Abstract
The matter of photosymbiosis in Paleozoic corals remains unresolved as it is not possible to directly check for the presence of algal symbionts in fossil corals. However, present-day photosymbiotic corals are characterised by a number of features that can be evaluated in fossil corals as well, such as large, highly integrated colonies, growth banding, and platy growth forms in mesophotic conditions. The present study aims to evaluate these features in heliolitid corals. Heliolitids were relatively highly integrated, compared to other Paleozoic corals and could produce large colonies, over 1 m in diameter. In this study, heliolitid corals from different outcrops from the Silurian (~ 444 to 419 ma) of Gotland (Sweden) were analysed, and additional Devonian (~ 419 to 359 ma) specimens from Belgium, Poland and Morocco, featuring cyclic growth banding, were also included. Overall, over 60% of studied specimens from Lower Visby Formation in Ireviken and over 80% from Eke Formation in Lau Käldu are platy or tabular. Those outcrops represent environments that were mesophotic. Specimens from the shallower Upper Visby Formation in Ygne are dominated by branching forms instead. The apparent light-related skeletal plasticity, high colony integration, growth banding, and the absence of heliolitids in deep water environments implies that some of these corals were most likely photosymbiotic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tabulate corals are very common reef builders of the Paleozoic era (~ 539 to 252 ma [million years ago]). One of the characteristic early- and mid-Paleozoic groups of tabulates are the heliolitids. Whereas their taxonomy has been studied since the nineteenth century (e.g. Goldfuss 1826; Rominger 1876; Lindström 1899), their paleoecology received much less attention. In the more modern literature (of approximately last 30 years), the works detailing the ecological preferences and adaptations of heliolitid corals are rather scarce (e.g. Noble and Lee 1990; Young and Scrutton 1991; Król et al. 2018a, 2021). Although they are known as a very widespread and important fauna of Silurian and Devonian reefal communities, the question of their symbiosis with algae remains open. While many groups of Paleozoic corals were studied in relation to their photosymbiosis (e.g. Coates and Jackson 1987; Scrutton 1998; Wood 1998; Copper 2002; Jakubowicz et al. 2015; Zapalski 2014; Zapalski et al. 2017a, b; Zapalski and Berkowski 2019; Bridge et al. 2022), heliolitids as a group were not a subject of a detailed analysis of this kind. The aim of the present work is to fill this gap and provide some insight into the likelihood of heliolitid corals possessing algal symbionts.
Coates and Jackson (1987) demonstrated correlation between growth form, corallite size and colony integration in relation to the presence of zooxanthellae in scleractinians and also analysed Paleozoic corals stating that while evidence is equivocal, it still advocates for presence of algal symbionts in tabulate corals. Wood (1998) and Copper (2002) also suggested that at least some of tabulates were zooxanthellate. Zapalski et al. (2017a) provided further evidence for photosymbiosis in selected taxa of tabulate corals, this time based upon statistical analysis of skeletal features (corallite diameter, colony integration and others), as well as on the basis of platy morphologies and tabulates forming mesophotic coral ecosystems (Zapalski et al. 2017b). Zapalski and Berkowski (2019) described the oldest mesophotic coral ecosystems from the Silurian (~ 430 ma) of Gotland, formed by platy, presumably photosymbiotic tabulate corals.
The reason why the claim of Paleozoic corals living in symbiosis with algae is disputed, stems from the fact that their analyses are based exclusively on skeletal material, while the fossilisation potential of symbiotic algae is non-existent. In order to prove, or disprove the claim absolutely, a well-preserved soft tissue of the coral would be required. In fossil corals, cases of any soft tissue being preserved whatsoever are extremely rare and the soft parts are not preserved well enough to be examined for the presence of algal cells (Copper 1985; Dixon 2010). For these reasons, whereas the direct proof of photosymbiosis (or lack thereof) in Paleozoic corals is effectively impossible to obtain, the research must turn to more indirect methods. Since the skeletons are the only parts of the corals that are preserved, one must rely solely on the analysis of the corallum and the surrounding sediment. In this paper, the following hypothesis will be examined: if heliolitid corals were photosymbiotic, their skeletons should exhibit the same typical features of zooxanthellate corals established for modern taxa. Those features include compound growth, large colonies, small polyps, corallum structure typical for highly integrated colonies, seasonal cyclic growth patterns, and skeletal plasticity, especially resulting in platy growth forms in low-light environments (Barbeitos et al. 2010; Coates and Jackson 1987; Scrutton and Powell 1980; Young and Kershaw 2005; Stanley and Helmle 2010; Stanley and Lipps 2011; Zapalski et al. 2017a, b).
The studied Silurian specimens from Gotland come from different environments ranging from deep water biostromes of the Lower Visby beds in Ireviken (described as mesophotic coral ecosystems by Zapalski and Berkowski 2019) and from more intermediate Upper Visby beds in Ygne, to shallow coral-stromatoporoid facies of the Eke Formation in Lau Käldu (Fig. 1). Some specimens from Middle Devonian strata of Belgium, Morocco and Poland are included as well. Together, these records should provide additional clues in the investigation of the possible photosymbiosis in heliolitids. Establishing whether they were more likely photosymbiotic or asymbiotic would be crucial to ascertain just how closely analogous they are to modern corals. That in turn can be helpful in studying and managing present-day reefs, as heliolitids faced multiple climate crises of the Middle Paleozoic.
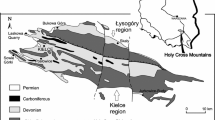
Study localities. A—Ireviken; B—Ygne, C—Lau Käldu; D—Dinant Synclinorium (Olloy-sur-Viroin and Wancennes, Belgium); E—Dziewki and Skały (southern Poland); F—Ouihlane (Morocco). L&U refers to Lower and Upper Visby Beds. Map of Gotland after Calner et al. (2004). The boxed area in the inset represents the location of the expanded Gotland map
Geological background
The Silurian deposits cropping out in Gotland range from latest Llandovery to Ludlow (~ 438 to 423 ma). The Visby Formation constitutes the oldest part of the profile, encompassing upper Telychian—lower Sheinwoodian interval (~ 435 to 430 ma; Calner et al. 2004). It corresponds with the time of the so-called Ireviken event, a minor extinction of graptolites, trilobites and conodonts, which seemingly did not affect coral-stromatoporoid communities (Munnecke et al. 2003). Lower Visby beds cropping out at Ireviken (Fig. 1A) and Ygne (Fig. 1B) consist of regular alteration of nodular limestones (2–5 cm) and marls (c. 10 cm) (Munnecke and Samtleben 1996). In places, halysitid biostromes occur, rich in tabulate and rugose corals and stromatoporoids.
Lower and Upper Visby beds are separated by a distinct biohorizon consisting of the large solitary rugose coral Phaulactis. The Upper Visby beds are distinguishable by dominance of detrital limestone and visibly less marl. Their bedding is also less regular and beds are commonly cut by erosional channels. Corals, stromatoporoids, bryozoans, crinoids, and brachiopods are more abundant than in the Lower Visby beds, and calcareous algae start to occur (Riding 1979). The Upper Visby beds are considered to represent a shallower, high-energy environment in contrast to the deeper, quieter conditions of Lower Visby beds. The entire Visby Formation is considered as shallowing-upward, with the Upper Visby beds representing a highstand systems tract (Calner et al. 2004).
The Eke Formation is dated to Ludlow (upper Ludfordian, ~ 423 ma). It represents the time of another minor extinction affecting graptolites and conodonts—the Lau event (Urbanek 1993). The Eke Formation is a c. 12-m-thick, relatively homogenous succession of shallow water, pack-, wack- and grainstones with oncoids, alternating with marls. In the northernmost outcrops, such as Lau Käldu (Fig. 1C), argillaceous bioherms and coarse-grained crinoidal grainstones and rudstones occur (Calner et al. 2004). The exposed parts of the Lau Käldu outcrop are c. 3 m high, with an approximately 1-m-thick coral-stromatoporoid biostromal layer at the top. The overlying sediments are covered by vegetation, but the sudden shift in the slope inclination from very steep to mild, just above the exposed coral-stromatoporoid biostrome, suggests a change in lithology.
Outcrops near the village of Dziewki in SW Poland (Fig. 1E) have been attributed to Givetian (~ 388 to 383 ma) on the basis of the occurrence of the index brachiopod species Stringocephalus burtini. These are small, isolated outcrops, but the presence of over 200 m-thick Givetian succession in the area was confirmed by drill core data. In the outcrops, it is represented by thickly bedded limestones, which are dolomitised to varying degrees. They contain abundant stromatoporoids, tabulate and rugose corals. This part of the succession can be interpreted as a shallow water, possibly back-reef type of environment (Śliwiński 1964; Racki et al. 1993).
The Skały Formation in the Holy Cross Mountains (SE Poland; Fig. 1E) is represented by marly shales interbedded with very fossiliferous limestones and marls with well-preserved brachiopods, crinoids, corals and bryozoans. The formation encompasses the Eifelian-Givetian boundary (~ 388 ma) with heliolitids and other tabulates in the Givetian part of the succession. It was deposited in a carbonate ramp setting, with coral-bearing facies in its shallower parts (Racki et al. 2022).
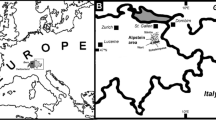
The Wancennes locality (Fig. 1D) along the southern margin of the Dinant Synclinorium (Belgium) exposes a large bioherm mostly built by stromatoporoids and tabulate corals. Rugose corals, chaetetid sponges, heliolitids, brachiopods and other invertebrates are very abundant and diverse and indicate a lower Eifelian (~ 393 to 388 ma) age. The reef-crest facies yields very large (up to 1 m) domal and hemisphaerical stromatoporoids and massive colonies of Heliolites porosus reaching 60 cm in width but most commonly 20–30 cm wide. The growth forms of the stromatoporoids and corals, the associated matrix (well-washed crinoidal rudstone) and depositional sequence within the reef point to a shallow water, very dynamic environment within the fair weather wave zone (Denayer 2023).
The Olloy-sur-Viroin locality, also located along the southern margin of the Dinant Synclinorium, is a small outcrop of the uppermost Emsian-lowermost Eifelian Eau Noire Formation (~ 393 ma). This formation is transitional between the siliciclastic-dominated upper Emsian sequence and the carbonate Eifelian deposits and displays mixed facies of bioclastic calcareous siltstone and shaly limestone rich in brachiopods, bryozoans, crinoids, solitary rugose corals, including Cystiphylloides spp. and Calceola sandalina. Tabulate corals are uncommon: small domal Favosites spp., thin laminar alveolitids and a single colony of Heliolites. The sedimentological context for the Eau Noire Formation points to a low-energy environment still under siliciclastic influx (Bertrand et al. 1993). The corals display typical constriction-rejuvenescence cyclic growth suggesting intermittent (potentially seasonal) sediment inputs.
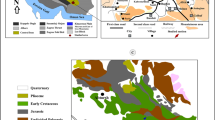
The Ouihlane section, located in southern Morocco (Fig. 1F), encompasses a thick succession of shallow marine to basinal deposits of age ranging from upper Emsian to lower Givetian (~ 398 to 386 ma). During that time, it was positioned in the northern parts of the Mader basin, on the north-western margin of Gondwana (Kaufmann 1998; Jakubowicz et al. 2019). The upper Eifelian part of the succession includes two coral-stromatoporoid horizons. The studied specimens come from the upper horizon, interpreted as a debris flow deposit. It consists of abundant heliolitids and other massive tabulates, massive stromatoporoids, and solitary, dissepimented rugose corals (Schröder and Kazmierczak 1999; Berkowski et al. 2023).
Material and methods
The local nature protection regulations of Gotland prohibit collecting in situ fossil specimens from the geological outcrops. For this reason, the studied material was collected from rock debris instead. Since the studied corals are predominantly delicate, platy forms, when collected from debris, they are mostly fragmented. In addition, the conditions in Ygne in particular (a steep cliff, parts of which often collapse) make studying specimens from the outcrop significantly more challenging. Both Lower and Upper Visby beds can be observed in the outcrop. Therefore, it is not possible to attribute them to the right geological formation with absolute certainty. For that reason, Lower and Upper Visby beds from Ygne are described together, while field observations provide the clues to the differences between the heliolitid assemblages within them.
Heliolitids are here understood as corals belonging to suborder Heliolitina sensu Hill (1981). A total of 266 specimens were collected from the Silurian outcrops of Gotland and 9 additional Devonian specimens from Belgium, Poland and Morocco are included. Though commonly fragmented, the specimens were otherwise mostly very well preserved, facilitating taxonomic identification on the basis of observation of the corallum surface. For more detailed analyses of colony development, 12 thin sections and 13 polished slabs were made. Studied specimens are housed at the Institute of Geology of Adam Mickiewicz University in Poznań (samples K1827, K2101‐K2151, K2220-K2227, W1803) and at the University of Liège (samples PRCI/18, OTB/17, WPEIII/B.5).
The corallite diameter measurements were taken to correctly attribute specimens to the species described from the studied outcrops by other authors. Colony dimensions were measured to attribute the correct growth forms. The terminology of the following coral growth forms was taken from Stearn (1982): “domal” (upwardly convex, broad base), “bulbous” (upwardly convex, narrowing towards the base), and “branching” (dendroid and stick-like). For coralla with a flat or undulated top surface, the term “platy” is used, if the W:H (width to height) ratio is equal or exceeds 4:1 (Rosen et al. 2002). If the W:H ratio is between 3:1 and 4:1, the term “tabular” of Stearn (1982) is used instead (cf. Young and Scrutton 1991). Coralla which do not fit any of the above-mentioned descriptions are labelled as “irregular”. Cyclic growth banding described from the studied material is distinguished from growth interruption surfaces (sensu Miller and West 1997) on the basis of the following criteria: bands should appear in semi-regular intervals in the corallum, should not feature breaks in growth, form zones of dense skeletal elements in longitudinal section (as opposed to lines), and occur independently from sediment intercalations and epibiont infestations. Low- and high-density bands ratio (L/H) was calculated for the specimens displaying cyclic growth banding (Young and Kershaw 2005). Colony integration is described according to Coates and Jackson (1987), as a measure of the connection between the polyps, reflected in the skeletal structures.
Results
Heliolitid specimens collected from the Silurian outcrops of Gotland belong predominantly to the species Stelliporella parvistella (61% of specimens). Other species, in a decreasing order of abundance, include Heliolites interstinctus, H. megastoma, Propora tubulata, H. daintreei, and H. spongodes (see Fig. 2). S. parvistella and H. interstinctus are at least moderately common in all localities, constituting 10% or more of each heliolitid assemblage. Basic measurements and features of the skeletal structure are summarised in Table 1. For the detailed morphometric descriptions of the above-mentioned species from Gotland, see Young & Scrutton (1991, Appendix B). All the Middle Devonian specimens from Belgium, Morocco and Poland were identified as H. porosus. For the detailed description of the species, see Iven (1980).
Colony surfaces of the studied heliolitid species from Gotland (photographs) and their abundance in the respective outcrops (pie charts). Sp—Stelliporella parvistella (A), Pt—Propora tubulata (B), Hi—Heliolites interstinctus (C), Hd—H. daintreei (D), Hm—H. megastoma (E), Hs—H. spongodes (F). N = 266. Scale bars are 2 mm
Tabular and platy coralla are the most abundant among the heliolitids from the studied outcrops in Gotland. They constitute 63% of all collected specimens. Branching and domal colonies are moderately common, whereas irregular and bulbous colonies are rarer (Fig. 3). The studied Devonian Heliolites porosus coralla are all domal in shape.
Stelliporella parvistella specimens are predominantly tabular and platy (61%), but it is also the only species with relatively abundant (21%) branching coralla (Fig. 4). On the other hand, it only rarely exhibits domal (3%) or bulbous (2%) forms, otherwise common in heliolitids. Twenty-seven per cent of S. parvistella coralla exhibit a distinct “rising” mode of growth, when a tabular colony extends vertically in multiple places, forming a kind of tumour-like protrusions, which seem to be early stages of branching growth (Fig. 5D–E).
Growth patterns of studied heliolitid corals. A—a polished longitudinal slab of a domal corallum of Propora tubulata with a cyclic growth pattern (Ireviken; scale bar = 1 cm); B–C—branching coralla; B—dashed outline of a Stelliporella parvistella corallum (Ygne; scale bar = 1 cm); C—a branch of Heliolites interstinctus (Lau Käldu; scale bar = 1 mm); D–E—an irregular, “rising” corallum of S. parvistella (Ygne; scale bars = 1 cm); D—side view; E—top view; F–I—platy coralla; F–G—H. interstinctus (Ireviken, scale bars = 1 cm); F—top view; G—bottom view; H–I—S. parvistella (Lau Käldu, scale bars = 1 cm); H—top view; I—side view
Lower Visby beds in Ireviken
In the field, heliolitid colonies in Ireviken occur either solitarily, embedded in the marly sediment, or in associations with other tabulates, mainly the halysitid corals, forming small biostromes (see Calner et al. 2000; Zapalski and Berkowski 2019). The heliolitids commonly settled on the halysitids; however, the reverse, halysitids growing on heliolitid colonies, also occur. In some cases, halysitids bioimmured in heliolitid coralla can also be found. Associated fauna includes solitary rugose corals, crinoids, bryozoans, gastropods and brachiopods.
Lower Visby beds cropping out at Ireviken differ from the other studied outcrops in Gotland by bearing the relatively fewest Stelliporella parvistella specimens (only 12% of Ireviken specimens) and by a high abundance of Propora tubulata and Heliolites interstinctus coralla (30% and 24% of Ireviken specimens, respectively) (Fig. 2).
Tabular and platy coralla (Fig. 5F–G) constitute nearly two-thirds (64%) of all Ireviken heliolitids. The remaining specimens are mainly domal (28%; Fig. 5A) and bulbous (6%), with rare irregular coralla. No branching heliolitids were found. These data correspond well with the most common growth forms of the most abundant species in Ireviken (P. tubulata and H. interstinctus; see Fig. 3).
Lower and Upper Visby beds in Ygne
The heliolitids and the associated fauna from the Lower Visby beds cropping out in Ygne are largely similar to those observed in Ireviken; however, there are significantly fewer specimens of Propora tubulata and the heliolitid-halysitid associations are also a lot less common. Upper Visby deposits are discriminated by the more lithified, less marly sediment with more abundant crinoids, brachiopods and stromatoporoids. Heliolitids also seem to be more numerous in this formation. Stelliporella parvistella is significantly more common in Upper Visby beds, with numerous branching coralla (Fig. 5B). Corals and stromatoporoids are commonly fragmented and overturned.
The majority (51%) of collected specimens from Ygne are of Stelliporella parvistella, and based on the field observations, vast majority of them can be attributed to the Upper Visby beds. This locality also bears most abundant specimens of Heliolites megastoma (27% of specimens from Ygne), which can be attributed to both the Lower and the Upper Visby beds.
The overall distribution of heliolitid growth forms is most even in Ygne (Fig. 3), likely because of the mixing of Upper and Lower Visby specimens in the debris. The most abundant are the branching coralla (34%) of S. parvistella, which are most likely exclusively from the Upper Visby beds. Domal (18%), bulbous (11%), platy (16%) and tabular (9%) coralla are fairly common in the whole profile.
Eke Formation in Lau Käldu
The Lau Käldu biostrome is mainly composed of platy alveolitid and heliolitid corals and stromatoporoids, accompanied by small solitary rugose corals, crinoid columnals, brachiopods and branching bryozoans. The fossils are embedded in coarse-grained limestone, with coral and stromatoporoid skeletons preserved in situ, in close proximity or superimposed on each other within the biostromal level. In some cases, encrusting organisms can be found on the undersides of the platy heliolitids.
The heliolitid assemblage of Lau Käldu is the least diversified of the three in Gotland presented in this study. Eighty-four per cent of specimens are attributed to Stelliporella parvistella, whereas Heliolites interstictus comprises 14%, and H. dantreei only 2% of all heliolitid specimens from Lau Käldu (Fig. 2).
The vast majority of the heliolitids from this assemblage exhibit platy (59%; Fig. 5H-I) and tabular (22%) growth forms. The remaining specimens are mostly irregular (11%) or branching (7%; Fig. 5C). Irregular coralla in this case are mostly intermediate, “rising” forms, which are platy, but forming irregular bulbs and branches on top of the plate.
Cyclic growth banding
Several heliolitid specimens exhibit apparent banding of the skeleton visible in longitudinal sections (Fig. 6). Those include five specimens of Propora tubulata and nine specimens of Heliolites porosus. The P. tubulata specimens (K2101, K2117, K2120/1, K2120/2, K2125; Fig. 5A, 6A) come from the Lower Visby beds at Ireviken. The H. porosus specimens come from the uppermost Emsian-lowermost Eifelian deposits of Olloy-sur-Viroin (specimen OTB/17, Fig. 6D), and the Eifelian reef of Wancennes (WPEIII/B.5, PRCI/18; Fig. 6C) in Belgium, the Givetian carbonate facies of Dziewki (K1827) and Skały (W1803; Fig. 6B) in Poland, as well as the Eifelian of Ouihlane in the Anti-Atlas of Morocco (K2220, K2224, K2226, K 2227; Fig. 6E,F).
Cyclic growth banding in longitudinal sections; A—a thin section of Propora tubulata, high-density bands in the coenenchyma consist of more, thicker spines (Ireviken; scale bar = 5 mm); B—a thin section of Heliolites porosus (Skały; scale bar = 2 mm); C—a polished slab of H. porosus (Wancennes); D—a thin section of Heliolites porosus, high-density (HD) and low-density (LD) bands are indicated (Olloy-sur-Viroin; scale bar = 2 mm); E–F—polished slabs of Heliolites porosus (Ouihlane; scale bars = 5 mm)
Banding is mostly visible in the coenenchyme, rather than in the corallites (Fig. 6B,D). In case of Heliolites porosus, the visually recognisable high-density bands are characterised by narrower tabulae spacing in the coenenchymal tubules (“siphonopores”). In Propora tubulata, which has a vesicular, dissepimented coenenchyme, the dissepiments are closer together and are smaller in the high-density bands. They are also more abundantly covered by spines. In some cases for both species the tabulae spacing of corallites is also narrower in the high-density bands, but this feature is not consistently present in the studied material. The high and low-density bands are also comprised of darker and lighter skeletal matter, respectively. The light and dark band pairings in P. tubulata are between 1.5 and 5.5 mm thick. In H. porosus, they are 1.5–7.0 mm thick, with the largest specimen (PRCI/18, 19.3 cm-high; Fig. 6C) having 24 band pairings between 3.0 and 7.0 mm, with an average thickness of 4.9 mm. If they were treated as seasonal bands, the rate of growth of studied specimens H. porosus would be 3.4 mm per year on average, while for P. tubulata it would be slower, with an average of 2.9 mm per year. The mean L/H ratio of P. tubulata is 1.7, and for H. porosus it is 2.2.
Discussion
Compound growth and colony integration
It has been observed that in modern corals, highly integrated colonies with small polyps are typical for zooxanthellate corals (Coates and Jackson 1987; Rosen et al. 2002; Barbeitos et al. 2010; Frankowiak et al. 2016). Within the broad range of colony integration levels shown by modern corals, heliolitids with common colony tissue represent relatively high colony integration (Fig. 7), possibly the highest among Paleozoic corals. Although this pattern is not always consistent (Frankowiak et al. 2016), it has been regarded as a good indicator of photosymbiosis (Coates and Oliver 1973; Zapalski 2014; Zapalski et al. 2017b; Swain et al. 2018).
Main taxonomic groups of tabulate corals and their levels of colony integration, as defined in quotations by Coates and Jackson (1987). Drawings (after Hill 1981) represent typical skeletal structures: a auloporid corallum; b syringoporid corallum; c favositid skeleton, transverse section; d alveolitid skeleton, transverse section; e pachyporid branch, longitudinal section; f heliolitid skeleton with a tubular coenenchyme, transverse section; g heliolitid skeleton with a vesicular coenenchyme, transverse section
Aside from heliolitids, the overwhelming majority of Paleozoic corals either formed double-walled cerioid and phaceloid colonies, or displayed solitary habitus (Hill 1981). In contrast, plocoid colonies like the ones formed by heliolitids, can be considered to be relatively highly integrated on the basis of the abundant coenenchyme, implying the presence of interpolypoidal tissue. Additionally, heliolitid coralla commonly have abundant growth interruption surfaces (Król et al. 2018a, 2021), which can be seen as a record of colony-wide response to negative stimuli. Their high abundance could also imply a relatively high level of integration between individual polyps, as the whole colony would react to stimuli that might affect only a part of it. Colony-wide response to stimuli strengthens the colony fitness and resistance to unfavourable conditions (Swain et al. 2018), but contrary interpretations that high integration levels make isolation from damaging factors impossible are also known (Baird and Marshall 2002). Either way, colony-wide response to external stimuli is characteristic for zooxanthellates (Baird and Marshall 2002; Swain et al. 2018). Healed lesions are also commonly known in zooxanthellate corals (Meesters et al. 1996), but to date have not been described from azooxanthellates. Some of the rejuvenations, very common in heliolitids (Król et al. 2018a, 2021), could result from a similar regenerative mechanism.
The colonial mode of growth of heliolitids is apparent and the corallites of the species covered in this study are mostly below 1.75 mm in diameter (Table 1). Only Heliolites megastoma corallites can reach up to 2.6 mm (see Young and Scrutton 1991, Appendix B), which is still relatively small. Only a few other known heliolitid species not included in this study had corallites attaining sizes over 2.5 mm—e.g. Cyrtophyllum elegantum and C. vulgaris (2.8 and 3.0 mm at maximum, respectively; Barskaya 1965), Plasmopora cf. petaliformis (2.7 mm at maximum; Noble and Young 1984), Plasmoporella marginata and P. densa (2.8 and 3.0 mm at maximum, respectively; Dixon and Jell 2012). Modern zooxanthellate corals mostly have corallites below 5 mm in diameter (Coates and Jackson 1987), but larger corallites also commonly occur (Veron 2000). An evolutionary correlation between corallite size and photosymbiosis in sclarctinians has been recently presented by Dimitrijević et al. (2023).
Additionally, heliolitid colonies could attain relatively large sizes (Table 1 herein; Tessitore et al. 2013; Zapalski et al. 2017b; Król et al. 2018a) sporadically reaching even 1 m in diameter. In recent zooxanthellates, plurimetric colonies are very common (Veron 2000; Brandt 2009), whereas azooxanthellates form rather small colonies (Cairns and Kitahara 2012; Zapalski et al. 2017a). Hence, although small colonies are common in both zooxanthellate and azooxenthellate corals, large colonies indicate the likelihood that corals were photosymbiotic.
Growth forms
Studied heliolitid corals represent a moderate spectrum of growth forms. The majority of them are thin platy and tabular, but branching, domal, bulbous and irregular morphologies also commonly occur. There are intermediate forms between them as well, like the “rising” coralla, which seem to mark a shift from platy to branching growth (Fig. 5D–E). The latter in particular bear resemblance to modern Porites rus and P. sillimaniani, in which similar mode of growth has been associated with changing light levels (Muko et al. 2000; Veron 2000). Similar growth forms in tabulate coral Roseoporella sp. have been mentioned by Majchrzyk et al. (2022; see also Majchrzyk et al. 2023) from the Givetian reef of Aferdou el Mrakib in Morocco, with a similar interpretation related to photosymbiosis. This was based on the observation that modern zooxanthellate corals commonly exhibit remarkable skeletal plasticity, when it comes to adapting to different levels of light availability (Fricke and Meischner 1985; Todd 2008; Lesser et al. 2009; Ow & Todd 2010).
The aspect of skeletal plasticity is particularly apparent in the case of assemblages of platy colonies, which are typical for mesophotic coral ecosystems (MCEs), where corals adapt to the conditions of restricted light availability by increasing the surface of light harvesting (e.g. Fricke and Meischner 1985; Rosen et al. 2002; Kahng et al. 2012). Such a growth form is exclusively an adaptation to depleted light conditions and does not reflect bathymetry, but the optical quality of water. Therefore, such forms can occur either in deeper water habitats (“blue” MCEs) or in shallow, but turbid environments, sometimes referred to as “brown” MCEs (Renema 2019; Zapalski et al. 2021). Since the thin platy morphologies are otherwise rather disadvantageous for corals, due to susceptibility to sediment fouling and mechanical damage, fossil assemblages of platy corals are commonly interpreted as indicative of MCEs (Rosen et al. 2002; Morsilli et al. 2012; Novak et al. 2013; Santodomingo et al. 2016; Zapalski et al. 2017a, b; Król et al. 2018b; Zapalski and Berkowski 2019; Majchrzyk et al. 2022, 2023). Tabular colonies are here functionally interpreted as platy colonies that most likely grew during periods of increased sedimentation rate, resulting in the need to grow more vertically upwards to stay above the sediment (cf. Philcox 1971).
The Lower Visby beds were interpreted by Zapalski and Berkowski (2019) as a deep water MCE. Nearly two-thirds of heliolitids collected from the Lower Visby beds at Ireviken exhibit platy and tabular growth forms. Heliolitids collected at Ygne consist of relatively the fewest (although still abundant, 25%) platy and tabular specimens, but they come from a mixture of deeper Lower Visby and shallower Upper Visby deposits rubble. Lau Käldu, belonging to the shallow-water Eke Formation (Calner et al. 2004), could be interpreted as a case of shallow, but turbid “brown” MCE (Renema 2019; Rosen et al. 2002). It is a small biostrome composed of densely packed platy tabulates and stromatoporoids, embedded in a coarse-grained limestone, where over 80% of heliolitids are either platy or tabular.
The platy growth form can also be advantageous for capturing space and an adaptation for more efficient heterotrophic feeding or to soft substrates, as the so-called “snow shoe” survival strategy (Gibson and Broadhead 1989; Yound and Scrutton 1991; Scrutton 1997, 1998; Insalaco 1996). However, these seem to be unlikely reasons for such high abundance of platy heliolitids in the studied outcrops. Firstly, heliolitids occurring abundantly in Middle Devonian reefal assemblages characterised by intense competition for space did not develop platy morphologies, but instead relied on competitive overgrowth (Jakubowicz et al. 2019; Król et al. 2018a, 2021). Secondly, heliolitids commonly settled on the skeletons of other organisms, when the soft substrate was impeding their growth (Król et al. 2018a, 2021). That strategy has not been observed as a common occurrence in the studied specimens, while platy growth was. Additionally, heliolitid assemblages of Aferdou el Mrakib (Givetian, Morocco) and Wellin (Eifelian, Belgium) described by Król et al. (2018a, 2021), which developed on soft sediment, very rarely exhibited platy growth forms. The majority of the specimens studied in this paper belong to Stelliporella parvistella, which had an exceptional capability for skeletal plasticity among heliolitids (Young and Scrutton 1991). In Lower Visby beds and in Lau Käldu it is predominantly platy and tabular. Meanwhile, S. parvistella from another outcrop in Gotland in Blåhäll, where soft substrate was prevalent, developed mostly branching growth forms, as an adaptation to high sedimentation rate instead (Young and Scrutton 1991; Calner et al. 2000). Similar to heliolitids from the Middle Devonian of Aferdou el Mrakib and Wellin, this species did not need to rely on the “snow shoe” strategy to survive on soft-sedimented seafloor. Zapalski and Berkowski (2019) also addressed the soft substrate adaptation possibility of platy tabulates from Lower Visby beds and ruled it out.
Seasonal banding
As mentioned above, heliolitids tend to record remarkably abundant growth interruption surfaces. However, generally in corals when they appear throughout the corallum in a longitudinal section, it can be challenging to differentiate between growth interruptions and cyclic growth banding. Growth interruptions record an unfavourable event and can be distinguished either by their more local occurrence in the corallum or by the direct record of such an event in the fossil record. They commonly consist of sediment intercalations and encrusters and are commonly followed by rejuvenations. Growth interruption surfaces usually occur irregularly in the corallum (Miller and West 1997; Król et al. 2018a, 2021). It is, however, important to note that they can be partly erased by diagenesis. Cyclic skeletal banding on the other hand should be more regular and should occur without signs of polyp mortality, sediment influxes, or infestations. It can be distinguished when forming zones (rather than surfaces) of darker and lighter skeleton, usually with cyclic condensation of skeletal elements and/or alteration of thick vs. thin skeletal elements (Berkowski and Belka 2008). It is commonly treated as a potential indicator of photosymbiosis (Scrutton and Powell 1980; Coates and Jackson 1987; Young and Kershaw 2005; Stanley 2006; Stanley and Helmle 2010; Stanley and Lipps 2011; Zapalski 2014).
Apparent regular banding visible in the sections of a few of the specimens studied here is considered as true cyclic growth banding, as it does not consist of encrusters, only sporadically includes small amounts of sediment, and is not followed by rejuvenations (Fig. 6). Banding seems to appear with relative regularity, and the skeletal elements of the coenenchyme are visibly condensed in regular intervals. The darker and lighter colour of the skeleton in the bands is also preserved in some cases. Therefore, they are here interpreted as cyclic banding, possibly related to seasonal changes in environmental conditions. Young and Kershaw (2005) also reported density banding in Propora tubulata and Heliolites interstinctus from Visby beds. They also pointed out that aside from light intensity, regular changes in parameters such as salinity and temperature can also result in density banding, and that density banding can be both accentuated and de-accentuated by diagenetic processes. The studied specimens do not seem to show any signs of diagenesis which could make the banding appear postmortem. It could be possible that the rarity of skeletal banding in heliolitids might be related to diagenesis, however, as they are commonly strongly recrystallised. If the observed band couplings were to be interpreted as yearly growth increments, it would suggest relatively slow growth rates. However, the average L/H ratios of 1.7 (P. tubulata) and 2.2 (H. porosus) seem to be comparable with the typical values displayed by modern photosymbiotic taxa (0.9–4.6 range; Young and Kershaw 2005 and the refrences therein). Nevertheless, as banding occurred in a very small percentage of the specimens in this study, it can only be treated as a secondary argument for the possibility of heliolitid photosymbiosis, and not a crucial one.
Bathymetry
Tabulate corals in general are very common in all sorts of Paleozoic benthic communities. Widespread groups like favositids and alveolitids, which according to some studies could also be photosymbiotic, occurred in both shallow and deep water settings including cryptic environments and vicinities of hydrothermal vents (Brachert et al. 1992; Jakubowicz et al. 2014; Król et al. 2016, 2018a, c; Berkowski et al. 2019; Zapalski and Berkowski 2019). The same, however, does not hold true for heliolitid corals.
Heliolitids preferred to live in relatively shallow environments, at least in comparison with other Paleozoic corals (e.g. Król 2018a, 2021; Zapalski et al. 2021). Tabulates did not usually form wave-resistant reef frameworks, developing in very shallow, high-energy environments (Lecompte 1958; Philcox 1971; Embry and Klovan 1972; Scrutton 1998, 1999). The extremely shallow depths, where scleractinians thrive on modern reefs, were at that time occupied by stromatoporoid sclerosponges instead, by some considered as possibly photosymbiotic (Coates and Jackson 1987; Machel and Hunter 1994), but arguments in either way are inconclusive (Kershaw et al. 2018). Unlike other tabulates, heliolitids show a preference for a fore reef, equivalent of annularis reef zones sensu Geister (1977, 1980)—where light availability was still most likely favourable for algal symbionts, but there was less hydrodynamic stress. A good example of such an environment is the Aferdou el Mrakib reef in Morocco, where heliolitids were rare and more platy in the deeper water setting during earlier stages of reef formation, but became extremely abundant (and more domal in shape) in shallower water in later stages of reef development (Król et al. 2018a; Jakubowicz et al. 2019; Majchrzyk et al. 2022).
In the studied outcrops, heliolitids show the most diversity in Upper Visby deposits, which were relatively shallow with a moderate hydrodynamic setting (Calner 2004). In the deposits of Lower Visby beds, the platy growth forms dominate, and heliolitids are also common, but not as abundant as halysitids, for example (Stel 1978; Zapalski and Berkowski 2019). Lau Käldu biostrome, comprised predominantly of densely packed stromatoporoids and platy heliolitids embedded in a coarse-grained crinoidal sediment, most likely developed in a shallow and turbid environment, as mentioned above. This seems to support the hypothesis of shallow-water preference of heliolitids, which in turn would be favourable for algal symbionts.
Conclusions
As presented in this paper and by other authors, heliolitid corals could form relatively large colonies, comprised of small polyps (mostly under 2.5 mm in diameter). On the basis of the plocoid structure of their colonies, as well as the abundance of colony-wide responses to negative stimuli recorded in growth interruption surfaces, heliolitids can be considered to be highly integrated. This seems to be true particularly in comparison with other Paleozoic corals, which were predominantly cerioid double-walled, phaceloid, or solitary.
Some heliolitids exhibit cyclic skeletal banding, which seems to be very similar to seasonal banding in modern photosymbiotic corals. It is visible only in a few of the studied specimens, but that might be due to diagenesis. The banding is represented by darker and lighter colour of skeleton in individual bands, which can be removed by diagenetic processes that coral fossils are susceptible to.
In the three studied outcrops in Gotland, three geological formations occur: the Lower Visby beds (in Ireviken and in Ygne), the Upper Visby beds (in Ygne), and the Eke beds (in Lau Käldu). Coral assemblages of Lower Visby beds and Eke beds are here interpreted as mesophotic coral ecosystems. Specimens coming from Ireviken and Lau Käldu are in overwhelming majority platy and tabular. Lower percentages of such coralla from Ygne might result from the mixing with fossils from the Upper Visby beds, deposited in a shallower, more high-energy environment. Heliolitids occurring in assemblages recognised as soft-bottom communities did not develop flat, platy morphologies, making this type of growth form most likely a response to low-light availability either due to depth or turbidity.
Unlike other groups of tabulates like favositids and alveolitids, which are also considered as possibly photosymbiotic by some other researchers, heliolitids do not seem to occur in deep water facies and cryptic environments. They typically preferred relatively shallow environments instead, where light could more easily penetrate the water column.
Overall, different individual characteristics described above could be attributed to causes other than photosymbiosis. However, the possibility of all these features typical for zooxanthellate corals, being exhibited by heliolitids by sheer coincidence, seems extremely doubtful. Therefore, we can conclude that at the very least some species of heliolitid corals, like Stelliporella parvistella, were most likely photosymbiotic.
References
Baird AH, Marshall PA (2002) Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser 237:133–141
Barbeitos M, Romano S, Lasker H (2010) Repeated loss of coloniality and symbiosis in scleractinian corals. Proc Natl Acad Sci USA 107(26):11877–11882
Barskaya VF (1965) Ordovician and Silurian corals from Gornyy Altay region. Int Geol Rev 7(5):898–909
Berkowski B, Belka Z (2008) Seasonal growth bands in Famennian rugose coral Scruttonia kunthi and their environmental significance. Palaeogeogr Palaeoclimatol Palaeoecol 265:87–92
Berkowski B, Jakubowicz M, Belka Z, Król JJ, Zapalski MK (2019) Recurring cryptic ecosystems in Lower to Middle Devonian carbonate mounds of Hamar Laghdad (Anti-Atlas, Morocco). Palaeogeogr Palaeoclimatol Palaeoecol 523:1–17. https://doi.org/10.1016/j.palaeo.2019.03.011
Berkowski B, Król JJ, Jakubowicz M, Zapalski MK (2023) Early life strategies and juvenile mortality in Favosites (Anthozoa, Tabulata) from the Middle Devonian of the Mader Basin (Anti-Atlas, Morocco). Palaeogeogr Palaeoclimatol Palaeoecol 625:111684. https://doi.org/10.1016/j.palaeo.2023.111684
Bertrand M, Coen-Aubert M, Dumoulin V, Préat A, Tourneur FJ (1993) Sédimentologie et paléoécologie de l’Emsien supérieur et de l’Eifelien inférieur des régions de Couvin et de Villers-la-Tour (bord sud du Synclinorium de Dinant, Belgique). Neues Jahrb Geol Paläontol 188(2):177–211
Brachert TC, Buggisch W, Flügel E, Hüssner HM, Joachimski MM, Tourneur FJ, Walliser OH (1992) Controls of mud mound formation: the Early Devonian Kess-Kess carbonates of the Hamar Laghdad, Antiatlas. Morocco Geologische Rundschau 81(1):15–44
Brandt ME (2009) The effect of species and colony size on the bleaching response of reef-building corals in the Florida Keys during the 2005 mass bleaching event. Coral Reefs 28:911–924. https://doi.org/10.1007/s00338-009-0548-y
Bridge TCL, Baird AH, Pandolfi JM, McWilliam MJ, Zapalski MK (2022) Functional consequences of Palaeozoic reef collapse. Sci Rep 12:1–11. https://doi.org/10.1038/s41598-022-05154-6
Cairns SD, Kitahara MV (2012) An illustrated key to the genera and subgenera of the Recent azooxanthellate Scleractinia (Cnidaria, Anthozoa), with an attached glossary. Zookeys 227:1–47. https://doi.org/10.3897/zookeys.227.3612
Calner M, Sandström O, Mötus M-A (2000) Significance of a Halysitid-Heliolitid Mud-facies Autobiostrome from the Middle Silurian of Gotland, Sweden. Palaios 15:511–523
Calner M, Jeppsson L, Munnecke A (2004) The Silurian of Gotland - Part I: Review of the stratigraphic framework, event stratigraphy, and stable carbon and oxygen isotope development. Erlanger Geol Abh Sonderband 5:113–131
Coates AG and Oliver WA Jr (1973) Coloniality in zoantharian corals. In: Boardman RS, Cheetham AH, Oliver WA Jr (eds) Animal colonies. Development through function and time. Pp 3–27
Coates AG, Jackson JBC (1987) Clonal growth, algal symbiosis, and reef formation by corals. Paleobiology 13(4):363–378
Copper P (1985) Fossilized polyps in 430-Myr-old Favosites corals. Nature 316:142–144
Copper P (2002) Reef development at the Frasnian/Famennian mass extinction boundary. Palaeogeogr Palaeoclimatol Palaeoecol 181:27–65
Denayer J (2023) From mud to limestone: birth and growth of a giant reef in the Eifelian (Middle Devonian) of S Belgium. Palaeogeogr Palaeoclimatol Palaeoecol (in press)
Dimitrijević D, Raja NB, Kiessling W (2023). Corallite sizes of reef corals: decoupling of evolutionary and ecological trends. Paleobiology 1–11. https://doi.org/10.1017/pab.2023.28
Dixon OA (2010) Fossilized polyp remains in Silurian Heliolites (Anthozoa, Tabulata) from Nunavut, Arctic Canada. Lethaia 43:60–72. https://doi.org/10.1111/j.1502-3931.2009.00173.x
Dixon OA, Jell JS (2012) Heliolitine tabulate corals from Late Ordovician and possibly early Silurian allochthonous limestones in the Broken River Province, Queensland, Australia. Alcheringa 36:69–98. https://doi.org/10.1080/03115518.2011.582807
Embry AF, Klovan JE (1972) Absolute Water Depth Limits of Late Devonian Paleoecological Zones. Geol Rundsch 61(2):672–686
Frankowiak K, Kret S, Mazur M, Meibom A, Kitahara MV, Stolarski J (2016) Fine-Scale Skeletal Banding Can Distinguish Symbiotic from Asymbiotic Species among Modern and Fossil Scleractinian Corals. PLoS ONE 11(1):e0147066. https://doi.org/10.1371/journal.pone.0147066
Fricke H, Meischner D (1985) Depth limits of Bermudan scleractinian corals: a submersible survey. Mar Biol 88:175–187
Geister J (1980) Calm-water reefs and rough-water reefs of the Caribbean Pleistocene. Acta Palaeontol Pol 25:541–556
Geister J (1977) The influence of wave exposure on the ecological zonation of Caribbean coral reefs. In: Taylor DL (ed) Proceedings of Third International Coral Reef Symposium Vol. 2: Geology. Rosenstiel School of Marine and Atmospheric Science, Miami, Florida, pp. 23–29.
Gibson MA, Broadhead TW (1989) Species-specific growth responses of favositid corals to soft-bottom substrates. Lethaia 22:287–299
Goldfuss A (1826) Petrefacta Germaniae. Anrz & Co., Dusseldorf
Hill D (1981) Part F - Coelenterata—Suplement 1—Rugosa and Tabulata. In: Teichert C, Ashlock V, Keim JD, McCormick L, Williams RB (eds) Treatise on Invertebrate Paleontology. The Geological Society of America, Boulder and the University of Kansas, Lawrence, pp F380–F762
Insalaco E (1996) Upper Jurassic microsolenid biostromes of northern and central Europe: facies and depositional environment. Palaeogeogr Palaeoclimatol Palaeoecol 121:169–194
Iven C (1980) Alveolitiden und Heliolitiden aus dem Mittel- und Oberdevon des Bergischen Landes (Rheinisches Schiefer Gebirge). Palaeontogr Abt A 167:121–179
Jakubowicz M, Berkowski B, Belka Z (2014) Cryptic coral-crinoid “hanging gardens” from the Middle Devonian of southern Morocco. Geology 42(2):119–122. https://doi.org/10.1130/G35217.1
Jakubowicz M, Berkowski B, López Correa M, Jarochowska E, Joachimski MM, Belka Z (2015) Stable Isotope Signatures of Middle Palaeozoic Ahermatypic Rugose Corals—Deciphering Secondary Alteration, Vital Fractionation Effects, and Palaeoecological Implications. PLoS ONE 10(9):e0140199. https://doi.org/10.1371/journal.pone.0136289
Jakubowicz M, Król JJ, Zapalski MK, Wrzołek T, Wolniewicz P, Berkowski B (2019) At the southern limits of the Devonian reef zone: Palaeoecology of the Aferdou el Mrakib reef (Givetian, eastern Anti-Atlas, Morocco). Geol J 54(1):10–38. https://doi.org/10.1002/gj.3152
Kahng SE, Hochberg EJ, Apprill A, Wagner D, Luck DG, Perez D, Bidigare RR (2012) Efficient light harvesting in deep-water zooxanthellate corals. Mer Ecol Prog Ser 455:65–77. https://doi.org/10.3354/meps09657
Kaufmann B (1998) Facies, stratigraphy and diagenesis of Middle Devonian reef and mud-mounds in the Mader (eastern Anti-Atlas, Morocco). Acta Geol Pol 48:43–106
Kershaw S, Munnecke A, Jarochowska E (2018) Understanding Palaeozoic Stromatoporoid Growth Earth-Sci Rev 187:53–76. https://doi.org/10.1016/j.earscirev.2018.08.003
Król JJ, Zapalski MK, Jakubowicz M, Berkowski B (2016) Growth strategies of the tabulate coral Favosites bohemicus on unstable, soft substrates: An example from the Hamar Laghdad (Lower Devonian, Anti-Atlas, Morocco). Palaeogeogr Palaeoclimatol Palaeoecol 449:531–540. https://doi.org/10.1016/j.palaeo.2016.02.047
Król JJ, Jakubowicz M, Zapalski MK, Berkowski B (2018a) Massive tabulates in competition for space: A case study from Aferdou el Mrakib (Middle Devonian, Anti-Atlas, Morocco). Palaeogeogr Palaeoclimatol Palaeoecol 497:105–116. https://doi.org/10.1016/j.palaeo.2018.02.009
Król JJ, Kołodziej B, Bucur II (2018b) Coral reefs near the Eocene-Oligocene boundary in the northern Transylvanian Basin, Romania: Composition and paleoenvironmental interpretation. Geol J 53(2):565–579. https://doi.org/10.1002/gj.2913
Król JJ, Zapalski MK, Berkowski B (2018c) Emsian tabulate corals of Hamar Laghdad (Morocco): taxonomy and ecological interpretation. Neues Jahrb Geol Paläontol 290(1–3):75–102. https://doi.org/10.1127/njgpa/2018/0773
Król JJ, Denayer J, Wolniewicz P, Zapalski MK (2021) Heliolitid corals and their competitors: a case study from the Wellin patch reefs, Middle Devonian. Belgium Lethaia 54(4):540–557. https://doi.org/10.1111/let.12421
Lecompte M (1958) Les Recifs Paleozoiques en Belgique. Geol Rundsch 47(1):384–401
Lesser MP, Slattery M, Leichter JJ (2009) Ecology of mesophotic coral reefs. J Exp Mar Biol Ecol 375:1–8. https://doi.org/10.1016/j.jembe.2009.05.009
Lindström G (1899) Remarks on the Heliolitidae. Kungliga Svenska Vetenskapsakademiens Handlingar 32(1):1–140
Machel HG, Hunter IG (1994) Facies models for middle to late devonian Shallow-Marine carbonates, with comparisons to modern reefs: a guide for facies analysis. Facies 30:155–176. https://doi.org/10.1007/BF02536895
Majchrzyk A, Jakubowicz M, Berkowski B, Bongaerts P, Zapalski MK (2022) In the shadow of a giant reef: Palaeoecology of mesophotic coral communities from the Givetian of Anti-Atlas (Morocco). Palaeogeogr Palaeoclimatol Palaeoecol 602(1):111177. https://doi.org/10.1016/j.palaeo.2022.111177
Majchrzyk A, Jakubowicz M, Bongaerts P, Zapalski MK (2023) Different times, similar mechanism? Convergent patterns in light-induced phenotypic plasticity in Devonian and modern corals. Coral Reefs 42:893–903. https://doi.org/10.1007/s00338-023-02394-4
Meesters EH, Wesseling I, Bak RPM (1996) Partial mortality in three species of reef-building corals and the relation with colony morphology. Bull Mar Sci 58(3):838–852
Miller KB, West RR (1997) Growth-interruption surfaces within chaetetid skeletons: records of physical disturbance and depositional dynamics. Lethaia 29:289–299
Morsilli M, Bosellini FR, Pomar L, Hallock P, Aurell M, Papazzoni CA (2012) Mesophotic coral buildups in a prodelta setting (Late Eocene, southern Pyrenees, Spain): a mixed carbonate–siliciclasticsystem. Sedimentology 59:766–794. https://doi.org/10.1111/j.1365-3091.2011.01275.x
Muko S, Kawasaki K, Sakai K, Takasu F, Shigesada N (2000) Morphological plasticity in the coral Porites sillimaniani and its adaptive significance. Bull Mar Sci 66:225–239
Munnecke A, Samtleben C (1996) The formation of micritic limestones and the development of limestone-marl alternations in the Silurian of Gotland, Sweden. Facies 34:159–176
Munnecke A, Samtleben C, Bickert T (2003) The Ireviken Event in the lower Silurian of Gotland, Sweden—relation to similar Palaeozoic and Proterozoic events. Palaeogeogr Palaeoclimatol Palaeoecol 195(1–2):99–124. https://doi.org/10.1016/S0031-0182(03)00304-3
Noble JPA, Lee D-J (1990) Ontogenies and astogenies and their significance in some favositid and heliolitid corals. J Paleontol 64:515–523
Noble JPA, Young GA (1984) The Llandovery-Wenlock heliolitid corals from New Brunswick, Canada. J Paleontol 58:867–884
Novak V, Santodomingo N, Rosler A, Di Martino E, Braga JC, Taylor PD, Johnson KG, Renema W (2013) Environmental reconstruction of a late Burdigalian (Miocene) patch reef in deltaic deposits (East Kalimantan, Indonesia). Palaeogeogr Palaeoclimatol Palaeoecol 374:110–122. https://doi.org/10.1016/j.palaeo.2013.01.009
Ow YX, Todd PA (2010) Light-induced morphological plasticity in the scleractinian coral Goniastrea pectinata and its functional significance. Coral Reefs 29(3):797–808. https://doi.org/10.1007/s00338-010-0631-4
Philcox ME (1971) Growth forms and role of colonial coelenterates in reefs of the Gower Formation (Silurian), Iowa. J Paleontol 45:338–346
Racki G, Wrzołek T, Słupik A, Nowak B (1993) Nowe dane o dewonie antykliny Siewierza na podstawie wiercenia WB-12. Prace Naukowe Uniwersytetu Śląskiego Geologia 12–13:110–125
Racki G, Wójcik K, Halamski AT, Narkiewicz M (2022) Middle Devonian Skały Formation in the Holy Cross Mountains (Poland)—formal description and subdivision based on new field data. Ann Soc Geol Pol 92(4):425–444. https://doi.org/10.14241/asgp.2022.18
Renema W (2019) Large benthic foraminifera in low-light environments. In: Loya Y, Puglise K, Bridge T (eds) Mesophotic Coral Ecosystems 12. Springer, Cham, pp 553–561
Riding R (1979) Calcareous algae. In: Jaanusson V, Laufeld S, Skoglund R (eds) Lower Wenlock faunal and floral dynamics—Vattenfallet section, Gotland. Sveriges Geologiska Undersökning, Uppsala pp 54–60
Rominger CL (1876) Paleontology. Fossil corals (Vol. 3). Geol Surv Michigan 3:1–161
Rosen BR, Aillud GS, Bosellini FR, Clack NJ, Insalaco E, Valldeperas FX, Wilson MEJ (2002) Platy coral assemblages: 200 million years of functional stability in response to the limiting effects of light and turbidity. In: Moosa MK, Soemodihardjo S Soegiarto, Rominmohtarto AK, Nontji A, Soekarno S, Suharsono L (eds) Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia. Ministry of Environment, Indonesian Institute of Sciences and International Society for Reef Studies pp. 255–264
Santodomingo N, Renema W, Johnson KG (2016) Understanding the murky history of the Coral Triangle: Miocene corals and reef habitats in East Kalimantan (Indonesia). Coral Reefs 35(3):765–781. https://doi.org/10.1007/s00338-016-1427-y
Schröder S, Kazmierczak M (1999) The Middle Devonian, coral reef’’ of Ouihlane (Morocco) - New data on the geology and rugose coral fauna. Geol Palaeontol 33:93–115
Scrutton CT (1997) The Palaeozoic corals, I: origins and relationships. Proc Yorkshire Geol Soc 51:177–208
Scrutton CT (1998) The Palaeozoic corals, II: structure, variation and palaeoecology. Proc Yorkshire Geol Soc 52:1–57
Scrutton CT (1999) Palaeozoic corals: their evolution and palaeoecology. Geol Today 15:184–193
Scrutton CT, Powell JH (1980) Periodic development of dimetrism in some favositid corals. Acta Palaeontol Pol 25:477–491
Śliwiński S (1964) Geologia obszaru siewierskiego. Wydawnictwa Geologiczne, Warsaw, 74 pp.
Stanley GD Jr (2006) Photosymbiosis and the evolution of modern coral reefs. Science 312(5775):857–858
Stanley GD Jr, Helmle KP (2010) Middle Triassic Coral Growth Bands and Their Implication for Photosymbiosis. Palaios 25:754–763. https://doi.org/10.2110/palo.2010.p10-039r
Stanley GD Jr, Lipps JH (2011) Photosymbiosis: The driving force for reef success and failure. Paleontological Society Papers 17:33–60
Stearn CW (1982) The shapes of Paleozoic and modern reefbuilders: a critical review. Paleobiology 8:228–241
Stel JH (1978) Environment and quantitative morphology of some Silurian tabulates from Gotland. Scripta Geol 47:1–75
Swain TD, Bold EC, Osborn PC, Baird AH, Westneat MW, Backman V, Marcelino LA (2018) Physiological integration of coral colonies is correlated with bleaching resistance. Mar Ecol Prog Ser 586:1–10. https://doi.org/10.3354/meps12445
Tessitore L, Schemm-Gregory M, Korn D, Wild FRWP, Naglik C, Klug C (2013) Taphonomy and palaeoecology of the green Devonian gypidulid brachiopods from the Aferdou El Mrakib, eastern Anti-Atlas, Morocco. Swiss J Paleontol 132:23–44. https://doi.org/10.1007/s13358-012-0050-y
Todd PA (2008) Morphological plasticity in scleractinian corals. Biol Rev 83:315–337
Urbanek A (1993) Biotic crises in the history of Upper Silurian graptoloids: A Palaeobiological model. Hist Biol 7:29–50. https://doi.org/10.1080/10292389309380442
Veron JEN (2000) Corals of the World, vol 3. Australian Institute of Marine Science pp. 490
Wood R (1998) The ecological evolution of reefs. Annu Rev Ecol Evol Syst 29:179–206
Young GA, Kershaw S (2005) Classification and controls of internal banding in Palaeozoic stromatoporoids and colonial corals. Palaeontology 48:623–651
Young GA, Scrutton CT (1991) Growth form in Silurian heliolitid corals: the influence of genetics and environment. Paleobiology 17:369–387
Zapalski MK (2014) Evidence of photosymbiosis in Palaeozoic tabulate corals. Proc R Soc Lond B 281:20132663
Zapalski MK, Berkowski B (2019) The Silurian mesophotic coral ecosystems: 430 million years of photosymbiosis. Coral Reefs 38:137–147
Zapalski MK, Nowicki J, Jakubowicz M, Berkowski B (2017a) Tabulate corals across the Frasnian/Famennian boundary: architectural turnover and its possible relation to ancient photosymbiosis. Palaeogeogr Palaeoclimatol Palaeoecol 487:416–429
Zapalski MK, Wrzołek T, Skompski S, Berkowski B (2017b) Deep in shadows, deep in time: the oldest mesophotic coral ecosystems from the Devonian of the Holy Cross Mountains (Poland). Coral Reefs 36:847–860
Zapalski MK, Baird AH, Bridge T, Jakubowicz M, Daniell J (2021) Unusual shallow water Devonian coral community from Queensland and its recent analogues from the inshore Great Barrier Reef. Coral Reefs 40:417–431
Acknowledgements
Prof. Stanisław Skompski and Prof. Piotr Łuczyński (University of Warsaw) are thanked for their help during field work. Michał Jankowiak (Adam Mickiewicz University) is acknowledged for the preparation of thin sections. Dr Stephen Kershaw (Brunel University London) and Dr Robert J. Elias (University of Manitoba) are thanked for their detailed reviews which helped significantly improve the final manuscript. This paper is a contribution to the National Science Centre of Poland research project no. 2017/27/N/ST10/01776 (to JJK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Król, J.J., Berkowski, B., Denayer, J. et al. Deducing photosymbiosis in extinct heliolitid corals. Coral Reefs 43, 91–105 (2024). https://doi.org/10.1007/s00338-023-02450-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02450-z