Abstract
Drivers of reef decline are well known both today and in the geological past. Considerably less is known about the preconditions for a pantropical expansion of coral reefs. The geological record of reef building is characterised by considerably long intervals with very limited reef expansion and geologically brief (< 20 million years) episodes of prolific, pantropical reef growth. Here, we propose a new "co-occurrence hypothesis" (COH), which posits that reefs thrive when fast-growing hypercalcifiers co-occur with encrusting organisms such as calcifying microbes or coralline algae to construct wave-resistant structures. While there is little evidence of the effect of abiotic drivers on reef proliferation, we find that positive co-occurrence patterns are significantly more common in reefal as compared to non-reefal communities, suggesting that biological interactions are more relevant in reefs than in non-reefs. Supporting COH, we also show that reefs after the end-Permian mass extinction became more modular in nature with limited membership in reef assemblages during reef booms than in typical periods of reef growth (background intervals). Modularity in reefs may have led to the stabilisation of reef ecosystems, giving them the ability to recover from small perturbations, promoting reefal carbonate accretion and prolific reef growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reefs are highly complex and biodiverse ecosystems, accounting for at least one-quarter of total marine diversity in an area limited only to shallow tropical marine waters (< 0.2% of the seafloor) (Reaka-Kudla 1997; Knowlton et al. 2010). Reef-building corals may be the foundation for the structurally complex habitats that are observed in modern coral reefs, but it is the complex interactions among multiple organisms, such as crustose coralline algae (CCA), sponges and other encrusters, that result in the hydrodynamically stable structures that sustain the abundant and diverse reef species (Weiss and Martindale 2017; Teichert et al. 2020). CCA are key carbonate producers, and they also act as consolidators and binders of loose sediment in reefs. Sponges, even when not calcifying, may also act as transient binders of reefal debris, thereby facilitating reef development (Wulff and Buss 1979). The geological record similarly contains evidence of the importance of microbial activities in reef frameworks (Riding 2002; Yao et al. 2016; Huang et al. 2019), in producing frameworks, as well as encrusting and binding reefal sediment.
The geological record of reefs, broadly defined as laterally constrained limestone structures built by sessile aquatic organisms, is generally characterised by considerably long (> 50 million years) intervals with very limited reef expansion separated by shorter (< 20 million years) episodes of prolific, pantropical reef growth, referred to here as ‘reef booms’, or reef crises (Kiessling 2009). Notably, most major reef crises experienced by post-Cambrian metazoan reefs have been associated with hyperthermal events marked by rapid warming that also coincided with changes in the carbon and water cycles, anoxic conditions and ocean acidification (Pandolfi and Kiessling 2014; Kiessling et al. 2022). While we have a good understanding of the causes of reef crises in the geological record, less is known about the geological intervals during which reefs have been significantly more widespread and productive than normal. On geological time scales, such reef booms were nearly as rapid as reef declines, but they have received much less scientific attention. The reef boom over the last ~ 23 million years is just one of several major reef booms which occurred during the last 540 million years and this one has already lasted longer than most previous phases of reef expansions. The expansion of reefs during this period has previously been attributed to (1) the greater availability of low-nutrient habitats, resulting from a decrease in equatorial upwelling (Perrin and Kiessling 2012), (2) suitable climatic conditions and (3) an increase in shallow marine substrate area in the tropics as a result of plate tectonics (Jones et al. 2022).
Previous hypotheses for successful reef building have also focused on the invention or efficacy of photosymbiosis and the biodiversity of potential reef builders (Kiessling 2005, 2009, 2010; Stanley and van de Schootbrugge 2009). However, the evolution of photosymbiosis in ancient reef builders remains somewhat speculative (Zapalski 2014). Previous analyses also have suggested that reef proliferation is not strongly cross-correlated with earth system parameters such as temperature or ocean chemistry (Kiessling 2002, 2009). Biotic interactions might thus be more important. Specifically, ecological interactions among reef-building organisms may be important to uphold the structure and functioning of the reef framework.
The structure of ecological communities is heavily determined by the specialisation of different taxa and the number as well as the nature of the connections that are shared among these taxa (Bascompte et al. 2003; Bastolla et al. 2009; Sanders et al. 2018). These interaction networks are the product of evolutionary and ecological processes that act at both the individual and collective levels (Thompson 2005) and have an influence on community persistence (Cenci et al. 2018). The stability of communities can be attributed to the network architecture of a community, more specifically the degree of modularity in an ecological network, i.e. the extent to which the network can be divided into structurally and functionally independent subgroups or ‘modules` (Grilli et al. 2016; Landi et al. 2018). In changing environments, the success of a community is not only dependent on the response of individual species to environmental fluctuations but the degree of asynchronicity in these responses (Yachi and Loreau 1999). Modularised communities buffer against declines and extinctions as not all species occupy the exact same niche space and hence are likely to respond differently to environmental disturbances (Yachi and Loreau 1999). Modularised communities tend to be insured against these disturbances through this asynchronicity in their responses to disturbances. On the other hand, in ecosystems without this asynchronicity, i.e. where species all respond similarly, the whole ecosystem behaves as a single species and is more prone to collapse (Landi et al. 2018). At the organismal level, many reef builders, such as scleractinian corals, bryozoans or hypercalcified sponges, tend to be highly modular organisms that depend on colonial growth allowing them to build large reefal frameworks with architectural complexity, flexibility and diversity, making them tolerant to some disturbances (Wood et al. 1992; Wood 1995, 1999).
Here, we test the hypothesis that reef proliferation was self-organised in the sense that certain assemblages of potential reef builders self-enforce the construction of large reef structures. Our "co-occurrence hypothesis" posits that reefs thrive when fast-growing hypercalcifiers co-occur with encrusting organisms such as calcifying microbes or coralline algae to construct hydrodynamically stable structures. We examine the environmental conditions that allow these community assemblages to persist during reef booms and the structure and nature of interactions among reef-building organisms, focusing on the co-occurrence of these organisms in reefs during periods of prolific reef growth.
Materials and methods
Data
We downloaded data on all reefs occurring during the Phanerozoic (~ 541 million years) from the PaleoReefs Database (PARED) (Kiessling and Krause 2021) on 30 October 2021. All reefs that were categorised as ‘mud mounds’ were excluded from the original dataset, because they are probably governed by different ecological factors than true reefs. In total, there were 3159 observations comprising reefs categorised as ‘true reef’, ‘reef mound’ and ‘biostrome’. The biotic composition for each reef provided in PARED comprises one of 96 types of biotic associations where reef-building groups are defined at the supra-ordinal level (Table S1). Reef-building groups were extracted from these associations and re-categorised in standard groups to ensure consistency among the different biotic associations (Table S1). In the case of red-algae, reefs with red algae listed as being the main or secondary reef builder were classified as containing CCA, following Teichert et al. (2020).
Cumulative reef volume in a geological time interval was used as a proxy for reef proliferation. The PARED dataset contains information on fossil reef thickness, width and extension (Table 1). Only 3% of the data comprised full data of actual sizes (width, thickness and extension) of the reefs. However, most reef sites were categorised in size classes (Table 1). Ordinal and ratio data can be empirically related. Mean reef volume for each size class was calculated to fill missing values and the reef volume was computed following a previous approach (Kiessling et al. 2000). In the case where size classes are not reported, we used an arbitrary, conservative estimate. For example, if no reef thickness was reported, we assumed a thickness of 10 m, which is a conservative estimate for a reef to be reported in the literature (the mean thickness of all measured reef sites is ~ 75 m). If reef width was unknown, we conservatively estimated it to be the same as reef thickness and if lateral extent was unknown, we assumed 100 m (mean extent of measured reefs is ~ 2000 m). The number of observed reefs in the geological record is of course strongly dependent on sampling and preservation, but the biological pattern of waxing and waning is likely maintained in the rock record (Kiessling 2006, 2008). Reef booms were then categorised as intervals where the total volume is above the 75th percentile and reef depressions as intervals where the total volume is below the 25th percentile. Background reef intervals were those where the total reef volume is between the 25th and 75th percentile.
Taxonomic occurrences of reef builders were compiled from the Paleobiology Database (PBDB, https://paleobiodb.org/) on 30 October 2021 and assigned to groups matching the same classification as in Table S1. The environmental data and palaeo-coordinates were obtained from the PBDB and the occurrences were categorised as being in “shallow” or “deep” water and in “reef” or “non-reef” settings based on criteria used in previous studies (Kiessling and Aberhan 2007; Kiessling 2010; Table S2). The occurrences were also categorised as being “tropical” (located between 35° N and 35° S) or “non-tropical”. To allow for a comparison between reefs and non-reefs, shallow, tropical, non-reef occurrences were retained, along with all of the reef occurrences. In total, there were 51,848 reefal occurrences and 24,436 non-reefal occurrences of potential reef builders. Both the PARED and PBDB data were resolved to the level of stratigraphic stages of the 2020 Geological Time Scale (GTS 2020; Gradstein et al. 2020). To re-investigate the influence of abiotic factors on reef proliferation, we also compiled environmental data based on palaeogeographical reconstructions and geochemical proxies (Table 2). All the abiotic variables were recalibrated to GTS 2020 to avoid any biases that may incur due to different timescales used.
Influence of abiotic factors on reef proliferation
We applied a multivariate linear regression model to investigate the relationship between reef proliferation and the selected abiotic variables (Table 2). As the variables exhibited positive autocorrelations for multiple lags (Table 3), we used an AutoRegressive Integrated Moving Average (ARIMA) algorithm to identify and remove the effect of any autocorrelation on the observations. We used the auto.arima() function from the ‘forecast’ package (Hyndman and Khandakar 2008) for this purpose. ARIMA performs back-fitting optimisation routines to account for temporal autocorrelation and this allows the correction of the non-stationary characteristics of a time series. The auto.arima() function automates this process by testing multiple permutations of different model specifications and returns the best-fitting ARIMA model. We used a stepwise model selection framework based on minimising values of Akaike information criteria (AIC) to select the most parsimonious model. The predictor variables were included as single interval time-lagged variables, i.e. all predictor variables were shifted backward by one time interval to investigate the cross-correlations between reef volume and the predictor variables. The same modelling approach was applied without including any time lag in the models for comparative purposes.
Quantifying co-occurrence of taxa
We used a probabilistic model (Veech 2013) implemented in the R package ‘cooccur’ (Griffith et al. 2016) to explore the associations between pairs of different taxonomic groups across all sites and time intervals. This approach makes use of a presence-absence matrix to calculate the expected frequency of two taxa occurring together given that they are independently distributed of each other across different sites, in our case, across different reefs. The expected probability (pexp) of two taxa co-occurring is then compared to the observed probability of co-occurrence (pobs). A positive association between two taxa is said to occur when pobs > pexp, and a negative association when pexp > pobs. The association is said to be random when there is no significant difference between pexp and pobs.
To investigate co-occurrence beyond just two groups of organisms, we also carried out a modularity analysis by creating a unipartite taxonomic network where any two groups that co-occur more frequently than expected by chance were linked. In these networks, modules correspond to subsets of taxa whose probability of co-occurring across reefs is higher when compared to the probability of co-occurring with other groups in the wider reef-building community. We used the modularity() in the ‘igraph’ R package (Csardi and Nepusz 2006) to identify sub-communities within the network structure. The importance of co-occurring potentially reef-building organisms in a reef was also examined by comparing the co-occurrence patterns between reefal and non-reefal sites using the PBDB data. These occurrences were further binned into 0.01 × 0.01 degree grid cells. The type of association (positive, random or negative) between reef-building organisms in these hexagonal cells and each time bin were then evaluated. This analysis was repeated for different cell sizes, namely 0.05°, 0.1°, 0.5°, 1° and 5°, for comparison purposes.
Results
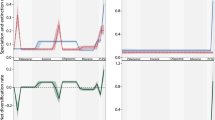
The total volume of reefs, excluding purely microbial reefs, remained relatively low in the Cambrian and Ordovician, with reef volume values remaining close to or below the 25th percentile (considered as reef depressions) of reef volume data across the Phanerozoic (Fig. 1). The first reef boom appeared in the Silurian, which was followed by the longest episode of a reef boom across the Devonian. Otherwise, reef booms were relatively short-lived, lasting ~ 20 million years or less. The reef booms identified here differ from previous patterns reported in the literature, e.g. Kiessling (2009) as (1) reef volume rather than the number of reefs are used to characterise intervals of reef proliferation, (2) mud mounds were not included in our analyses, and (3) we used a statistical definition for the reef booms—those intervals where the total volume is above the 75th percentile were classified. In total, we counted 13 intervals with reef booms across the Phanerozoic and a similar number of reef depressions, and 23 background intervals. All intervals with reef depressions were omitted from subsequent analyses as we aimed to explain the causes of reef booms relative to background times.
Total reef volume across 10-myr time bins over the Phanerozoic. Background intervals (in dark red) are intervals where the reef volume lies between the 25th and 75th percentile, reef booms (in orange) are intervals above the 75th percentile and reef depressions (in grey) are intervals where the reef volume was below the 25th percentile. Intervals of reef depressions (shaded grey area) were omitted from subsequent analyses
We first investigated the relationship between preserved reef volume and reconstructed environmental factors, namely continental shelf area, sea-surface temperature, 87Sr/86Sr, δ34S and δ13C (Table 2), using a multivariate framework. The model with the lowest AIC (AIC = 1954.38, Table 2) with an adjusted R2 of 0.032 (p = 0.12) only included one predictor variable: 87Sr/86Sr (Table 4). The model with temperature and 87Sr/86Sr as predictor variables, however, was chosen as the “best” model as it had a higher R2 of 0.038 but only marginally higher AIC (AIC = 1954.98) (Table 4). All other models similarly showed low or negligible explanatory power (Table 4). Temperature showed a negative relationship with reef volume, where reef volume decreased with increasing temperature, whereas the opposite is true for 87Sr/86Sr (Table 5).
There are more positive co-occurrences of potential reef builders in reefal sites than in non-reefal sites at a spatial resolution of 0.01 degrees (Fig. 2a). Similar patterns are observed at coarser resolutions but are not statistically significant for resolutions of greater or equal to one degree (Fig. 2b–f). Co-occurrences were also compared between intervals of reef booms and background intervals. During background intervals, modularity in reefs remained low. There was a tendency to have more reef builders co-occurring in background intervals as compared to reef boom intervals (Fig. 3a). Over the Phanerozoic, pooled modularity values of the interaction networks of reef-building organisms during reef booms were significantly higher than those during background times (Fig. 3a, b). Modularly structured reefs only became common in post-Permian reef-building communities during the Mesozoic and Cenozoic (Fig. 4), with modularity values significantly higher during reef booms than during background intervals (Fig. 3c, d, Table 6). Individual intervals of reef booms showed high modularity but could not be statistically differentiated from random (Table 6), most likely due to limited sampling in individual time slices.
The mean proportion of random, positive and negative co-occurrences in reef and non-reef environments during the Phanerozoic for cell sizes of a 0.01, b 0.05, c 0.1, d 0.5, e 1 and f 5 degrees, respectively. Error bars represent 95% confidence intervals. W represents the Wilcoxon test static and p the association p value
Network interactions between reef builders during a background intervals and b reef booms across the Phanerozoic, c reef booms during the Palaeozoic and d reef booms during the Mesozoic and Cenozoic. Subcommunities identified by the modularity analysis are highlighted orange, green and grey. Interactions that occur between subcommunities are shown in black. The values m and p in each panel represent the modularity value and associated p value (with the null hypothesis being that the graph is randomly structured), respectively
Discussion
Environmental control on reef proliferation
We did not find any strong associations between global abiotic variables and reef volume, which suggests that global environmental fluctuations may have had little effect in driving long-term reefal carbonate production, and hence reef booms, across the Phanerozoic (Table 4). This is consistent with previous studies that showed a decoupling between carbonate productivity and environmental factors over the Phanerozoic (Kiessling 2002, 2005). Abiotic factors, individually or in combination, can alter the internal dynamics of ecosystems by influencing the competition balance among key groups (Conversi et al. 2015), such as reef-building organisms in this case, leading to a decrease in ecological resilience and the restructuring of a community, e.g. phase shifts from coral to algal dominance in modern reefs as a result of anthropogenic stress (Fung et al. 2011). Small changes in physical or chemical conditions may be enough to give one group an edge over the another. For example, in modern reefs, the degradation of coral reefs as a result of environmental disturbances leading to large-scale coral mortality can lead to phase shifts where benthic organisms such as macroalgae, sea urchins or sponges become the dominant life form, inhibiting coral recovery (Norström et al. 2009). Similarly, in the geological record, there were changes in the prevalence of reef builders, which may have been triggered by environmental changes. For example, the long-term replacement of dominant microbes and sponges as the dominant reef builders by corals in the Triassic is thought to be triggered by climatic changes (Kiessling 2010; Dal Corso et al. 2020). Another example is the shift from corals to rudists in Cretaceous reefs (Gili et al. 1995; Scott 1995; Pandolfi and Kiessling 2014). This shift has been attributed to a series of environmental changes that favoured rudists.
Cooperative competition
Our results show that positive associations between organisms were more common and negative associations less common in reefs compared to non-reefs, whereas no difference was observed in the number of random associations between reefs and non-reefs (Fig. 2). These associations are only observed at finer spatial resolutions, suggesting that they tend to be localised in reefs and that co-occurrence matters at the scale of individual reef sites but not beyond. Competition for space is severe in reefs and can be considered one of the reasons why reefs have topographical relief in the first place (Fagerstrom et al. 2000). Spatial competition may lead to overgrowth or overtopping of corals over one another, as a result of limited space and availability of hard substrates on the seafloor (Muscatine and Porter 1977; Fagerstrom et al. 2000). The survival strategies of competing organisms can be reflected in their mode of life, habitat, morphology or growth rates (Fagerstrom et al. 2000). In space-limited environments, the winners typically are colonial, sessile and large, have fast growth rates and/or have morphological flexibility such as corals, calcareous sponges or bryozoans. For example, some reef builders have developed fast growth rates that can exclude competitors through upward growth or the overtopping of other reef builders (López-Victoria et al. 2006), whereas others are compensated for their losses through indirect effects, such as grazing (Harborne et al. 2016; Wilson et al. 2021).
In the face of competition, reef-building organisms have also developed several mechanisms for coexistence, which we refer to as cooperative competition, a concept adopted from game theory (Fort 2020; Herring 2021). Within ecological game theory models, several organisms interact, directly or indirectly, with each other, while acting in their own interests (which could be in conflict with the interests of, or commonly shared by, other organisms) (Fort 2020). Ecological interactions can be in the form of positive cooperative interactions such as mutualism or conflict-based interactions such as pure competition. In some cases where both types of interaction occur between two organisms, ecological dynamics may result in a state of cooperative equilibrium (Herring 2021). This cooperative equilibrium seems to be especially prevalent after the end-Permian mass extinction (Fig. 3b), which significantly altered the taxonomic and functional composition of reefs, with the rise of scleractinian corals as the main reef builders. In pre-Triassic reefs, hypercalcifying calcareous sponges are observed together with corals and microbes (Fig. 3c), e.g. during the Silurian–Devonian and in the Middle–Late Permian, while corals in modern oceans mostly co-occur with red algae, more specifically CCA; 85% of Mesozoic and Cenozoic reefs listed as being composed of red algae had CCA as reef builders. Biological interactions between these different organisms on reefs, although individually competitive, might lead to cooperative equilibrium within a reef, leading to its success. The positive role of CCA on coral larval recruitment (Ritson-Williams et al. 2014, 2016) or as consolidators of modern reefs (Weiss and Martindale 2017; Teichert et al. 2020) is well documented, but many CCA, especially thick crust CCA, compete for space with corals in reefs and this may lead to overgrowth in some cases (Buenau et al. 2012). However, as a result of preferential grazing pressure from herbivorous fishes, they tend to grow in crevices in coral reefs for protection and end up acting as binders or consolidators of reefs and facilitating coral reef formation. Similar cases of cooperative competition, where competition between taxonomic groups has beneficial results for all competitors involved (Fort 2020; Herring 2021), are also observed in the past.
Limited membership in modern reefs
Reefs during reef booms (Fig. 1) were characterised by higher modularity than those in background intervals during the Mesozoic and Cenozoic (Figs. 3, 4). The end-Permian mass extinction wiped out all the Palaeozoic corals and led to a complete restructuring of reef-building communities. Scleractinian corals expanded during the Triassic as the main reef builders replacing calcifying sponges and microbes in reef environments, leading to the reef boom during the Norian that has been suggested to be due to the spread of photosymbiosis (Kiessling 2010), but could also be the consequence of climatic changes (Sun et al. 2020). Scleractinian corals generally aligned with calcareous sponges or more recently with CCA during reef booms, while reefs with non-coral reef builders were commonly associated with microbes (Fig. 3), such as the siliceous sponge-microbial biotic associations observed in the Upper Jurassic (Reolid 2011). The alignments among these reef-building organisms which are all primary producers and contributors to the reef carbonate budget (Kiessling et al. 2000; Lange et al. 2020; Teichert et al. 2020), and therefore promote reefal carbonate accretion and prolific reef growth.
Just like competitive cooperation, modularity can also lead to the stabilisation of ecological networks, giving them the ability to recover from small perturbations (Grilli et al. 2016). During reef booms, the interactions between specific reef-building organisms that are simultaneously competing with each other, as well as favourable environmental conditions, contribute to the increased volume of reefs. Modularity in reefs is not only confined to the higher level organisation of a reef but also at lower taxonomic scales and even at the organismal level (Wood et al. 1992; Wood 1995, 1999). Limited membership among reef-building communities has also been suggested, where similar taxonomic composition is observed in reefs of different ages, despite evidence of environmental disturbances during the Pleistocene (Pandolfi 1996). The evenness of reef-building communities is known to decrease as the number of reefs globally increased during the Phanerozoic (Kiessling 2009) whereby reef expansion can be interpreted to be associated with limited community membership. The Pleistocene is part of the most recent interval of reef boom observed in the geological record (Fig. 1). The persistence of a particular reef composition can also be attributed to the limited community membership observed in the reef (Pandolfi 1996). Increased modularity of the community structure during intervals of reef booms (Fig. 3) indicates close interactions between certain reef-building groups. Limited membership thus confirms the alignment of the same organisms in reefs over time, in spite of environmental perturbations, suggesting that such membership in reef-building communities and subcommunities is crucial not only for building successful reefs but also for surviving environmental disturbances. At the organismic level, modular organisation in reef-building organisms, i.e. the ability to grow vertically or laterally, as is observed in many coral and sponge taxa, provides several advantages to the individual, such as regeneration capabilities and the ability to grow to larger sizes, resulting in modular organisms with greater longevity than solitary forms of the same group (Wood et al. 1992; Wood 1995). Modular organisms are also longer-lived and are better able to survive overgrowths than solitary organisms. These advantages may scale up to the community level as observed here and may explain why massive reefs are able to persist and flourish on long time scales.
Conclusions
Consistent with previous research, this study finds little effect of global abiotic variables on reef volume across the Phanerozoic. Reef-building communities have undergone complex evolution and transitions, with various reef-building organisms being dominant at different times over the Phanerozoic. Instead, we show support for the co-occurrence hypothesis whereby the co-occurrence of and interactions among reef builders are considered crucial for the success of a reef. Positive associations between reef builders are more common in reefs than in non-reefs, although this is only relevant at the scale of the individual reef. While these reef builders may compete with each other for, e.g. space, in reef environments, we propose that reef-building organisms have developed cooperative competition to coexist while building large reefal structures. This is especially true for reef-building communities that emerged in the aftermath of the end-Permian mass-extinction, which coincided with the rise of scleractinian corals as the main reef- builders. Post-Permian reef-building communities were not only highly modularised but also had limited membership in terms of taxonomic composition during reef booms. Reef-builders, such as scleractinian corals, specifically aligned with, e.g. calcareous sponges or more recently with CCA, conditions that favoured reefal carbonate accretion and prolific reef growth.
Data availability
The source code and data used to produce the results and analyses presented in this manuscript are available from: https://doi.org/10.17605/osf.io/ud25t.
References
Bascompte J, Jordano P, Melián CJ, Olesen JM (2003) The nested assembly of plant–animal mutualistic networks. PNAS 100:9383–9387. https://doi.org/10.1073/pnas.1633576100
Bastolla U, Fortuna MA, Pascual-García A et al (2009) The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature 458:1018–1020. https://doi.org/10.1038/nature07950
Bellwood DR, Hughes TP (2001) Regional-scale assembly rules and biodiversity of coral reefs. Science 292:1532–1535. https://doi.org/10.1126/science.1058635
Buenau KE, Price NN, Nisbet RM (2012) Size dependence, facilitation, and microhabitats mediate space competition between coral and crustose coralline algae in a spatially explicit model. Ecol Model 237–238:23–33. https://doi.org/10.1016/j.ecolmodel.2012.04.013
Cenci S, Song C, Saavedra S (2018) Rethinking the importance of the structure of ecological networks under an environment-dependent framework. Ecol Evol 8:6852–6859. https://doi.org/10.1002/ece3.4252
Conversi A, Dakos V, Gårdmark A et al (2015) A holistic view of marine regime shifts. Philos Trans R Soc B Biol Sci 370:20130279. https://doi.org/10.1098/rstb.2013.0279
Csardi G, Nepusz T (2006) The igraph software package for complex network research. InterJ Complex Syst 1695:1–9
Dal Corso J, Bernardi M, Sun Y et al (2020) Extinction and dawn of the modern world in the Carnian (Late Triassic). Sci Adv 6:eaba0099. https://doi.org/10.1126/sciadv.aba0099
Fagerstrom JA, West RR, Kershaw S, Cossey PJ (2000) Spatial competition among clonal organisms in extant and selected Paleozoic reef communities. Facies 42:1–24. https://doi.org/10.1007/BF02562563
Fort H (2020) Combining niche and game theories to address interspecific cooperation in ecological communities. Community Ecol 21:13–24. https://doi.org/10.1007/s42974-020-00006-7
Fung T, Seymour RM, Johnson CR (2011) Alternative stable states and phase shifts in coral reefs under anthropogenic stress. Ecology 92:967–982. https://doi.org/10.1890/10-0378.1
Gili E, Skelton PW, Vicens E, Obrador A (1995) Corals to rudists—an environmentally induced assemblage succession. Palaeogeogr Palaeoclimatol Palaeoecol 119:127–136. https://doi.org/10.1016/0031-0182(95)00064-X
Gradstein FM, Ogg JG, Smith AG (2004) A geologic time scale 2004. Cambridge University Press, Cambridge
Gradstein FM, Ogg JG, Schmitz MD, Ogg GM (2012) The geologic time scale 2012. Elsevier, Amsterdam
Gradstein FM, Ogg JG, Schmitz MD, Ogg GM (2020) Geologic time scale 2020. Elsevier, Amsterdam
Griffith DM, Veech JA, Marsh CJ (2016) cooccur: probabilistic species co-occurrence analysis in R. J Stat Softw 69:1–17. https://doi.org/10.18637/jss.v069.c02
Grilli J, Rogers T, Allesina S (2016) Modularity and stability in ecological communities. Nat Commun 7:12031. https://doi.org/10.1038/ncomms12031
Harborne AR, Nagelkerken I, Wolff NH et al (2016) Direct and indirect effects of nursery habitats on coral-reef fish assemblages, grazing pressure and benthic dynamics. Oikos 125:957–967. https://doi.org/10.1111/oik.02602
Herring J (2021) Cooperative equilibrium in biosphere evolution: reconciling competition and cooperation in evolutionary ecology. Acta Biotheor 69:629–641. https://doi.org/10.1007/s10441-021-09409-z
Huang W-T, Zhang Y-L, Guan C-Q et al (2019) Role of calcimicrobes and microbial carbonates in the Late Carboniferous (Moscovian) mounds in southern Guizhou, South China. J Palaeogeogr 8:26. https://doi.org/10.1186/s42501-019-0041-7
Hyndman RJ, Khandakar Y (2008) Automatic time series forecasting: the forecast package for R. J Stat Softw 26:1–22. https://doi.org/10.18637/jss.v027.i03
Jones LA, Mannion PD, Farnsworth A et al (2022) Climatic and tectonic drivers shaped the tropical distribution of coral reefs. Nat Commun 13:3120. https://doi.org/10.1038/s41467-022-30793-8
Kiessling W (2001) Paleoclimatic significance of Phanerozoic reefs. Geology 29:751–754. https://doi.org/10.1130/0091-7613(2001)029%3c0751:PSOPR%3e2.0.CO;2
Kiessling W (2005) Long-term relationships between ecological stability and biodiversity in Phanerozoic reefs. Nature 433:410–413. https://doi.org/10.1038/nature03152
Kiessling W (2006) Towards an unbiased estimate of fluctuations in reef abundance and volume during the Phanerozoic. Biogeosciences 3:15–27. https://doi.org/10.5194/bg-3-15-2006
Kiessling W (2008) Sampling-standardized expansion and collapse of reef building in the Phanerozoic. Fossil Rec 11:7–18. https://doi.org/10.1002/mmng.200700008
Kiessling W (2009) Geologic and biologic controls on the evolution of reefs. Annu Rev Ecol Evol Syst 40:173–192. https://doi.org/10.1146/annurev.ecolsys.110308.120251
Kiessling W (2010) Reef expansion during the Triassic: spread of photosymbiosis balancing climatic cooling. Palaeogeogr Palaeoclimatol Palaeoecol 290:11–19. https://doi.org/10.1016/j.palaeo.2009.03.020
Kiessling W, Aberhan M (2007) Environmental determinants of marine benthic biodiversity dynamics through Triassic-Jurassic time. Paleobiology 33:414–434. https://doi.org/10.1666/06069.1
Kiessling W, Flügel E, Golonka J (2000) Fluctuations in the carbonate production of Phanerozoic reefs. Geol Soc Lond Spec Publ 178:191–215. https://doi.org/10.1144/GSL.SP.2000.178.01.13
Kiessling W, Krause MC (2021) PARED—an online database of Phanerozoic reefs. https://www.paleo-reefs.pal.uni-erlangen.de/
Kiessling W, Kohler TA, Cramer W, et al (2022) Cross-chapter box PALEO: vulnerability and adaptation to past climate changes. In: Pörtner H-O, Roberts DC, Tignor M et al (eds) Climate change 2022: impacts, adaptation and vulnerability. contribution of working group II to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Kiessling W (2002) Secular variations in the Phanerozoic reef ecosystem. In: Kiessling W, Flügel E, Golonka J (eds) Phanerozoic Reef Patterns. SEPM Special Publication, vol 72, pp 625–690, Tulsa, USA
Knowlton N, Brainard RE, Fisher R et al (2010) Coral reef biodiversity. Life in the world’s oceans: diversity distribution and abundance. 65–74. https://doi.org/10.1002/9781444325508.ch4
Kocsis ÁT, Scotese CR (2021) Mapping paleocoastlines and continental flooding during the Phanerozoic. Earth Sci Rev 213:103463. https://doi.org/10.1016/j.earscirev.2020.103463
Landi P, Minoarivelo HO, Brännström Å et al (2018) Complexity and stability of ecological networks: a review of the theory. Popul Ecol 60:319–345. https://doi.org/10.1007/s10144-018-0628-3
Lange ID, Perry CT, Alvarez-Filip L (2020) Carbonate budgets as indicators of functional reef “health”: a critical review of data underpinning census-based methods and current knowledge gaps. Ecol Indic 110:105857. https://doi.org/10.1016/j.ecolind.2019.105857
López-Victoria M, Zea S, Weil E (2006) Competition for space between encrusting excavating Caribbean sponges and other coral reef organisms. Mar Ecol Prog Ser 312:113–121. https://doi.org/10.3354/meps312113
Muscatine L, Porter JW (1977) Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27:454–460. https://doi.org/10.2307/1297526
Norström AV, Nyström M, Lokrantz J, Folke C (2009) Alternative states on coral reefs: beyond coral–macroalgal phase shifts. Mar Ecol Prog Ser 376:295–306. https://doi.org/10.3354/meps07815
Ogg JG, Ogg G, Gradstein FM (2016) A concise geologic time scale: 2016. Elsevier, Amsterdam
Pandolfi JM (1996) Limited membership in pleistocene reef coral assemblages from the Huon Peninsula, Papua New Guinea: constancy during global change. Paleobiology 22:152–176. https://doi.org/10.1017/S0094837300016158
Pandolfi JM, Kiessling W (2014) Gaining insights from past reefs to inform understanding of coral reef response to global climate change. Curr Opin Environ Sustain 7:52–58. https://doi.org/10.1016/j.cosust.2013.11.020
Perrin C, Kiessling W (2012) Latitudinal trends in cenozoic reef patterns and their relationship to climate. In: Carbonate systems during the Oligocene–Miocene climatic transition. Wiley, Hoboken, pp 17–33
Prokoph A, Shields GA, Veizer J (2008) Compilation and time-series analysis of a marine carbonate δ18O, δ13C, 87Sr/86Sr and δ34S database through Earth history. Earth Sci Rev 87:113–133. https://doi.org/10.1016/j.earscirev.2007.12.003
Prokoph A, El Bilali H, Ernst R (2013) Periodicities in the emplacement of large igneous provinces through the Phanerozoic: relations to ocean chemistry and marine biodiversity evolution. Geosci Front 4:263–276. https://doi.org/10.1016/j.gsf.2012.08.001
Reaka-Kudla ML (1997) The global biodiversity of coral reefs: a comparison with rain forests. In: Reaka-Kudla ML, Wilson DE, Wilson EO (eds) Biodiversity II: Understanding and protecting our biological resources. Joseph Henry Press, Washington, D.C., p 551
Reolid M (2011) Interactions between microbes and siliceous sponges from Upper Jurassic buildups of external prebetic (SE Spain). In: Reitner J, Quéric N-V, Arp G (eds) Advances in stromatolite geobiology. Springer, Berlin, pp 343–354
Riding R (2002) Structure and composition of organic reefs and carbonate mud mounds: concepts and categories. Earth Sci Rev 58:163–231. https://doi.org/10.1016/S0012-8252(01)00089-7
Ritson-Williams R, Arnold SN, Paul VJ, Steneck RS (2014) Larval settlement preferences of Acropora palmata and Montastraea faveolata in response to diverse red algae. Coral Reefs 33:59–66. https://doi.org/10.1007/s00338-013-1113-2
Ritson-Williams R, Arnold SN, Paul VJ (2016) Patterns of larval settlement preferences and post-settlement survival for seven Caribbean corals. Mar Ecol Prog Ser 548:127–138. https://doi.org/10.3354/meps11688
Sanders D, Thébault E, Kehoe R, van Veen FJF (2018) Trophic redundancy reduces vulnerability to extinction cascades. PNAS 115:2419–2424. https://doi.org/10.1073/pnas.1716825115
Scott RW (1995) Global environmental controls on Cretaceous reefal ecosystems. Palaeogeogr Palaeoclimatol Palaeoecol 119:187–199. https://doi.org/10.1016/0031-0182(94)00068-9
Song H, Wignall PB, Song H et al (2019) Seawater temperature and dissolved oxygen over the past 500 million years. J Earth Sci 30:236–243. https://doi.org/10.1007/s12583-018-1002-2
Stanley GD, van de Schootbrugge B (2009) The evolution of the coral-algal symbiosis. In: van Oppen MJH, Lough JM (eds) Coral bleaching: patterns, processes, causes and consequences. Springer, Berlin, pp 7–19
Sun YD, Orchard MJ, Kocsis ÁT, Joachimski MM (2020) Carnian-Norian (Late Triassic) climate change: evidence from conodont oxygen isotope thermometry with implications for reef development and Wrangellian tectonics. Earth Planet Sci Lett 534:116082. https://doi.org/10.1016/j.epsl.2020.116082
Teichert S, Steinbauer M, Kiessling W (2020) A possible link between coral reef success, crustose coralline algae and the evolution of herbivory. Sci Rep 10:17748. https://doi.org/10.1038/s41598-020-73900-9
Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press
Veech JA (2013) A probabilistic model for analysing species co-occurrence. Glob Ecol Biogeogr 22:252–260. https://doi.org/10.1111/j.1466-8238.2012.00789.x
Weiss A, Martindale RC (2017) Crustose coralline algae increased framework and diversity on ancient coral reefs. PLoS ONE 12:e0181637. https://doi.org/10.1371/journal.pone.0181637
Wilson MW, Gaines SD, Stier AC, Halpern BS (2021) Variation in herbivore grazing behavior across Caribbean reef sites. Mar Biol 168:53. https://doi.org/10.1007/s00227-021-03844-9
Wood R (1995) The changing biology of reef-building. Palaios 10:517–529. https://doi.org/10.2307/3515091
Wood R (1999) Reef evolution. Oxford University Press, Oxford
Wood RA, Zhuravlev AYu, Debrenne F (1992) Functional biology and ecology of Archaeocyatha. Palaios 7:131–156. https://doi.org/10.2307/3514925
Wulff JL, Buss LW (1979) Do sponges help hold coral reefs together? Nature 281:474–475. https://doi.org/10.1038/281474a0
Yachi S, Loreau M (1999) Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc Natl Acad Sci 96:1463–1468. https://doi.org/10.1073/pnas.96.4.1463
Yao L, Aretz M, Chen J et al (2016) Global microbial carbonate proliferation after the end-Devonian mass extinction: mainly controlled by demise of skeletal bioconstructors. Sci Rep 6:39694. https://doi.org/10.1038/srep39694
Zapalski MK (2014) Evidence of photosymbiosis in Palaeozoic tabulate corals. Proc R Soc B 281:20132663. https://doi.org/10.1098/rspb.2013.2663
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (KI 806/17-1) and the Australian Research Council (DP130100250) and is embedded in the Research Unit TERSANE (FOR 2332: temperature-related stressors as a unifying principle in ancient extinctions).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
NBR, JMP and WK designed research; NBR performed research and analyses and wrote the manuscript with input from JMP and WK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raja, N.B., Pandolfi, J.M. & Kiessling, W. Modularity explains large-scale reef booms in Earth’s history. Facies 69, 15 (2023). https://doi.org/10.1007/s10347-023-00671-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10347-023-00671-w