an attractive and intuitive solution, may not be enough or the best approach. Viewing reefs as the outcome of collective calcification of a range of organisms, including algae, with scleractinian corals offering a boost under the right circumstances, offers a new perspective on what we may need to do to preserve and sustain reefs (cf. Adey 1998). While there may be strong socioeconomic incentives for coral restoration (Hein et al. 2019), if we are primarily interested in maintaining coral reef accretion and structure, restoration efforts that raise and outplant colonies of corals that are highly susceptible to marine heatwaves, and have limited contributions to in-situ accretion, are likely to be an extraordinarily expensive and ultimately futile undertaking (Hughes et al. 2023).
It should be acknowledged that restoration practitioners may focus on restoring corals as an indirect approach to sustain or restore other reef functions, such as the productivity of fish communities. However, such indirect approaches invariably rely on the existence of strong links between fishes and coral cover, which can be context-dependent and weaker than often assumed (e.g. Wismer et al. 2019a, 2019b; Muruga et al. 2024). Indeed, reef fish productivity appears to be more closely related to structural complexity, rather than coral cover (e.g. Rogers et al. 2018; Morais and Bellwood 2019; Hamilton et al. 2022). Given that the structure provided by many fast-growing corals is likely to be relatively short-lived (due to the sensitivity of such corals to disturbance [Loya et al. 2001; Hughes et al. 2017b] and the fact that their skeletons rapidly erode after death [Morais et al. 2022]), then restoring these fast-growing corals may prove to be a relatively inefficient way of restoring reef functions such as fish productivity. Clearly there is a need to better understand the roles that corals and structural complexity play in supporting fish populations, especially in respect to separating co-occurrence from more causal, functional, interdependence.
How do we currently manage reef functions?
To date, a coral-centric view of coral reefs has largely shaped our approaches to managing functions, both directly and indirectly. Historically, we have aimed to secure ‘healthy’ high-coral cover reefs by managing the behaviour of local human populations (e.g. fishing activities). Certain functional groups of fishes were identified as critical to maintaining resilient reefs with high coral cover, such as herbivorous parrotfishes, that are thought to control the growth of competitive algae (Bellwood et al. 2004; Hughes et al. 2007; Graham et al. 2013). To maintain these fishes on reefs, we implemented actions that managed human behaviour (such as establishing no-take marine protected areas [MPAs]), in the belief that this would conserve these fishes and ultimately the ecosystem benefits they delivered (Hughes et al. 2007; Mumby and Steneck 2008). However, the links from management action to the ultimate goal of ecosystem resilience and functioning appear to have been too indirect and multifaceted to deliver easily discernible, consistent, ecosystem-scale benefits (reviewed in Bruno et al. 2019). This became especially evident in the wake of climate change and associated marine heatwaves, which do not respond to MPA boundaries delineated on paper (Jones et al. 2004; Hughes et al. 2017b; Bates et al. 2019; Graham et al. 2020).
The overarching goal of preserving coral cover on reefs has also spawned some of the largest scale and most well-funded attempts to directly manage a coral reef ecosystem function: reducing coral consumption by the corallivorous crown-of-thorns starfish (CoTS) in the Indo-Pacific (Fig. 2d) (Great Barrier Reef Marine Park Authority 2020). By 2018 over 1 million CoTS had been killed on the GBR (Pratchett et al. 2019), with the Australian Government investing ~ $70 million US in direct funding to the culling program between 2012 and 2022 (Pratchett and Cumming 2019), with an additional ~ $109 million US recently awarded (David and Bathgate 2022). The GBR program is, however, dwarfed by the most extensive culling effort undertaken to date in Japan, where ~ 13 million CoTS were removed from reefs in southern Japan in the 1970s and 1980s (Yamaguchi 1986). The cost of this activity was over 600 million yen in 1970; which is equivalent to more than $2.5 billion US today [Pratchett and Cumming 2019]). Despite the popularity of these interventions, an important conundrum has remained unresolved for decades (e.g. Kenchington and Kelleher 1992): are these active management interventions ecologically justifiable?
The conundrum of CoTS control revolves around the fact that these animals are a native species, in their natural habitat, expressing natural behaviour, and they are part of the biodiversity for which coral reefs are renowned. On the GBR, CoTS have been present for 1000 s of years (Fabricius and Fabricius 1992), with regular outbreaks occurring since at least the early-mid 1900s (Moran et al. 1992; Pratchett et al. 2014a). Indeed, CoTS have probably been interacting with coral reefs, and delivering the function of coral consumption, for 10 million years (Hall et al. 2017; Yuasa et al. 2021). Yet, because of their capacity to eat corals, CoTS have been labelled a pest (Hall et al. 2017) (with the Merriam-Webster dictionary defining a pest as: “a plant or animal detrimental to humans or human concerns [such as agriculture or livestock production]”). This pejorative view of CoTS is reinforced by the belief that outbreaks are caused by, or exacerbated by, anthropogenic activities. In this respect, in 1992 the first chairman and chief executive officer of the Great Barrier Reef Marine Park Authority (GBRMPA), Graeme Kelleher, noted: “The Authority’s policy on controlling COTS is not to interfere on a large scale unless it can be shown that outbreaks are caused or exacerbated by human activity” (Kelleher 1992). Although it was clearly considered fundamental for science to establish if anthropogenic activities caused or exacerbated CoTS outbreaks before decisions were made to actively regulate population densities, we are still far from a conclusive answer (Pratchett et al. 2017, 2021). Indeed, after reviewing 30 years of research and > 1200 research articles, Pratchett et al. (2017) concluded that the question “Why [CoTS] outbreaks occur and whether they are natural or unnatural phenomena?” was largely unresolved. Nevertheless, despite a lack of clear scientific evidence that humans have modified CoTS outbreaks, the decision to systematically cull CoTS populations has already been made (discussed in Pratchett et al. 2017).
But, what does the mass killing of CoTS mean from a functional perspective? At face value the outcomes appear obvious: a decrease in coral consuming starfishes will yield higher coral cover (Plagányi et al. 2020; Westcott et al. 2020; Castro‐Sanguino et al. 2023). However, if we consider that CoTS are a natural component of many coral reefs, we can dive deeper and ask: could coral consumption by CoTS be an important function on reefs? In answering this question, the argument could be made that CoTS preferentially target fast growing, competitively dominant, ‘weedy’ corals (albeit often moving onto other coral species once they have exhausted the supply of their preferred prey) (De’ath and Moran 1998; Pratchett et al. 2009; Pratchett 2010; Kayal et al. 2012; Keesing et al. 2019). It has even been suggested (by Porter 1972) that such consumption of highly competitive, fast-growing corals may release slower-growing species from overgrowth and shading and increase long-term benthic diversity (note Pratchett 2010 found overall coral diversity declined during a moderate outbreak of CoTS). In this respect, it is interesting to note that over evolutionary timescales, the fast-growing Acroporidae has been associated with the greatest rates of extinction in non-acroporid corals (Siqueira et al. 2022). Moreover, by consuming fast-growing corals, CoTS: (a) release benthic space for increased benthic productivity, which is critical in coral reef food webs (Russ and St. John 1988; Klumpp and McKinnon 1989; Hatcher 1990), (b) provide the preferred habitat for many reef crustacea, dead coral (Kramer et al. 2014; Fraser et al. 2021), and (c) free up space for other critical benthic coverings, such as crustose coralline algae (Adey 1998; Cornwall et al. 2023).
We are currently entering a period in which fast growing corals, especially Acropora, are likely to be subjected to unstable boom-and-bust dynamics (Wilson et al. 2019; Pratchett et al. 2020; Morais et al. 2021). Indeed, while many fast-growing corals are highly vulnerable to heat stress, their comparatively high growth rates may ultimately (and counterintuitively) bestow an ecological advantage. Specifically, by heating the planet, we may artificially increase the competitive advantage of fast-growing corals (e.g. Acropora) in some contexts, with such corals being some of the few that can ‘recover’ during the much shorter ‘recovery windows’ between the large disturbance events that coral reefs now experience (Hughes et al. 2018; Lough et al. 2018; Morais et al. 2023). Could it be that CoTS moderate the instability of these boom-and-bust periods via coral consumption akin to the important role fires play in some Australian native forests (as noted by Kenchington and Kelleher 1992)? Are we in effect, actively preventing the establishment of diverse, resilient reefs by culling Acropora consuming CoTS? Alternatively, could large fluctuations in CoTS densities, combined with their variable effects on coral assemblages, add to instability in Acropora abundance and overall coral cover? Answering these critical ecological questions is essential if we wish to understand how unstable, disturbed, coral reefs function, and the roles of CoTS therein.
In a nutshell, CoTS may perform a key function on coral reefs. Ecological evidence (or rather the lack thereof) warrants, at the very least, further investigation into their true functional role, despite the current established view that CoTS are detrimental to reef functioning because they decrease live coral cover. Could it be that across the Indo-Pacific we have spent the equivalent of billions of dollars trying to prevent a natural and functionally important process? In this context, the prescience of Graeme Kelleher’s call for caution (in the same article as above) is striking: “there is the possibility that our massive interference in what might be a natural element of the Great Barrier Reef system could have major unforeseen ecological effects” and that the “conclusion is that the risks from adopting a policy of massive destruction of crown-of-thorns starfish are very significant and that the only potential benefits may turn out to be costs in the long run” (Kelleher 1992). Changing priorities of the management agency, GBRMPA, have since overturned these initial considerations (cf. Morrison 2017).
Currently it is unclear if CoTS do, or do not, perform a key function on reefs. Nevertheless, we have already labelled these organisms as pests, a defamatory characterization that is facilitated by their toxic spines, hostile appearance, and the religious overtones of their common name. Such value-laden terms can cloud scientific judgement (Johns and DellaSala 2017; Morton 2017) and may mean that we do not approach the study of CoTS with an open mind, nor entertain the possibility of them performing a key function on reefs. These subconscious biases can be critical in shaping how we study coral reefs and, as scientists, we must be aware of their potential to shape our decisions of what to study and why (Bellwood et al. 2020). Our perceptions of the parrotfish Bolbometopon muricatum, relative to CoTS may be indicative of such potential biases.
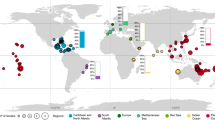
After CoTS, B. muricatum are arguably the single largest predator of corals on Indo-Pacific reefs (when present in their original, natural, densities; Bellwood et al. 2003, 2012; McCauley et al. 2014). In contrast to CoTS, they actively reduce the coral reef framework and net storage of calcium carbonate. B. muricatum can erode the reef at a rate of 5.69 tonnes per individual per year, with intact parrotfish populations capable of consuming the reef at a rate that matches local accretion; thereby maintaining a dynamic carbonate balance (Bellwood et al. 2003) (Fig. 3). Yet, despite consuming a vast quantity of coral and reef structure each year, B. muricatum are widely considered to perform important, beneficial, ecosystem functions on reefs via the removal of carbonate and the production of sand (Bellwood et al. 2004; Perry et al. 2015; Morgan and Kench 2016; Thomson et al. 2021).
Bioerosion by the excavating parrotfish Bolbometopon muricatum. A The spatial distribution of mean bioerosion by B. muricatum at 35 coral reef locations within their natural range (denoted by the grey area) based on the capacity of each individual B. muricatum to erode 5.69 tonnes of carbonate per year (Bellwood et al. 2003). B B. muricatum bioerosion rates compared to coastal human population densities from within a 100 km radius buffer. C B. muricatum (photograph: JP Krajewski). B. muricatum abundance data were sourced from the publicly available Reef Life Survey dataset (Edgar and Stuart-Smith 2014; Edgar et al. 2020). Only locations with 20 or more transects at shallow depths (< 15 m) were included. Coastal population data were sourced from Center for International Earth Science Information Network–CIESIN–Columbia University (2018)
However, during the mid-1900s B. muricatum were so heavily fished on most reefs across the Indo-Pacific that there are now few reefs left that support natural densities of these parrotfish (Bellwood et al. 2012). Thus, across an entire ocean basin, we have directly modified the critical process of bioerosion—few reefs across the Indo-Pacific experience the high levels of bioerosion that they evolved under (Fig. 3). In response, the overharvesting of B. muricatum is widely viewed as a key conservation issue, with a growing push for better management strategies to conserve the individuals that remain (Donaldson and Dulvy 2004; Comeros-Raynal et al. 2012; Hamilton et al. 2016, 2019; Friedlander et al. 2023). The contrast between a ‘conservation priority’ (B. muricatum) and a ‘pest’ (CoTS), that both deliver, fundamentally, a similar ecosystem function (in terms of coral mortality), is remarkable.
Ultimately, there is no doubt that coral loss is a concerning trend that is reshaping the world’s reefs (Fig. 2) and that human activity is the fundamental driver of these trends. Scientists and managers alike recognize the critical need to halt and reverse the decline of coral reefs (De’ath et al. 2012; Bellwood et al. 2019a; Bruno et al. 2019). The only problem is that we may be making questionable decisions and for the wrong reasons, especially if our decision-making process is grounded not on robust scientific evidence but is instead shaped by aesthetic, sentimental, economic, or political values. Indeed, it seems startling to think that during an era of the twenty-first century, when humanity’s effort to put a person on the moon is becoming a distant memory, our greatest achievement in directly managing an ecosystem function on coral reefs is the mass killing of a naturally occurring echinoderm. Will such approaches hold up to scrutiny when future generations ask us why we managed coral reefs the way we did?
Future outlook and conclusions
Considering reefs from a functional perspective may change our perceptions, including the way we perceive both corals and their predators. Going forward, studies that have an objective focus on ecosystem functions will be critical in developing and appraising our decision-making processes when it comes to reef conservation. In this respect, a first step may be to carefully consider if our coral-centric view of the world’s coral reefs is justified and if corals will be the best indicators for coral reef conservation in the future. It has been repeatedly noted that many reefs are not typically dominated by corals (based on 2D planar extent) (e.g. Vroom 2011; Bruno et al. 2014; Tebbett et al. 2023d), but instead are, in the words of Adey (1998), “algal structured and mediated ecosystems”. Such algae, including crustose coralline algae and turfs, are typically the most abundant component of reefs now, and are likely to be even more so into the future (Agostini et al. 2021; Cornwall et al. 2023; Tebbett et al. 2023c, 2023d). Some algae play pivotal functional roles on reefs, including a key role in facilitating reef growth (Kench et al. 2022; Cornwall et al. 2023) and in shaping the settlement of reef organisms (Evans et al. 2020; Ricardo et al. 2021; Doll et al. 2023). However, despite their prevalence, and the fact that they are central to how coral reefs operate, such algae have been largely overlooked in coral reef science, conservation, and restoration endeavours (especially relative to corals). Ultimately, in our efforts to date, we may have focused on studying a vulnerable and conspicuous subset of the reef’s inhabitants, in a subset of places, with the high likelihood that this has shaped our perceptions of how coral reefs operate (Bellwood et al. 2020). Realigning our efforts to understand other functionally important, yet largely overlooked, components of coral reefs may hold the key to helping us navigate reefs through a changing world.
Beyond realigning scientific efforts, the second major step consists of fully appreciating the markedly different contexts in which coral reefs exist (Fig. 1) and ensuring the different biases we may have with regards to reefs in different contexts do not cloud our judgement (cf. Bellwood et al. 2019b). Indeed, it is becoming increasingly clear that lessons learnt about how reefs function in one context or location, may not translate to reefs in different contexts (Roff and Mumby 2012; Brandl et al. 2019a; Tebbett et al. 2023c). This means there is no ‘one size fits all’ approach to reef management, with the goals of any management strategy dependent on how the specific ecosystem in question functions given the social and environmental context within which it exists (Bellwood et al. 2019a; Williams et al. 2019). Moreover, examining coral reefs in extreme and marginal contexts can yield key insights into how reefs operate outside typical domains, and may yield functionally informative insights that question widely held perceptions (although this is invariably dependent on how analogous marginal/extreme reefs are to disturbed coral reefs) (Burt et al. 2020; Schoepf et al. 2023; Tebbett et al. 2023a). For example, the Galápagos Islands are renowned for their unique, endemic diversity. In this special issue, however, Tebbett et al. (2024) showed that the largely overlooked surgeonfish Prionurus laticlavius in the Eastern Galápagos Islands disproportionally contributed to the productivity of herbivorous fish assemblages, underpinning productivity that was more than threefold higher compared to typical, high-diversity reef systems. Importantly, such intriguing results are not out of the ordinary, with functional studies on coral reefs having a long history of revealing fascinating and unexpected insights into how these ecosystems operate across a variety of contexts (e.g. Bellwood et al. 2006; McWilliam et al. 2018; Brandl et al. 2019b; Tebbett et al. 2020; Schiettekatte et al. 2022a).
Overall, this special issue has built on the work of pioneering reef researchers (e.g. Odum and Odum 1955; Bardach 1961; Randall 1961; Smith and Kinsey 1976; Hamner et al. 1988) and helped showcase the potential for functional studies to contribute to our understanding of coral reefs. Studies of reef functions, grounded in empirical data, and at appropriate scales, will be pivotal in critically appraising our perceptions and approaches to reef science, management, conservation, and governance in the Anthropocene. Ultimately, robust, independent, scientific investigation will be key in helping us navigate reefs through the current coral reef crisis.
References
Adey WH (1975) The algal ridges and coral reefs of St. Croix: their structure and Holocene development. Atoll Res Bull 185:1–67
Adey WH (1978) Coral reef morphogenesis: a multidimensional model. Science 202:831–837
Adey WH (1998) Coral reefs: algal structured and mediated ecosystems in shallow, turbulent, alkaline waters. J Phycol 34:393–406
Adey WH, Macintyre IG (1973) Crustose coralline algae: a re-evaluation in the geological sciences. Geol Soc Am Bull 84:883–904
Agostini S, Harvey BP, Milazzo M, Wada S, Kon K, Floc’h N, Komatsu K, Kuroyama M, Hall-Spencer JM (2021) Simplification, not “tropicalization”, of temperate marine ecosystems under ocean warming and acidification. Glob Change Biol 27(4771):4784
Alevizon W, Richardson R, Pitts P, Serviss G (1985) Coral zonation and patterns of community structure in Bahamian reef fishes. Bull Mar Sci 36:304–318
Anderson GRV, Ehrlich AH, Ehrlich PR, Roughgarden JD, Russell BC, Talbot FH (1981) The community structure of coral reef fishes. Am Nat 117:476–495
Australian Institute of Marine Science (AIMS) (2015a) AIMS Long-term Monitoring Program: Video and Photo Transects (Great Barrier Reef). Accessed 20th July 2023. https://doi.org/10.25845/5c09bc4ff315c
Australian Institute of Marine Science (AIMS) (2015b) AIMS Long-term Monitoring Program: Crown-of-thorns starfish and benthos Manta Tow Data (Great Barrier Reef). Accessed 20th July 2023. https://doi.org/10.25845/5c09b0abf315a
Bak RPM (1994) Sea urchin bioerosion on coral reefs: place in the carbonate budget and relevant variables. Coral Reefs 13:99–103
Bak RPM, Luckhurst BE (1980) Constancy and change in coral reef habitats along depth gradients at Curacao. Oecologia 47:145–155
Bardach JE (1959) The summer standing crop of fish on a shallow Bermuda reef. Limnol Oceanogr 4:77–85
Bardach JE (1961) Transport of calcareous fragments by reef fishes. Science 133:98–99
Bates AE, Cooke RSC, Duncan MI, Edgar GJ, Bruno JF, Benedetti-Cecchi L, Côté IM, Lefcheck JS, Costello MJ, Barrett N, Bird TJ, Fenberg PB, Stuart-Smith RD (2019) Climate resilience in marine protected areas and the ‘protection paradox.’ Biol Conserv 236:305–314
Bellwood DR (1995) Direct estimate of bioerosion by two parrotfish species, Chlorurus gibbus and C. sordidus, on the Great Barrier Reef. Australia Mar Biol 121:419–429
Bellwood DR, Hoey AS, Choat JH (2003) Limited functional redundancy in high diversity systems: resilience and ecosystem function of coral reefs. Ecol Lett 6:281–285
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827–833
Bellwood DR, Hughes TP, Hoey AS (2006) Sleeping functional group drives coral-reef recovery. Curr Biol 16:2434–2439
Bellwood DR, Hoey AS, Hughes TP (2012) Human activity selectively impacts the ecosystem roles of parrotfishes on coral reefs. Proc R Soc B Biol Sci 279:1621–1629
Bellwood DR, Pratchett MS, Morrison TH, Gurney GG, Hughes TP, Álvarez-Romero JG, Day JC, Grantham R, Grech A, Hoey AS, Jones GP, Pandolfi JM, Tebbett SB, Techera E, Weeks R, Cumming GS (2019a) Coral reef conservation in the Anthropocene: confronting spatial mismatches and prioritizing functions. Biol Conserv 236:604–615
Bellwood DR, Streit RP, Brandl SJ, Tebbett SB (2019b) The meaning of the term ‘function’ in ecology: a coral reef perspective. Funct Ecol 33:948–961
Bellwood DR, Hemingson CR, Tebbett SB (2020) Subconscious biases in coral reef fish studies. Bioscience 70:621–627
Bohnsack JA, Bannerot SP (1986) A stationary visual census technique for quantitatively assessing community structure of coral reef fishes. NOAA Technical Report NMFS. National Oceanic and Atmospheric Administration/National Marine Fisheries Service
Bosence DWJ (1983) Coralline algal reef frameworks. J Geol Soc 140:365–376
Brandl SJ, Rasher DB, Côté IM, Casey JM, Darling ES, Lefcheck JS, Duffy JE (2019a) Coral reef ecosystem functioning: eight core processes and the role of biodiversity. Front Ecol Environ 17:445–454
Brandl SJ, Tornabene L, Goatley CHR, Casey JM, Morais RA, Côté IM, Baldwin CC, Parravicini V, Schiettekatte NMD, Bellwood DR (2019b) Demographic dynamics of the smallest marine vertebrates fuel coral-reef ecosystem functioning. Science 364:1189–1192
Bruno JF, Precht WF, Vroom PS, Aronson RB (2014) Coral reef baselines: how much macroalgae is natural? Mar Pollut Bull 80:24–29
Bruno JF, Côté IM, Toth LT (2019) Climate change, coral loss, and the curious case of the parrotfish paradigm: why don’t marine protected areas improve reef resilience? Annu Rev Mar Sci 11:307–334
Buddemeier RW, Smith SV (1988) Coral reef growth in an era of rapidly rising sea level: predictions and suggestions for long-term research. Coral Reefs 7:51–56
Burt JA, Camp EF, Enochs IC, Johansen JL, Morgan KM, Riegl B, Hoey AS (2020) Insights from extreme coral reefs in a changing world. Coral Reefs 39:495–507
Carpenter RC (1986) Partitioning herbivory and its effects on coral reef algal communities. Ecol Monogr 56:345–364
Castro-Sanguino C, Bozec Y, Condie SA, Fletcher CS, Hock K, Roelfsema C, Westcott DA, Mumby PJ (2023) Control efforts of crown-of-thorns starfish outbreaks to limit future coral decline across the Great Barrier Reef. Ecosphere 14:e4580
Center for International Earth Science Information Network - CIESIN - Columbia University (2018) Gridded Population of the World, Version 4 (GPWv4): Population Count, Revision 11. Palisades, New York: NASA Socioeconomic Data and Applications Center (SEDAC). Accessed 26th July 2023. https://doi.org/10.7927/H4JW8BX5
Chappell J (1980) Coral morphology, diversity and reef growth. Nature 286:249–252
Chave KE, Smith SV, Roy KJ (1972) Carbonate production by coral reefs. Mar Geol 12:123–140
Choat JH (1969) Studies on the biology of labroid fishes (Labridae and Scaridae) at Heron Island, Great Barrier Reef. PhD dissertation, University of Queensland
Collins WP, Bellwood DR, Morais RA (2023) Small coral reef fishes with large ecological footprints. Coral Reefs. https://doi.org/10.1007/s00338-023-02384-6
Comeros-Raynal MT, Choat JH, Polidoro BA, Clements KD, Abesamis R, Craig MT, Lazuardi ME, McIlwain J, Muljadi A, Myers RF, Nañola CL Jr, Pardede S, Rocha LA, Russell B, Sanciangco JC, Stockwell B, Harwell H, Carpenter KE (2012) The likelihood of extinction of iconic and dominant herbivores and detritivores of coral reefs: the parrotfishes and surgeonfishes. PLoS ONE 7:e39825
Cornwall CE, Diaz-Pulido G, Comeau S (2019) Impacts of ocean warming on coralline algal calcification: meta-analysis, knowledge gaps, and key recommendations for future research. Front Mar Sci 6:186
Cornwall CE, Comeau S, Kornder NA, Perry CT, van Hooidonk R, DeCarlo TM, Pratchett MS, Anderson KD, Browne N, Carpenter R, Diaz-Pulido G, D’Olivo JP, Doo SS, Figueiredo J, Fortunato SAV, Kennedy E, Lantz CA, McCulloch MT, González-Rivero M, Schoepf V, Smithers SG, Lowe RJ (2021) Global declines in coral reef calcium carbonate production under ocean acidification and warming. Proc Natl Acad Sci U S A 118:e2015265118
Cornwall CE, Harvey BP, Comeau S, Cornwall DL, Hall-Spencer JM, Peña V, Wada S, Porzio L (2022) Understanding coralline algal responses to ocean acidification: meta-analysis and synthesis. Glob Change Biol 28:362–374
Cornwall CE, Carlot J, Branson O, Courtney TA, Harvey BP, Perry CT, Andersson AJ, Diaz-Pulido G, Johnson MD, Kennedy E, Krieger EC, Mallela J, McCoy SJ, Nugues MM, Quinter E, Ross CL, Ryan E, Saderne V, Comeau S (2023) Crustose coralline algae can contribute more than corals to coral reef carbonate production. Commun Earth Environ 4:105
Cortés J (1997) Biology and geology of eastern Pacific coral reefs. Coral Reefs 16:S39–S46
Darwin C (1842) On the structure and distribution of coral reefs: being the first part of the geology of the voyage of the beagle under the command of captain fitzroy, RN during the years 1832 to 1836. Smith Elder, London
David P, Bathgate R (2022) Budget Review 2022–23. Research Paper Series, Parliament of Australia, Canberra, Australia, pp 75–59
De’ath G, Moran PJ (1998) Factors affecting the behaviour of crown-of-thorns starfish (Acanthaster planci L.) on the Great Barrier Reef: 2: feeding preferences. J Exp Mar Biol Ecol 220(107):126
De’ath G, Fabricius KE, Sweatman H, Puotinen M (2012) The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci USA 109(17995):17999
DiFiore B, Queenborough S, Madin E, Paul V, Decker M, Stier A (2019) Grazing halos on coral reefs: predation risk, herbivore density, and habitat size influence grazing patterns that are visible from space. Mar Ecol Prog Ser 627:71–81
Doherty PJ, Williams DMCB (1988) The replenishment of coral reef fish populations. Oceanogr Mar Biol Annu Rev 26(487):551
Doll PC, Uthicke S, Caballes CF, Diaz-Pulido G, Abdul Wahab MA, Lang BJ, Jeong SY, Pratchett MS (2023) Settlement cue selectivity by larvae of the destructive crown-of-thorns starfish. Biol Lett 19:20220399
Donaldson TJ, Dulvy NK (2004) Threatened fishes of the world: Bolbometopon muricatum (Valenciennes 1840) (Scaridae). Environ Biol Fishes 70:373
Done T (1982) Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs 1:95–107
Done T (1983) Coral zonation: its nature and significance. In: Barnes DJ (ed) Perspectives on Coral Reefs. Brian Clouston, Manuka, pp 107–147
Done TJ, Ogden J, Wiebe W, Rosen B (1996) Biodiversity and ecosystem function of Coral Reefs. In: Mooney HA, Cushman J, Medina E, Sala O, Schulze E-D (eds) Functional roles of biodiversity: a global perspective. John Wiley & Sons, UK, pp 393–429
Edgar GJ, Stuart-Smith RD (2014) Systematic global assessment of reef fish communities by the Reef Life Survey program. Sci Data 1:140007
Edgar GJ, Cooper A, Baker SC, Barker W, Barrett NS, Becerro MA, Bates AE, Brock D, Ceccarelli DM, Clausius E, Davey M, Davis TR, Day PB, Green A, Griffiths SR, Hicks J, Hinojosa IA, Jones BK, Kininmonth S, Larkin MF, Lazzari N, Lefcheck JS, Ling SD, Mooney P, Oh E, Pérez-Matus A, Pocklington JB, Riera R, Sanabria-Fernandez JA, Seroussi Y, Shaw I, Shields D, Shields J, Smith M, Soler GA, Stuart-Smith J, Turnbull J, Stuart-Smith RD (2020) Establishing the ecological basis for conservation of shallow marine life using Reef life survey. Biol Conserv 252:108855
Enochs IC, Toth LT, Kirkland A, Manzello DP, Kolodziej G, Morris JT, Holstein DM, Schlenz A, Randall CJ, Maté JL, Leichter JJ, Aronson RB (2021) Upwelling and the persistence of coral-reef frameworks in the eastern tropical Pacific. Ecol Monogr 91:e01482
Evans RD, Wilson SK, Fisher R, Ryan NM, Babcock R, Blakeway D, Bond T, Dorji P, Dufois F, Fearns P, Lowe RJ, Stoddart J, Thomson DP (2020) Early recovery dynamics of turbid coral reefs after recurring bleaching events. J Environ Manage 268:110666
Fabricius KE, Fabricius FH (1992) Re-assessment of ossicle frequency patterns in sediment cores: rate of sedimentation related to Acanthaster planci. Coral Reefs 11:109–114
Ferrario F, Beck MW, Storlazzi CD, Micheli F, Shepard CC, Airoldi L (2014) The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nat Commun 5:3794
Fraser KM, Stuart-Smith RD, Ling SD, Edgar GJ (2021) High biomass and productivity of epifaunal invertebrates living amongst dead coral. Mar Biol 168:102
Friedlander AM, Bukurrou A, Filous A, Muller Karanassos C, Koike H, Koshiba S, Mereb G, Nestor V, Oleson KLL, Olsudong D, Oruetamor J, Otto EI, Polloi K, Rengiil G, Tellei E, Golbuu Y (2023) Assessing and managing charismatic marine megafauna in Palau: Bumphead parrotfish (Bolbometopon muricatum) and Napoleon wrasse (Cheilinus undulatus). Aquat Conserv Mar Freshw Ecosyst 33:349–365
Gahan J, Bellwood DR, Bellwood O, Schlaefer J (2023) Gelatinous versus non-gelatinous zooplankton: their value as food for planktivorous coral reef fishes. Coral Reefs. https://doi.org/10.1007/s00338-023-02395-3
Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbean corals. Science 301:958–960
Gherardi DFM, Bosence DWJ (2001) Composition and community structure of the coralline algal reefs from Atol das Rocas, South Atlantic, Brazil. Coral Reefs 19:205–219
Gischler E (2010) Indo-Pacific and Atlantic spurs and grooves revisited: the possible effects of different Holocene sea-level history, exposure, and reef accretion rate in the shallow fore reef. Facies 56:173–177
Gischler E (2014) Quaternary reef response to sea-level and environmental change in the western Atlantic. Sedimentology 62:429–465
Glynn PW, Feingold JS, Baker A, Banks S, Baums IB, Cole J, Colgan MW, Fong P, Glynn PJ, Keith I, Manzello D, Riegl B, Ruttenberg BI, Smith TB, Vera-Zambrano M (2018) State of corals and coral reefs of the Galápagos Islands (Ecuador): Past, present and future. Mar Pollut Bull 133:717–733
Goreau TF (1959) The ecology of Jamaican coral reefs I. species composition and zonation. Ecology 40:67–90
Goreau TF (1963) Calcium carbonate deposition by coralline algae and corals in relation to their roles as reef-builders. Ann N Y Acad Sci 109:127–167
Goreau TF, McClanahan T, Hayes R, Strong A (2000) Conservation of coral reefs after the 1998 global bleaching event. Conserv Biol 14:5–15
Graham NAJ, Bellwood DR, Cinner JE, Hughes TP, Norstrom AV, Nyström M (2013) Managing resilience to reverse phase shifts in coral reefs. Front Ecol Environ 11:541–548
Graham NAJ, Robinson JPW, Smith SE, Govinden R, Gendron G, Wilson SK (2020) Changing role of coral reef marine reserves in a warming climate. Nat Commun 11:2000
Great Barrier Reef Marine Park Authority (2020) Crown-of-thorns starfish strategic management framework. Great Barrier Reef Marine Park Authority, Townsville
Hall MR, Kocot KM, Baughman KW, Fernandez-Valverde SL, Gauthier MEA, Hatleberg WL, Krishnan A, McDougall C, Motti CA, Shoguchi E, Wang T, Xiang X, Zhao M, Bose U, Shinzato C, Hisata K, Fujie M, Kanda M, Cummins SF, Satoh N, Degnan SM, Degnan BM (2017) The crown-of-thorns starfish genome as a guide for biocontrol of this coral reef pest. Nature 544:231–234
Hamilton RJ, Almany GR, Stevens D, Bode M, Pita J, Peterson NA, Choat JH (2016) Hyperstability masks declines in bumphead parrotfish (Bolbometopon muricatum) populations. Coral Reefs 35:751–763
Hamilton RJ, Hughes A, Brown CJ, Leve T, Kama W (2019) Community-based management fails to halt declines of bumphead parrotfish and humphead wrasse in Roviana Lagoon, Solomon Islands. Coral Reefs 38:455–465
Hamilton M, Robinson JPW, Benkwitt CE, Wilson SK, MacNeil MA, Ebrahim A, Graham NAJ (2022) Climate impacts alter fisheries productivity and turnover on coral reefs. Coral Reefs 41:921–935
Hamner WM, Jones MS, Carleton JH, Hauri IR, Williams DM (1988) Zooplankton, planktivorous fish, and water currents on a windward reef face: Great Barrier Reef, Australia. Bull Mar Sci 42:459–479
Harrison HB, Bode M, Williamson DH, Berumen ML, Jones GP (2020) A connectivity portfolio effect stabilizes marine reserve performance. Proc Natl Acad Sci 117:25595–25600
Hatcher BG (1990) Coral reef primary productivity: a hierarchy of pattern and process. Trends Ecol Evol 5:149–155
Hatcher BG, Larkum AWD (1983) An experimental analysis of factors controlling the standing crop of the epilithic algal community on a coral reef. J Exp Mar Biol Ecol 69:61–84
Hay ME (1984) Patterns of fish and urchin grazing on Caribbean coral reefs: are previous results typical? Ecology 65:446–454
Hein MY, Birtles A, Willis BL, Gardiner N, Beeden R, Marshall NA (2019) Coral restoration: socio-ecological perspectives of benefits and limitations. Biol Conserv 229:14–25
Hiatt RW, Strasburg DW (1960) Ecological relationships of the fish fauna on coral reefs of the Marshall Islands. Ecol Monogr 30:65–127
Hoegh-Guldberg O, Pendleton L, Kaup A (2019) People and the changing nature of coral reefs. Reg Stud Mar Sci 30:100699
Hopley D, Smithers S (2008) Geomorphology of coral reefs with special reference to the Great Barrier Reef. In: Hutchings P, Kingsford M, Hoegh-Guldberg O (eds) The Great Barrier Reef: biology, environment and management. CSIRO Publishing, Australia, pp 9–24
Hughes TP, Baird AH, Dinsdale EA, Moltschaniwskyj NA, Pratchett MS, Tanner JE, Willis BL (1999) Patterns of recruitment and abundance of corals along the Great Barrier Reef. Nature 397:59–63
Hughes TP, Bellwood DR, Folke CS, McCook LJ, Pandolfi JM (2007) No-take areas, herbivory and coral reef resilience. Trends Ecol Evol 22:1–3
Hughes TP, Barnes ML, Bellwood DR, Cinner JE, Cumming GS, Jackson JBC, Kleypas J, van de Leemput IA, Lough JM, Morrison TH, Palumbi SR, Nes EHV, Scheffer M (2017a) Coral reefs in the Anthropocene. Nature 546:82–90
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TCL, Butler I, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin M, Figueira W, Gilmour J, Harrison HB, Heron SF, Hoey AS, Hobbs J-PA, Hoogenboom MO, Kennedy EV, Kuo C-Y, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm H, McWilliam M, Pandolfi JM, Pears R, Pratchett MS, Schoepf V, Simpson T, Skirving W, Sommer B, Torda G, Wachenfeld D, Willis BL, Wilson SK (2017b) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs JPA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett M, Schoepf V, Torda G, Wilson SK (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83
Hughes TP, Baird AH, Morrison TH, Torda G (2023) Principles for coral reef restoration in the Anthropocene. One Earth 6:656–665
Hutchings PA (1986) Biological destruction of coral reefs—a review. Coral Reefs 4:239–252
Johns D, DellaSala DA (2017) Caring, killing, euphemism and George Orwell: how language choice undercuts our mission. Biol Conserv 211:174–176
Johnson KG, Jackson JBC, Budd AF (2008) Caribbean reef development was independent of coral diversity over 28 million years. Science 319:1521–1523
Jones R (1968) Ecological relationships in Hawaiian and Johnston Island Acanthuridae (surgeonfishes). Micronesica 4:309–361
Jones GP, McCormick MI, Srinivasan M, Eagle JV (2004) Coral decline threatens fish biodiversity in marine reserves. Proc Natl Acad Sci U S A 101:8251–8253
Jones GP, Almany GR, Russ GR, Sale PF, Steneck RS, Van Oppen MJH, Willis BL (2009) Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs 28:307–325
Kayal M, Vercelloni J, Lison de Loma T, Bosserelle P, Chancerelle Y, Geoffroy S, Stievenart C, Michonneau F, Penin L, Planes S, Adjeroud M (2012) Predator crown-of-thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLoS ONE 7:e47363
Keeling CD, Piper SC, Bacastow RB, Wahlen M, Whorf TP, Heimann M, Meijer HA (2001) Exchanges of atmospheric CO2 and 13CO2 with the terrestrial biosphere and oceans from 1978 to 2000. I. Global aspects. Scripps Institution of Oceanography, San Diego
Keeling RF, Walker SJ, Piper SC, Bollenbacher AF (2023) Scripps CO2 Program (http://scrippsco2.ucsd.edu ). Scripps institution of oceanography (SIO) University of California, La Jolla, California
Keesing JK, Thomson DP, Haywood MDE, Babcock RC (2019) Two time losers: selective feeding by crown-of-thorns starfish on corals most affected by successive coral-bleaching episodes on western Australian coral reefs. Mar Biol 166:72
Kelleher G (1992) A management approach to the COTS question. In: Engelhardt U, Lassig B (eds) The possible causes and consequences of outbreaks of the crown-of-thorns starfish. Great Barrier Reef Marine Park Authority, Townsville, pp 157–159
Kench PS, Beetham EP, Turner T, Morgan KM, Owen SD, Mclean RF (2022) Sustained coral reef growth in the critical wave dissipation zone of a Maldivian atoll. Commun Earth Environ 3:9
Kenchington R, Kelleher G (1992) Crown-of-thorns starfish management conundrums. Coral Reefs 11:53–56
Kennedy EV, Perry CT, Halloran PR, Iglesias-Prieto R, Schönberg CHL, Wisshak M, Form AU, Carricart-Ganivet JP, Fine M, Eakin CM, Mumby PJ (2013) Avoiding coral reef functional collapse requires local and global action. Curr Biol 23:912–918
Kleypas JA, McManus JW, Meñez LAB (1999) Environmental limits to coral reef development: where do we draw the line? Am Zool 39:146–159
Kleypas JA, Buddemeier RW, Gattuso J-P (2001) The future of coral reefs in an age of global change. Int J Earth Sci 90:426–437
Klumpp DW, McKinnon AD (1989) Temporal and spatial patterns in primary production of a coral-reef epilithic algal community. J Exp Mar Biol Ecol 131:1–22
Kramer MJ, Bellwood DR, Bellwood O (2014) Benthic Crustacea on coral reefs: a quantitative survey. Mar Ecol Prog Ser 511:105–116
Kuffner IB, Toth LT (2016) A geological perspective on the degradation and conservation of western Atlantic coral reefs. Conserv Biol 30:706–715
Lau JD, Hicks CC, Gurney GG, Cinner JE (2019) What matters to whom and why? Understanding the importance of coastal ecosystem services in developing coastal communities. Ecosyst Serv 35:219–230
Leray M, Alldredge AL, Yang JY, Meyer CP, Holbrook SJ, Schmitt RJ, Knowlton N, Brooks AJ (2019) Dietary partitioning promotes the coexistence of planktivorous species on coral reefs. Mol Ecol 28:2694–2710
Littler MM, Doty MS (1975) Ecological components structuring the seaward edges of tropical pacific reefs: the distribution, communities and productivity of Porolithon. J Ecol 63:117–129
Littler MM, Littler DS (1984) Models of tropical reef biogenesis: the contribution of algae. Prog Phycol Res 3:323–364
Lough JM, Anderson KD, Hughes TP (2018) Increasing thermal stress for tropical coral reefs: 1871–2017. Sci Rep 8:6079
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
Lutzenkirchen LL, Duce SJ, Bellwood DR (2023) The global biogeography of reef morphology. Glob Ecol Biogeogr 32:1353–1364
Lutzenkirchen LL, Duce SJ, Bellwood DR (2024) Upscaling our understanding of reef functions: the relative contribution of ecology and remote sensing. Coral Reefs. https://doi.org/10.1007/s00338-024-02468-x
Madin EMP, Precoda K, Harborne AR, Atwood TB, Roelfsema CM, Luiz OJ (2019) Multi-trophic species interactions shape seascape-scale coral reef vegetation patterns. Front Ecol Evol 7:102
Madin JS, McWilliam M, Quigley K, Bay LK, Bellwood D, Doropoulos C, Fernandes L, Harrison P, Hoey AS, Mumby PJ, Ortiz JC, Richards ZT, Riginos C, Schiettekatte NMD, Suggett DJ, Van Oppen MJH (2023) Selecting coral species for reef restoration. J Appl Ecol 60:1537–1544
Magneville C, Claverie T, Villéger S (2023) Remote video surveys unveil the diurnal variability of trophic-based processes by fishes on coral reefs. Coral Reefs. https://doi.org/10.1007/s00338-023-02436-x
Manzello DP, Eakin CM, Glynn PW (2017) Effects of global warming and ocean acidification on carbonate budgets of eastern pacific Coral Reefs. In: Glynn PW, Manzello DP, Enochs IC (eds) Coral Reefs of the eastern tropical pacific. Springer, Dordrecht, pp 517–533
McCauley DJ, Young HS, Guevara R, Williams GJ, Power EA, Dunbar RB, Bird DW, Durham WH, Micheli F (2014) Positive and negative effects of a threatened parrotfish on reef ecosystems: varied impacts of a threatened parrotfish. Conserv Biol 28:1312–1321
McWilliam MJ, Chase TJ, Hoogenboom MO (2018) Neighbor diversity regulates the productivity of coral assemblages. Curr Biol 28:3634–3639
Montaggioni LF (2005) History of Indo-Pacific coral reef systems since the last glaciation: development patterns and controlling factors. Earth Sci Rev 71:1–75
Morais RA, Bellwood DR (2019) Pelagic subsidies underpin fish productivity on a degraded coral reef. Curr Biol 29:1521–1527
Morais J, Morais RA, Tebbett SB, Pratchett MS, Bellwood DR (2021) Dangerous demographics in post-bleach corals reveal boom-bust versus protracted declines. Sci Rep 11:18787
Morais J, Morais R, Tebbett SB, Bellwood DR (2022) On the fate of dead coral colonies. Funct Ecol 36:3148–3160
Morais J, Tebbett SB, Morais RA, Bellwood DR (2023) Natural recovery of corals after severe disturbance. Ecol Lett. https://doi.org/10.1111/ele.14332
Moran P, De’ath G, Baker V, Bass D, Christie C, Miller I, Miller-Smith B, Thompson A (1992) Pattern of outbreaks of crown-of-thorns starfish (Acanthaster planci L.) along the great barrier reef since 1966. Mar Freshw Res 43(555):568
Morgan KM, Kench PS (2016) Parrotfish erosion underpins reef growth, sand talus development and island building in the Maldives. Sediment Geol 341:50–57
Morrison TH (2017) Evolving polycentric governance of the Great Barrier Reef. Proc Natl Acad Sci 114:E3013–E3021
Morton SR (2017) On pessimism in Australian ecology. Austral Ecol 42:122–131
Motta PJ (1988) Functional morphology of the feeding apparatus of ten species of Pacific butterflyfishes (Perciformes, Chaetodontidae): an ecomorphological approach. Environ Biol Fishes 22:39–67
Mumby PJ, Steneck RS (2008) Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol Evol 23:555–563
Muruga P, Siqueira AC, Bellwood DR (2024) Meta-analysis reveals weak associations between reef fishes and corals. Nat Ecol Evol. https://doi.org/10.1038/s41559-024-02334-7
Nash MC, Opdyke BN, Troitzsch U, Russell BD, Adey WH, Kato A, Diaz-Pulido G, Brent C, Gardner M, Prichard J, Kline DI (2013) Dolomite-rich coralline algae in reefs resist dissolution in acidified conditions. Nat Clim Change 3:268–272
Obura DO, Aeby G, Amornthammarong N, Appeltans W, Bax N, Bishop J, Brainard RE, Chan S, Fletcher P, Gordon TAC, Gramer L, Gudka M, Halas J, Hendee J, Hodgson G, Huang D, Jankulak M, Jones A, Kimura T, Levy J, Miloslavich P, Chou LM, Muller-Karger F, Osuka K, Samoilys M, Simpson SD, Tun K, Wongbusarakum S (2019) Coral reef monitoring, reef assessment technologies, and ecosystem-based management. Front Mar Sci 6:580
Odum HT, Odum EP (1955) Trophic structure and productivity of a windward coral reef community on Eniwetok Atoll. Ecol Monogr 25:291–320
Pandolfi JM, Kiessling W (2014) Gaining insights from past reefs to inform understanding of coral reef response to global climate change. Curr Opin Environ Sustain 7:52–58
Perry CT, Alvarez-Filip L (2019) Changing geo-ecological functions of coral reefs in the Anthropocene. Funct Ecol 33:976–988
Perry CT, Kench PS, O’Leary MJ, Morgan KM, Januchowski-Hartley F (2015) Linking reef ecology to island building: parrotfish identified as major producers of island-building sediment in the Maldives. Geology 43:503–506
Perry CT, Alvarez-Filip L, Graham NAJ, Mumby PJ, Wilson SK, Kench PS, Manzello DP, Morgan KM, Slangen ABA, Thomson DP, Januchowski-Hartley F, Smithers SG, Steneck RS, Carlton R, Edinger EN, Enochs IC, Estrada-Saldívar N, Haywood MDE, Kolodziej G, Murphy GN, Pérez-Cervantes E, Suchley A, Valentino L, Boenish R, Wilson M, MacDonald C (2018) Loss of coral reef growth capacity to track future increases in sea level. Nature 558:396–400
Plagányi ÉE, Babcock RC, Rogers J, Bonin M, Morello EB (2020) Ecological analyses to inform management targets for the culling of crown-of-thorns starfish to prevent coral decline. Coral Reefs 39:1483–1499
Pomar L, Baceta JI, Hallock P, Mateu-Vicens G, Basso D (2017) Reef building and carbonate production modes in the west-central Tethys during the Cenozoic. Mar Pet Geol 83:261–304
Porter JW (1972) Predation by Acanthaster and its effect on coral species diversity. Am Nat 106:487–492
Pratchett MS (2010) Changes in coral assemblages during an outbreak of Acanthaster planci at Lizard Island, northern Great barrier Reef (1995–1999). Coral Reefs 29:717–725
Pratchett MS, Cumming GS (2019) Managing cross-scale dynamics in marine conservation: pest irruptions and lessons from culling of crown-of-thorns starfish (Acanthaster spp.). Biol Conserv 238:108211
Pratchett MS, Schenk TJ, Baine M, Syms C, Baird AH (2009) Selective coral mortality associated with outbreaks of Acanthaster planci L. in bootless Bay. Papua New Guinea Mar Environ Res 67:230–236
Pratchett MS, Caballes CF, Rivera-Posada JA, Sweatman HPA (2014a) Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr Mar Biol Annu Rev 52:133–200
Pratchett MS, Hoey AS, Wilson SK (2014b) Reef degradation and the loss of critical ecosystem goods and services provided by coral reef fishes. Curr Opin Environ Sustain 7:37–43
Pratchett M, Anderson K, Hoogenboom M, Widman E, Baird A, Pandolfi J, Edmunds P (2015) Spatial, temporal and taxonomic variation in coral growth: implications for the structure and function of coral reef ecosystems. Oceanogr Mar Biol Annu Rev 53:215–295
Pratchett MS, Caballes CF, Wilmes JC, Matthews S, Mellin C, Sweatman HPA, Nadler LE, Brodie J, Thompson CA, Hoey J, Bos AR, Byrne M, Messmer V, Fortunato SAV, Chen CCM, Buck ACE, Babcock RC, Uthicke S (2017) Thirty years of research on crown-of-thorns starfish (1986–2016): scientific advances and emerging opportunities. Diversity 9:41
Pratchett MS, Lang BJ, Matthews S (2019) Culling crown-of-thorns starfish (Acanthaster cf. solaris) on Australia’s great barrier reef: rationale and effectiveness. Aust Zool 40:13–24
Pratchett MS, McWilliam MJ, Riegl B (2020) Contrasting shifts in coral assemblages with increasing disturbances. Coral Reefs 39:783–793
Pratchett MS, Caballes CF, Cvitanovic C, Raymundo ML, Babcock RC, Bonin MC, Bozec Y-M, Burn D, Byrne M, Castro-Sanguino C, Chen CCM, Condie SA, Cowan Z-L, Deaker DJ, Desbiens A, Devantier LM, Doherty PJ, Doll PC, Doyle JR, Dworjanyn SA, Fabricius KE, Haywood MDE, Hock K, Hoggett AK, Høj L, Keesing JK, Kenchington RA, Lang BJ, Ling SD, Matthews SA, McCallum HI, Mellin C, Mos B, Motti CA, Mumby PJ, Stump RJW, Uthicke S, Vail L, Wolfe K, Wilson SK (2021) Knowledge gaps in the biology, ecology, and management of the pacific crown-of-thorns sea star Acanthaster sp. on Australia’s great barrier reef. Biol Bull 241:330–346
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raja NB, Pandolfi JM, Kiessling W (2023) Modularity explains large-scale reef booms in Earth’s history. Facies 69:15
Randall JE (1955) An analysis of the genera of surgeon fishes (Family Acanthuridae). Pac Sci 9:359–367
Randall JE (1961) Overgrazing of algae by herbivorous marine fishes. Ecology 42:812
Randall JE (1967) Food habits of reef fishes of the West Indies. Stud Trop Oceanogr 5:665–847
Randall JE, Allen GR, Steene RC (1990) Fishes of the great barrier reef and coral Sea. Crawford House Press, Bathurst
Rasser M, Riegl B (2002) Holocene coral reef rubble and its binding agents. Coral Reefs 21:57–72
Rees SA, Opdyke BN, Wilson PA, Fifield LK (2005) Coral reef sedimentation on Rodrigues and the Western Indian Ocean and its impact on the carbon cycle. Philos Trans R Soc Math Phys Eng Sci 363:101–120
Ricardo GF, Harper CE, Negri AP, Luter HM, Abdul Wahab MA, Jones RJ (2021) Impacts of water quality on Acropora coral settlement: the relative importance of substrate quality and light. Sci Total Environ 777:146079
Robertson DR, Polunin NVC, Leighton K (1979) The behavioral ecology of three Indian Ocean surgeonfishes (Acanthurus lineatus, A. leucosternon and Zebrasoma scopas): their feeding strategies, and social and mating systems. Environ Biol Fishes 4:125–170
Roff G (2020) Reef accretion and coral growth rates are decoupled in Holocene reef frameworks. Mar Geol 419:106065
Roff G (2021) Evolutionary history drives biogeographic patterns of coral reef resilience. Bioscience 71:26–39
Roff G, Mumby PJ (2012) Global disparity in the resilience of coral reefs. Trends Ecol Evol 27:404–413
Rogers A, Blanchard JL, Mumby PJ (2018) Fisheries productivity under progressive coral reef degradation. J Appl Ecol 55:1041–1049
Russ GR (1984a) Distribution and abundance of herbivorous grazing fishes in the central Great Barrier Reef. I. Levels of variability across the entire continental shelf. Mar Ecol Prog Ser 20:23–34
Russ GR (1984b) Distribution and abundance of herbivorous grazing fishes in the central Great Barrier Reef. II. Patterns of zonation of mid-shelf and outershelf reefs. Mar Ecol Prog Ser 20:35–44
Russ GR (1987) Is rate of removal of algae by grazers reduced inside territories of tropical damselfishes? J Exp Mar Biol Ecol 110:1–17
Russ GR, St. John J (1988) Diets, growth rates and secondary production of herbivorous coral reef fishes. In: Proceedings of 6th International Coral Reef Symposium, Vol 2, pp 37–43
Sale PF (2004) Connectivity, recruitment variation, and the structure of reef fish communities. Integr Comp Biol 44:390–399
Santavy DL, Horstmann CL, Sharpe LM, Yee SH, Ringold P (2021) What is it about coral reefs? Translation of ecosystem goods and services relevant to people and their well-being. Ecosphere 12:e03639
Schiettekatte NMD, Brandl SJ, Casey JM, Graham NAJ, Barneche DR, Burkepile DE, Allgeier JE, Arias-Gonzaléz JE, Edgar GJ, Ferreira CEL, Floeter SR, Friedlander AM, Green AL, Kulbicki M, Letourneur Y, Luiz OJ, Mercière A, Morat F, Munsterman KS, Rezende EL, Rodríguez-Zaragoza FA, Stuart-Smith RD, Vigliola L, Villéger S, Parravicini V (2022a) Biological trade-offs underpin coral reef ecosystem functioning. Nat Ecol Evol 6:701–708
Schiettekatte NMD, Conte F, French B, Brandl SJ, Fulton CJ, Mercière A, Norin T, Villéger S, Parravicini V (2022b) Combining stereo-video monitoring and physiological trials to estimate reef fish metabolic demands in the wild. Ecol Evol 12:e9084
Schoepf V, Baumann JH, Barshis DJ, Browne NK, Camp EF, Comeau S, Cornwall CE, Guzmán HM, Riegl B, Rodolfo-Metalpa R, Sommer B (2023) Corals at the edge of environmental limits: a new conceptual framework to re-define marginal and extreme coral communities. Sci Total Environ 884:163688
Scoffin TP (1992) Taphonomy of coral reefs: a review. Coral Reefs 11:57–77
Scoffin TP, Dixon JE (1983) The distribution and structure of coral reefs: one hundred years since Darwin. Biol J Linn Soc 20:11–38
Sheppard C (1980) Coral cover, zonation and diversity on reef slopes of Chagos Atolls, and population structures of the major species. Mar Ecol Prog Ser 2:193–205
Siqueira AC, Kiessling W, Bellwood DR (2022) Fast-growing species shape the evolution of reef corals. Nat Commun 13:2426
Siqueira AC, Muruga P, Bellwood DR (2023) On the evolution of fish–coral interactions. Ecol Lett. https://doi.org/10.1111/ele.14245
Skirving WJ, Heron SF, Marsh BL, Liu G, De La Cour JL, Geiger EF, Eakin CM (2019) The relentless march of mass coral bleaching: a global perspective of changing heat stress. Coral Reefs 38:547–557
Smith SV, Kinsey DW (1976) Calcium carbonate production, coral reef growth, and sea level change. Science 194:937–939
Stearn CW, Scoffin TP, Martindale W (1977) Calcium carbonate budget of a fringing reef on the west coast of Barbados: part I—zonation and productivity. Bull Mar Sci 27:479–510
Steneck RS (1983) Quantifying herbivory on coral reefs: just scratching the surface and still biting off more than we can chew. Symp Ser Undersea Res 1:103–111
Steneck RS (1988) Herbivory on coral reefs: a synthesis. In: proceedings on 6th International Coral Reef Symposium, Vol 1, pp 37–49
Stoddart DR (1969) Ecology and morphology of recent coral reefs. Biol Rev 44:433–498
Streit RP, Bellwood DR (2023) To harness traits for ecology, let’s abandon ‘functionality.’ Trends Ecol Evol 38:402–411
Sully S, Burkepile DE, Donovan MK, Hodgson G, van Woesik R (2019) A global analysis of coral bleaching over the past two decades. Nat Commun 10:1264
Talbot FH (1965) A description of the coral structure of Tutia Reef (Tanganyika Territory, East Africa) and its fish fauna. Proc Zool Soc Lond 145:431–470
Tebbett SB, Hoey AS, Depczynski M, Wismer S, Bellwood DR (2020) Macroalgae removal on coral reefs: realised ecosystem functions transcend biogeographic locations. Coral Reefs 39:203–214
Tebbett SB, Bellwood DR, Bassett T, Cuttler MVW, Moustaka M, Wilson SK, Yan HF, Evans RD (2023a) The limited role of herbivorous fishes and turf-based trophic pathways in the functioning of turbid coral reefs. Rev Fish Biol Fisheries. https://doi.org/10.1007/s11160-023-09823-1
Tebbett SB, Bennett S, Bellwood DR (2023b) A functional perspective on the meaning of the term ‘herbivore’: patterns versus processes in coral reef fishes. Coral Reefs. https://doi.org/10.1007/s00338-023-02378-4
Tebbett SB, Connolly SR, Bellwood DR (2023c) Benthic composition changes on coral reefs at global scales. Nat Ecol Evol 7:71–81
Tebbett SB, Crisp SK, Evans RD, Fulton CJ, Pessarrodona A, Wernberg T, Wilson SK, Bellwood DR (2023d) On the challenges of identifying benthic dominance on Anthropocene coral reefs. Bioscience 73:220–228
Tebbett SB, Yan HF, Lutzenkirchen LL, Siqueira AC, Bellwood DR (2024) Global patterns of herbivorous reef fish productivity: the role of Prionurus laticlavius in the Galápagos. Coral Reefs. https://doi.org/10.1007/s00338-024-02473-0
Teichert S, Steinbauer M, Kiessling W (2020) A possible link between coral reef success, crustose coralline algae and the evolution of herbivory. Sci Rep 10:17748
Thomson DP, Cresswell AK, Doropoulos C, Haywood MDE, Orr M, Hoey AS (2021) Hidden giants: the story of Bolbometopon muricatum at Ningaloo Reef. Fishes 6:73
Toth LT, Kuffner IB, Stathakopoulos A, Shinn EA (2018) A 3,000-year lag between the geological and ecological shutdown of Florida’s coral reefs. Glob Change Biol 24:5471–5483
Toth LT, Stathakopoulos A, Kuffner IB, Ruzicka RR, Colella MA, Shinn EA (2019) The unprecedented loss of Florida’s reef-building corals and the emergence of a novel coral-reef assemblage. Ecology 100:e02781
Toth LT, Courtney TA, Colella MA, Kupfner Johnson SA, Ruzicka RR (2022) The past, present, and future of coral reef growth in the Florida Keys. Glob Change Biol 28:5294–5309
Toth LT, Storlazzi CD, Kuffner IB, Quataert E, Reyns J, McCall R, Stathakopoulos A, Hillis-Starr Z, Holloway NH, Ewen KA, Pollock CG, Code T, Aronson RB (2023) The potential for coral reef restoration to mitigate coastal flooding as sea levels rise. Nat Commun 14:2313
Vargas-Ángel B, Richards CL, Vroom PS, Price NN, Schils T, Young CW, Smith J, Johnson MD, Brainard RE (2015) Baseline assessment of net calcium carbonate accretion rates on U.S. pacific reefs. PLoS ONE 10:e0142196
Vroom PS (2011) “Coral dominance”: a dangerous ecosystem misnomer? J Mar Biol 2011:164127
Wainwright PC (1988) Morphology and ecology: functional basis of feeding constraints in Caribbean labrid fishes. Ecology 69:635–645
Webster JM, Braga JC, Humblet M, Potts DC, Iryu Y, Yokoyama Y, Fujita K, Bourillot R, Esat TM, Fallon S, Thompson WG, Thomas AL, Kan H, McGregor HV, Hinestrosa G, Obrochta SP, Lougheed BC (2018) Response of the Great Barrier Reef to sea-level and environmental changes over the past 30,000 years. Nat Geosci 11:426–432
Weiss A, Martindale RC (2017) Crustose coralline algae increased framework and diversity on ancient coral reefs. PLoS ONE 12:e0181637
Westcott DA, Fletcher CS, Kroon FJ, Babcock RC, Plagányi EE, Pratchett MS, Bonin MC (2020) Relative efficacy of three approaches to mitigate crown-of-thorns starfish outbreaks on Australia’s Great Barrier Reef. Sci Rep 10:12594
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer Nature, Switzerland
Williams DM, Hatcher AI (1983) Structure of fish communities on outer slopes of inshore, mid-shelf and outer shelf reefs of the Great Barrier Reef. Mar Ecol Prog Ser 10:239–250
Williams DM, Sale PF (1981) Spatial and temporal patterns of recruitment of juvenile coral reef fishes to coral habitats within “one tree Lagoon”, Great Barrier Reef. Mar Biol 65:245–253
Williams GJ, Graham NAJ, Jouffray JB, Norström AV, Nyström M, Gove JM, Heenan A, Wedding LM (2019) Coral reef ecology in the Anthropocene. Funct Ecol 33:1014–1022
Wilson SK, Robinson JPW, Chong-Seng K, Robinson J, Graham NAJ (2019) Boom and bust of keystone structure on coral reefs. Coral Reefs 38:625–635
Wismer S, Tebbett SB, Streit RP, Bellwood DR (2019a) Spatial mismatch in fish and coral loss following 2016 mass coral bleaching. Sci Total Environ 650:1487–1498
Wismer S, Tebbett SB, Streit RP, Bellwood DR (2019b) Young fishes persist despite coral loss on the Great Barrier Reef. Commun Biol 2:456
Womersley HBS, Bailey A (1969) The marine algae of the Solomon Islands and their place in biotic reefs. Philos Trans R Soc Lond B Biol Sci 255:433–442
Woodhead AJ, Hicks CC, Norström AV, Williams GJ, Graham NAJ (2019) Coral reef ecosystem services in the Anthropocene. Funct Ecol 33:1023–1034
Yamaguchi M (1986) Acanthaster planci infestations of reefs and coral assemblages in Japan: a retrospective analysis of control efforts. Coral Reefs 5:23–30
Yuasa H, Kajitani R, Nakamura Y, Takahashi K, Okuno M, Kobayashi F, Shinoda T, Toyoda A, Suzuki Y, Thongtham N, Forsman Z, Bronstein O, Seveso D, Montalbetti E, Taquet C, Eyal G, Yasuda N, Itoh T (2021) Elucidation of the speciation history of three sister species of crown-of-thorns starfish (Acanthaster spp.) based on genomic analysis. DNA Res 28:dsab012
Acknowledgements
We thank the Reef Life Survey Team and their volunteers for collecting and supplying the data used for the study. These data are managed through, and were sourced from, Australia’s Integrated Marine Observing System (IMOS)—IMOS is enabled by the National Collaborative Research Infrastructure Strategy (NCRIS). We also thank the Australian Institute of Marine Science, and all other data sources, for publishing their data or making it publicly available; A Hoggett and JP Krajewski for photographs, and anonymous reviewers for insightful comments.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was funded by the Australian Research Council (DRB; grant number FL190100062).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bellwood, D.R., Brandl, S.J., McWilliam, M. et al. Studying functions on coral reefs: past perspectives, current conundrums, and future potential. Coral Reefs 43, 281–297 (2024). https://doi.org/10.1007/s00338-024-02474-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-024-02474-z