Abstract
Modern scleractinian corals can display high phenotypic plasticity, which reflects an interplay among various environmental controls, such as sediment input, water hydrodynamics or light intensity. In particular, the latter can strongly influence the morphology of coral species living across broad depth gradients. Light intensity was also a factor shaping the colonies of extinct Palaeozoic tabulate corals (Anthozoa: Tabulata). Based on gradual transitions in morphology observed in corals from the Middle Devonian (Givetian stage, ~ 385 Ma) mesophotic coral ecosystems (MCE) of the Aferdou el Mrakib reef (Anti-Atlas mountains, Morocco) and comparative material originating from different palaeobathymetric and biogeographical settings, we show that Devonian tabulate corals, such as Roseoporella and Alveolites, were characterised by high phenotypic plasticity and the ability to dramatically change their morphology depending on the inferred light conditions. Such a mechanism is similar to that observed in modern scleractinians, e.g. Porites sillimaniana. This recurring morphological theme suggests that Palaeozoic tabulate corals shared many functional characteristics of modern scleractinians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life has an impressive potential to adjust to environmental conditions. One of the key adaptations that enable organisms to cope with environmental variations is phenotypic plasticity. This term refers to the phenomenon that one genotype can be expressed as diversified phenotypes depending on the environmental factors influencing an organism during its ontogeny (e.g. DeWitt et al. 1998; Pigliucci 2005). It can be expressed by changes in morphological (sometimes described as morphological plasticity), physiological or behavioural characteristics (DeWitt et al. 1998; Pigliucci 2005; Fusco and Minelli 2010). Phenotypic plasticity is of major evolutionary importance and occurs across a broad spectrum of clades (Stearns 1989; Pigliucci 2005; Fusco and Minelli 2010), including the scleractinian corals (Bruno and Edmunds 1997; Shaish et al. 2007; Todd 2008; Barshis et al. 2010).
In scleractinian corals, phenotypic plasticity has been widely studied in terms of the impact of sediment input (Segal and Castro 2011), water movement (Todd 2008; Padilla-Gamiño et al. 2012), water chemistry (Tambutte et al. 2015) and light intensity (e.g. Graus and Macintyre 1976; Kühlmann 1983; Muko et al. 2000; Kaniewska et al. 2009; Kahng et al. 2019) and may be crucial to understand the potential adaptations of corals to climate change (e.g. Todd 2008; Torda et al. 2017; Fox et al. 2019; Voolstra et al. 2021). In particular, light availability can have a profound effect on the morphology and physiology of zooxanthellate (i.e. photosymbiotic) corals (e.g. Muko et al. 2000; Hoogenboom et al. 2008; Kahng et al. 2019). It is especially visible in mesophotic coral ecosystems (MCEs), where flattening of the coral morphologies becomes a vital adaptation for efficient light harvesting (e.g. Lesser et al. 2009; Kahng et al. 2012, 2019). Particularly vivid examples of light-connected phenotypic plasticity are members of the genus Porites (Muko et al. 2000; Padilla-Gamiño et al. 2012). For example, under light-depleted conditions, Porites sillimaniana produces a platy morphology, but with increasing light intensity it develops a branching growth form. Intermediate stages can display a platy colony base with some finger-like outgrowths growing perpendicular to the rest of the corallum (Muko et al. 2000).
Phenotypic plasticity is broadly studied in modern ecosystems, including coral reefs, and a number of attempts were also made regarding the fossil record of this phenomenon. Despite this concentrated research effort, unequivocal fossil examples of phenotypic plasticity remain rare, largely due to taphonomic biases or difficulties in distinguishing phenotypic plasticity from intraspecific variations or evolutionary innovations (see Lister 2021 and references therein). Because of the nature of the fossil record, the morphological traits of phenotypic plasticity are the easiest to recognise. It is no surprise then that the investigations are focussed, especially on colonial animals, which generally display high morphological plasticity and, due to the significant number of genetically identical individuals in the colony, the environmental control on their morphology can be relatively easily recognised (Budd et al. 1994; Hageman 1995; Lister 2021). The Palaeozoic colonial reef builders seem to be perfect candidates for such studies.
The Palaeozoic acme of the reef ecosystems falls within the Devonian (~ 419–359 Ma)—the time of the most extensive reef development in the Earth’s history, with the peak of the reef growth reached during the Givetian to early Frasnian (~ 388–378 Ma) and reefs present as far as ~ 50◦S (Fagerstrom 1994; Copper and Scotese 2003; Kiessling 2009; Jakubowicz et al. 2019). This exceptional development was facilitated by a number of favourable, co-occurring factors, including the warm climate (Copper 1994, 2002; Copper and Scotese 2003; Bridge et al. 2022; Scotese et al. 2021), high global sea level and associated expansion of broad shelf seas (Haq and Schutter 2008), favourable continent arrangement (Jakubowicz et al. 2019), as well as ecology of tabulate corals and their symbionts (Coates and Jackson 1987, see also discussion in Majchrzyk et al. 2022). The main role of massive, calcifying organisms, most notably stromatoporoids and tabulate corals, in building the middle Palaeozoic reefs had a substantial impact on the preservation of these structures in the fossil record (Wood 2001). For the tabulates, of significant importance for their success in the mid-Palaeozoic reefs was probably their cooperation with photosymbionts (e.g. Talent 1988; Zapalski 2014; Zapalski et al. 2017a, b; Zapalski and Berkowski 2019), in which they resembled the modern scleractinian corals.
Phenotypic plasticity of the Palaeozoic tabulate corals is still poorly understood. Previous studies showed that tabulates were able to develop a suite of ecological and morphological adaptations to high sediment input (Król et al. 2018), water movement (Scrutton 1998 and references therein; Zapalski et al. 2022) and light deficiency (e.g. Zapalski et al. 2017a, 2021). Like modern scleractinians, tabulate corals formed various types of communities depending on the light availability, including the construction of MCEs (Zapalski et al. 2017a; Zapalski and Berkowski 2019; Majchrzyk et al. 2022; Zatoń et al. 2022). The coenitid and alveolitid tabulates, common inhabitants of Devonian MCEs (Zapalski et al. 2017a; Majchrzyk et al. 2022), seem to have been well adapted to low-light conditions, although they were also present in abundance in shallow-water environments (Lecompte 1939, 1959; Stumm 1964). Therefore, these groups seem to be suitable for studies on light-induced phenotypic plasticity.
Here, we analyse the morphological variability of the Middle Devonian coenitid and alveolitid tabulate corals from different palaeobathymetric and biogeographic settings (Fig. 1), showing peculiar examples of the plasticity of these organisms, which strikingly resembles the light-inducted morphological plasticity of modern Porites corals. This pattern shows that light was already an important factor in the morphogenesis/astogenesis of Devonian reef-building corals, and suggests that the ecology of the Palaeozoic and modern corals was more similar than previously thought.
Material and methods
This research is mainly based on observations and material collected during field expeditions to Morocco in 2014 and 2020 (21 thin sections and 25 polished slabs were used) and supported by comparative studies on collections of fossils from Belgium (~ 200 examined specimens—mainly thin sections) and Poland (~ 100 studied specimens).
Due to the firm lithification of the fossil-bearing limestones, the fauna of the studied bioherms from Morocco could not be examined as fully extracted specimens. The tabulate corals from Morocco were identified using standard techniques based on thin section and polished slab observations. The observations were performed with a polarising microscope Nikon Eclipse LV100 Pol and a stereoscopic microscope NIKON SMZ1000. Microphotographs and scans were obtained using a Nikon DS-5Mc camera, working on NIS Elements software, and a Delta Optical DLT-CAM PRO camera, both connected with the microscopes.
Studied specimens are housed in the Museum of the Faculty of Geology, University of Warsaw (collection number: MWGUW ZI/106), the Institute of Palaeobiology of the Polish Academy of Sciences, Warsaw (collection numbers: ZPAL T. II; ZPAL T. XVII; ZPAL T. XVIII), and Royal Belgian Institute of Natural Sciences, Brussels (abbreviated RBINS; collections of the Devonian corals of Lecompte: 1933, 1939).
Geological settings
Aferdou el Mrakib, Morocco
The studied tabulate corals from Morocco were collected at Aferdou el Mrakib (eastern Anti-Atlas), one of the largest Devonian reefs (~ 1 km in diameter and 100 m in height; Kaufmann 1998) exposed in northern Africa (Fig. 1). The reef grew during latest Eifelian–earliest Givetian (~ 387–385 Ma) time in the semi-restricted, intra-shelf Mader Basin, located on the northern Gondwana margin (Kaufmann 1998; Jakubowicz et al. 2019; Zatoń et al. 2023). Aferdou el Mrakib was situated close to the southern limit of the extensive Devonian reef zone (~ 45° south of the equator) and represents one of the most poleward Palaeozoic reefs known to date (Jakubowicz et al. 2019; Majchrzyk et al. 2022). The Aferdou el Mrakib reef reveals many characteristics typical of Devonian coral-stromatoporoid reefs, including its ecological succession, the limited role of calcareous algae and development within the photic zone, emphasising the cosmopolitan character of the Middle Devonian reef ecosystem. However, the presence of some features distinctive from those of the tropical, most notably Laurussian Devonian reefs, like the dominance of heliolitid tabulate corals and the minor role of stromatoporoids, apparently reflects the high-latitude position of the reef and its development in the semi-restricted, high-turbidity basin (Król et al. 2018; Jakubowicz et al. 2019).
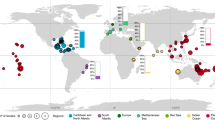
Location (a) and palaeogeographic position (b) of the collection areas: Anti-Atlas, Morocco (Aferdou el Mrakib reef), Holy Cross Mountains, Poland (Skały and Laskowa sections), and Ardennes, Belgium (Couvin, Rochefort and Wellin reefs). The Middle Devonian palaeogeographic framework adapted from Scotese (2001; slightly modified after Jakubowicz et al. 2019)
The material originates from the numerous small (up to 3 m in height) carbonate banks (bioherms) that developed at the base of the Aferdou el Mrakib reef (Jakubowicz et al. 2019; Majchrzyk et al. 2022). These buildups hosted a peculiar fauna dominated by platy tabulate corals (Roseoporella and Alveolites) and stromatoporoid sponges (Stromatoporella), with abundant solitary cystyphillid rugose corals. Based on the morphology of the tabulates and the palaeobathymetric and sedimentological data, these communities have recently been interpreted as MCEs, the most poleward ones known to date (Majchrzyk et al. 2022).
Holy Cross Mountains, Poland
The material from Poland originates from the Skały and Laskowa sections in the Holy Cross Mountains, Eifelian to Givetian in age. During the Devonian, this area was part of the tropical, southern shelf of Laurussia, located at ~ 20°–25°S (Golonka 2007; Fig. 1). The Devonian strata of the Holy Cross Mountains can be divided into two main palaeogeographical units: the Łysogóry palaeolow and the Kielce palaeohigh, with the Kostomłoty Transitional Zone separating them (Racki 1992). Reef communities are widely present in all these zones, with the shallowest-water ecosystems typical of the Kielce region (Racki 1992). The Skały (Łysogóry region) and Laskowa (Kostomłoty Zone) communities developed in deeper-water settings and, based on the taxonomic, morphological and palaeoecological constraints, have been interpreted as MCEs (Zapalski et al. 2017a; Zatoń et al. 2018). These communities are dominated by tabulate corals, most notably Alveolites, Roseoporella and Platyaxum, the rugose coral Phillipsastrea, and chaetetid sponges. Majchrzyk et al. (2022) compared the MCEs from the Holy Cross Mountains to their counterparts from the Aferdou el Mrakib reef and suggested development of the former under more light-depleted conditions.
Ardennes, Belgium
The Ardennes are the area with the most extensively studied Devonian reefs exposed in Europe (e.g. Lecompte 1959; Tsien et al. 1980; Burchette 1981; Copper and Scotese 2003; Hubert et al. 2007; Zapalski et al. 2007; Bridge et al. 2022). During the Devonian, this region was part of the tropical, southern shelf of Laurussia, located west of the Holy Cross Mountains at a similar latitude (Golonka 2007; Fig. 1). The origin of the extensive reef ecosystem took place here during the latest Emsian (~ 390 Ma; Mabille and Boulvain 2007; Denayer 2019). The reef growth continued throughout the Eifelian, reaching its acme during the Givetian to Frasnian (Boulvain et al. 2005; Da Silva et al. 2011). The Devonian reefs are exposed in the Ardennes within several tectonic units, especially close to the margins of the Dinant Synclinorium (Denayer 2019). The studied corals come from three main areas: Couvin (Mabille and Boulvain 2007), Rochefort (Boulvain et al. 2005; Da Silva et al. 2011) and Wellin (Król et al. 2021). The specimens are late Emsian to Frasnian (~ 390–370 Ma; mostly – especially the coenitids – Eifelian) in age. Unfortunately, due to a lack of detailed locality information in the RBINS collections, it was not possible to assign the studied specimens to particular sites and, therefore, to precisely determine their palaeoenvironmental backgrounds.
Results
Aferdou el Mrakib
All studied tabulates belong to the coenitid (Tabulata: Favositida: Coenitidae) genus Roseoporella which is also the most common coral in the MCEs of the Aferdou el Mrakib reef. The studied specimens form prostrate, mostly encrusting coralla, up to 2 cm in thickness and 15 cm in width. The coralla are unifacial, with the corallites at the proximal parts of the colonies oriented parallel or subparallel to the lower surface of the corallum (Fig. 2a). The walls are thick and connecting pores between the corallites are scarce. A peculiar feature of the studied Roseoporella coralla is the presence of finger-like outgrowths. The outgrowths are up to several centimetres high and grew upwards, approximately perpendicular to the base of the corallum (Fig. 2b). In some cases, additional, subordinate outgrowths deriving from the larger ones are present, giving the coralla a semi-branching appearance (Fig. 2b). The axial zone of the outgrows is very narrow; the corallites reach the corallum surface at an angle of ~ 20°. In the transverse section, corallites in the outgrowths reveal a specific, vortex-like arrangement (Fig. 2c). The sides of some of the outgrows, especially the large ones, are encrusted by Allonema (a probable foraminifer; Jarochowska and Munnecke 2014) (Fig. 2b). In some cases, the presence of numerous small outgrowths gives the coralla an “undulating” appearance (Fig. 2a). The specimens with such morphology are sometimes partially covered by sediment, with the outgrowths protruding above the sediment layer (Fig. 2a). Growth interruption surfaces are absent. These coralla are mostly preserved in situ, presumably in growth positions, and dislocated specimens are rare. Most commonly, Roseoporella encrust other tabulates or stromatoporoids, but specimens growing on soft substrate are also present.
Photomicrographs of Roseoporella sp. specimens from the mesophotic communities of the Givetian of the Aferdou el Mrakib reef, Morocco (collection number: MWGUW ZI/106). a longitudinal section of a specimen with small outgrowths projecting above the sediment layer (arrowed). b longitudinal section of a major outgrowth. Note the presence of encrusters (Allonema) on the sides of the outgrowth (marked with squares); c transverse section of several outgrowths (arrowed)
Holy Cross Mountains
As in the case of the material from Aferdou el Mrakib, all the studied tabulates from the Holy Cross Mountains belong to the genus Roseoporella. The size and morphology of these coralla are similar to those of their Moroccan counterparts. The coralla are mostly encrusting, up to 1–2 cm thick and 10–15 cm across. The Roseoporella specimens commonly encrust each other or other reef builders, forming a series of mutual overgrowths. The most notable difference between the morphology of Roseoporella from the Holy Cross Mountains and Morocco is the almost entire lack of the finger-like outgrowths observed in the former. Only a few of the examined specimens possess some outgrowths, typically relatively small (~ 2 cm) and sparse (most commonly 1–2 per specimen; Fig. 3 d-e).
Coenitid and alveolitid tabulate corals from the Ardennes (a–c, f–i; collections of the Devonian corals of Lecompte: 1933, 1939) and Holy Cross Mountains (d–e; collection numbers: ZPAL T. II, XVII, XVIII); a longitudinal section of platy Roseporella gradata (Eifelian, Rochefort); b transverse section of Roseporella media with several outgrowths (arrowed; Givetian, Wellin); c transverse section of a Roseporella media outgrowth (Givetian, Wellin); d top surface of Roseporella sp. with a single, broken outgrowth (arrowed; Givetian, Skały); e polished slab (longitudinal section) of Roseoporella sp. with a visible single outgrowth (arrowed; Givetian, Skały); f longitudinal section of branching Coenites clathratus (Eifelian, Couvin); g transverse section of a branch of Coenites clathratus (Eifelian, Couvin); h top surface of Alveolites suborbicularis with numerous broken outgrowths; i longitudinal section of an outgrowth of Alveolites tenuissimus (Frasnian, Couvin)
Ardennes
Because the original identification of most of the tabulates hosted at the RBINS was carried out in 1930s, the taxonomy of the corals from the Ardennes needed to be re-evaluated for the sake of the present study. Most of the examined specimens represent the species Roseoporella gradata and R. media, rather than Coenites gradatus and C. medius, as originally described (see Zapalski 2012). The studied coralla are mostly encrusting and prostrate, in some cases slightly thicker than the specimens from Morocco or Poland (Fig. 3a). Some rare specimens of Roseoporella (especially R. media) produced outgrowths morphologically similar to, although not as prominent as, those from Morocco (Fig. 3b-c). Roseoporella coralla commonly encrust each other or other organisms, with some specimens also apparently growing unattached in soft sediment (Fig. 3a). Branching morphotypes are represented by other coenitids, most notably Coenites clathratus (Fig. 3f-g). The branching coralla are composed of slender branches (typically < 1 cm in diameter), with narrow axial zones and corallites reaching the surface at an angle of ~ 25°. In transverse section, the corallites reveal a vortex-like arrangement similar to that observed in the outgrows of Roseoporella, although this characteristic is not as profound due to the slightly wider axial zone of the C. clathratus branches. Interestingly, some of the alveolitid corals, such as Alveolitella subaequalis, Alveolites tenuissimus and Alveolites saleei, produced intermediate, semi-branching morphotypes, to some extent similar to those displayed by the Moroccan specimens of Roseoporella (Fig. 3i), although with different corallite arrangement in the longitudinal sections of the branches (compare Figs. 2b and 3i).
Discussion
Morphological plasticity in modern Porites and Devonian Roseoporella
Morphological variations associated with light intensity are common among numerous scleractinian species present across broad depth gradients, for example Platygyra lamellina, Turbinaria reniformis and Goniastrea retiformis (Kahng et al. 2019). In general, zooxanthellate corals living under light-depleted conditions exhibit platy growth forms to collect light in the most efficient way (Lesser et al. 2009; Kahng el al. 2019). The genus Porites is characterised by particularly high morphological variability, and light intensity is one of the major factors influencing its growth pattern (Budd et al. 1994; Muko et al. 2000; Forsman et al. 2009; Padilla-Gamiño et al. 2012). Based on experimental data on Porites sillimaniana, Muko et al. (2000) presented a model linking the changes in the corallum shape during the colony growth to variations in light conditions. In well-irradiated environments, P. sillimaniana develops a fully branching morphology, while with decreasing light availability the number of branches decreases, and finally the corallum becomes entirely platy. Such growth forms are very peculiar for a branching coral species; under light-depleted conditions such corals usually do not develop fully platy coralla, but rather their branches become laterally flattened (Muir et al. 2015). P. sillimaniana is not the only species with such morphological plasticity; other representatives of the genus Porites, P. latistellata, P. rus and P. nigrescens, as well as Montipora stellata may develop similar morphotypes (Veron 2000; Padilla-Gamiño et al. 2012; Fig. 4). Recent studies showed that physiological adaptations in intermediate growth forms of Porites are also of major importance in the environmental acclimatisation of this genus. Padilla-Gamiño et al. (2012) showed that the platy areas of P. rus colonies had larger tissue biomasses and higher chlorophyll concentrations than the branching regions. Also, the δ13C signatures of the corals’ tissues suggested different rates of photosynthesis and/or capacity for heterotrophy between the platy and branching areas of the coralla (Padilla-Gamiño et al. 2012). Muko et al. (2000) and Padilla-Gamiño et al. (2012) showed that Porites can acclimatise to changes in environmental conditions on the short timescales of several weeks.
Examples of modern scleractinian coral assemblages with numerous intermediate forms of Porites cf. rus from Bougainville Reef (Coral Sea, Australia). a side view of the compound colonies of P. cf. rus; b. view on top surfaces of P. cf. rus colonies; c buildup made by P. cf. rus colonies; note the progressive decrease in the number of outgrowths with depth. Photographs courtesy of Tom Bridge
The relation between the light intensity and morphology of tabulate corals was recently recognised for several Palaeozoic MCEs (Zapalski et al. 2017a, 2021; Zapalski and Berkowski 2019; Majchrzyk et al. 2022; Zatoń et al. 2022). One of the taxa present in these MCEs were the representatives of the coenitid tabulate Roseoporella. The morphology of the Devonian Roseoporella skeletons studied here shows some variability, especially the corals from the Moroccan MCEs produced a range of morphologies. These corals can display a fully platy morphology, or possess additional outgrowths—a morphotypes highly similar to the intermediate growth form of Porites (Fig. 5). This pattern can be explained by the former presence of photosymbionts in Roseoporella and thus the coral’s morphological responses to changes in light availability. The variations in the coenitid morphology documented in the present study fit well to the model of Muko et al. (2000), suggesting a remarkably similar response to the light-limited conditions revealed by some modern scleractinians and Palaeozoic tabulates, i.e. the two groups of calcifying corals capable of forming large reefal buildups owing to their photosymbiotic nourishment. This pattern is in line with recent studies documenting a suite of functional characteristics shared by both tabulate and scleractinian corals, including the photosymbiosis (Zapalski 2014), and development of similar ecological and morphological adaptations to both typical (Zapalski et al. 2017a; Zapalski and Berkowski 2019; Majchrzyk et al. 2022) and “brown-water” mesophotic environments (Zapalski et al. 2021; for more information on “brown-water” communities see Renema 2019), as well as to environments with strong bottom currents (Zapalski et al. 2022).
Hoogenboom et al. (2008) demonstrated that variations in zooxanthellate coral morphology are especially visible at niche boundaries, at which the environmental conditions are often more stressful. Majchrzyk et al. (2022) showed that the communities from the base of the Moroccan Aferdou el Mrakib reef developed most likely at water depths between 20 and 60 m and represent an upper mesophotic ecosystem, which implies that the reef builders were probably exposed to intermediate to low-light conditions. In addition, changes in water clarity, and thus light availability, could have also been caused by variations in water turbidity or suspended sediment supply. The taxonomical structure of the Aferdou el Mrakib MCEs, with the presence of the cystiphyllid rugosans, a group well adapted to high sedimentation rates (Sorauf 2003; Berkowski 2012), may support the involvement of episodes of increased sediment influx. Accordingly, Król et al. (2018) and Jakubowicz et al. (2019) proposed that the dominance of the climax stage of the Aferdou el Mrakib reef growth by Heliolites tabulates reflects increased water turbidity in the reef-hosting marine basin. Such an environment seems particularly favourable for the development of the various morphologies observed in Roseoporella. An open question remains as to whether all of these morphotypes existed simultaneously, occupying slightly different micro-ecological niches, or rather we see the fossil record of numerous communities living at different times, undistinguishable because of the taphonomical biases and available stratigraphic resolution.
An alternative explanation for the origin of the finger-like outgrowths in Devonian Roseoporella, not involving changes in light conditions, might be a morphological response to episodes of high sediment input, preventing the corallum from entire burial. For the Aferdou el Mrakib reef, the presence of specimens partially covered by sediment, with some outgrowths projecting above the sediment layer, might favour such an explanation (Fig. 2a). However, the sides of the most prominent outgrows are commonly encrusted by Allonema (Fig. 2b), attesting to their significant relief over the basal, platy part of the colony. Therefore, the episodic sediment influx is unlikely to have been the dominant control on the morphological variability observed in the studied Roseoporella coralla.
The fossil material from the European sites fits well in the proposed model. Roseoporella is one of the most common taxa in the MCEs from the Holy Cross Mountains (Zapalski et al. 2017a). The taxonomic composition of these communities, with the very rare occurrence of stromatoporoids and green algae, showed that their development took place under more light-depleted conditions than it was the case for their Moroccan counterparts (Majchrzyk et al. 2022). The morphology of Roseoporella present in the MCEs from the Holy Cross Mountains is almost exclusively platy, with only a few specimens that produced some singular outgrowths. The platy morphology would be more beneficial under low-light conditions than the intermediate morphotypes characteristic of the Aferdou el Mrakib reef, where the light availability was probably less restricted (Majchrzyk et al. 2022). The morphological variability typifying Roseoporella from the Ardennes, similar to that observed at Aferdou el Mrakib, shows that the described pattern in the light-induced phenotypic plasticity was present in Devonian tabulates not only in restricted, peculiar environments, but was more widespread. We did not find fully branching specimens of Roseoporella in our material. Nevertheless, close relatives of Roseoporella, such as Coenites clathratus, may serve as a model of the potential branching morphotype of this coral, which developed under well-irradiated conditions (Fig. 5). To our knowledge, similar evidence of the morphological plasticity has not been presented so far for tabulate corals, except for very brief notes on the possible presence of some outgrowths in alveolitids (Lecompte 1939; Hill 1981) or photographs of such specimens (Lecompte 1939). Some of the specimens described by Lecompte (1939), present in the RBINS, indeed display similar intermediate, semi-branching morphotypes. These observations show that, as in the case of scleractinian corals, this type of light-controlled morphological variability was rather rare, but likely not restricted to a single tabulate genus, and probably also present in several species within the suborder Alveolitina.
Taxonomic implications of the morphological variability in tabulate corals
Our observations on the morphology of Roseoporella are of importance also for the taxonomy of the coenitid tabulates. For nearly two centuries (at least since the work by Eichwald 1829), the systematics of the Coenitidae was a subject of numerous, sometimes inconsistent changes, which created an ambiguous image of the taxonomy of this group (see details in Zapalski 2012). Corals currently identified as members of Roseporella were long attributed to different coenitid and alveolitid genera, such as Alveolites, Planocoenites or Coenites (e.g. Lecompte 1939; Stasińska 1959; Niko 2003). Roseoporella was distinguished from the other coenitids mainly based on its platy, prostrate colony shape and thus separated from the branching genus Coenites (Zapalski 2012). The high phenotypic plasticity of Roseoporella documented here may suggest that some coenitid genera were congeneric, as some specimens analysed in the present study display a range, or a combination, of morphologies considered indicative of the two different genera. However, the outgrowths described here for Roseoporella differ in some aspects from the branches of Coenites. Although the Coenites and Roseoporella corallites reveal a similar vortex-like arrangement when observed in transverse sections, the axial zone in the branches is usually broader than observed in the studied Roseoporella coralla. Some specimens of alveolitids, like Alveolitella subaequalis illustrated by Lecompte (1939), also highly resemble the analysed Roseoporella specimens. However, these specimens may have been incorrectly determined and may in fact represent coenitids. It cannot be excluded that the observed morphological diversity of Roseoporella could be an interspecific variation; however, in such a case we should rather observe two (or more) specific morphotypes representing different species, which is not the case in our material. Instead, especially in the Aferdou el Mrakib reef, we see gradual transitions between completely platy forms, morphotypes with single outgrowths and coralla with multiple outgrowths and semi-branching appearance, a pattern consistent with the observations of the intraspecific variations in modern scleractinian corals (Muko et al. 2000). In general, the morphological variability observed in Roseoporella emphasises the need of major reevaluation of the validity of some genera within the family Coenitidae. It cannot be excluded that, as it was suggested for the scleractinian genus Porites (Budd et al. 1994; Forsman et al. 2009), intraspecific morphological variations in tabulate genera such as Roseoporella and/or Coenites may be so profound and continuous that the classical, morphotype-based approach based on traditional taxonomical methods (such as visual examination or measurements) can be insufficient to distinguish between separate species. Unfortunately, the nature of the fossil record excludes or restricts using some of the methods applied in modern coral taxonomy, such as molecular studies, making the taxonomical assignment of tabulate corals even more challenging.
References
Barshis DJ, Stillman JH, Gates RD, Toonen RJ, Smith LW, Birkeland C (2010) Protein expression and genetic structure of the coral Porites lobata in an environmentally extreme Samoan back reef: Does host genotype limit phenotypic plasticity? Mol Ecol 19(8):1705–1720
Berkowski B (2012) Life strategies and function of dissepiments in rugose coral Catactotoechus instabilis from the Lower Devonian of Morocco. Acta Palaeontol Pol 57(2):391–400
Boulvain F, Demany B, Coen-Aubert M (2005) Frasnian carbonate buildups of southern Belgium: the Arche and Lion members interpreted as atolls. Geol Belg 8(1–2):69–91
Bridge TC, Baird AH, Pandolfi JM, McWilliam MJ, Zapalski MK (2022) Functional consequences of Palaeozoic reef collapse. Sci Rep 12:1386
Bruno JF, Edmunds PJ (1997) Clonal variation for phenotypic plasticity in the coral Madracis mirabilis. Ecology 78(7):2177–2190
Budd AF, Johnson KG, Potts DC (1994) Recognizing morphospecies in colonial reef corals: I. Landmark-based methods. Paleobiology 20(4):484–505
Burchette TP (1981) European Devonian reefs: a review of current concepts and models. SEPM Spl Publ 30:85–142
Coates AG, Jackson JB (1987) Clonal growth, algal symbiosis, and reef formation by corals. Paleobiology 13(4):363–378
Copper P (1994) Ancient reef ecosystem expansion and collapse. Coral Reefs 13(1):3–11
Copper P (2002) Silurian and Devonian reefs: 80 million yrs of global greenhouse between two ice ages. SEPM Spl Publ 72:181–238
Copper P, Scotese CR (2003) Megareefs in Middle Devonian supergreenhouse climates. Spl Paper Geol Soc Am 370:209–230
Da Silva AC, Kershaw S, Boulvain F (2011) Sedimentology and stromatoporoid palaeoecology of frasnian (upper devonian) carbonate mounds in southern Belgium. Lethaia 44(3):255–274
Denayer J (2019) Revised stratigraphy of the Eifelian (Middle Devonian) of S. Belgium: sequence stratigraphy, global events, reef development and basin structuration. Geologica Belgica 22(3–4):149–73
DeWitt TJ, Sih A, Wilson DS (1998) Cost of plasticity. Trends Ecol Evol 13(2):77–81
Eichwald CE (1829) Zoologia specialis quam expositis animalibus tum vivis, tum fossilibus potissimum Rossiaein universum et Poloniae in specie, in usum lectionum. Józef Zawadzki, Vilnus, vol 1
Fagerstrom JA (1994) The history of Devonian-Carboniferous reef communities: extinctions, effects, recovery. Facies 30(1):177–191
Forsman ZH, Barshis DJ, Hunter CL, Toonen RJ (2009) Shape-shifting corals: molecular markers show morphology is evolutionarily plastic in Porites. BMC Evol Biol 9(1):1–9
Fox RJ, Donelson JM, Schunter C, Ravasi T, Gaitán-Espitia JD (2019) Beyond buying time: the role of plasticity in phenotypic adaptation to rapid environmental change. Philos Trans R Soc B 374(1768):20180174
Fusco G, Minelli A (2010) Phenotypic plasticity in development and evolution: facts and concepts. Philos Trans R Soc B: Biol Sci 365(1540):547–556
Golonka J (2007) Phanerozoic paleoenvironment and paleolithofacies maps. Late Paleozoic Geologia 33(2):145–200
Graus RR, Macintyre IG (1976) Light control of growth form in colonial reef corals: computer simulation. Science 193(4256):895–897
Hageman SJ (1995) Observed phenotypic variation in a Paleozoic bryozoan. Paleobiology 21(3):314–328
Haq BU, Schutter SR (2008) A chronology of Paleozoic sea-level changes. Science 322(5898):64–68
Hill D (1981) Rugosa and Tabulata. In Teichert, C. Treatise on Invertebrate Paleontology. Pt f Coelenterata 2:379–762
Hoogenboom MO, Connolly SR, Anthony KRN (2008) Interactions between morphological and physiological plasticity optimize energy acquisition in corals. Ecology 89(4):1144–1154
Hubert BL, Zapalski MK, Nicollin JP, Mistiaen B, Brice D (2007) Selected benthic faunas from the Devonian of the Ardennes: an estimation of palaeobiodiversity. Acta Geol Pol 57(2):223–262
Jakubowicz M, Król J, Zapalski MK, Wrzołek T, Wolniewicz P, Berkowski B (2019) At the southern limits of the Devonian reef zone: Palaeoecology of the Aferdou el Mrakib reef (Givetian, eastern Anti-Atlas, Morocco). Geol J 54(1):10–38
Jarochowska E, Munnecke A (2014) The Paleozoic problematica Wetheredella and Allonema are two aspects of the same organism. Facies 60(2):651–662
Kahng SE, Hochberg EJ, Apprill A, Wagner D, Luck DG, Perez D, Bidigare RR (2012) Efficient light harvesting in deep-water zooxanthellate corals. Mar Ecol Prog Ser 455:65–77
Kahng SE, Akkaynak D, Shlesinger T, Hochberg EJ, Wiedenmann J, Tamir R, Tchernov D (2019) Light, temperature, photosynthesis, heterotrophy, and the lower depth limits of mesophotic coral ecosystems. In: Loya Y, Puglise K, Bridge T (eds) Mesophotic coral ecosystems. Springer, Cham, pp 801–828
Kaniewska P, Campbell PR, Fine M, Hoegh-Guldberg O (2009) Phototropic growth in a reef flat acroporid branching coral species. J Exp Biol 212(5):662–667
Kaufmann B (1998) Facies, stratigraphy and diagenesis of Middle Devonian reef- and mud-mounds in the Mader (eastern Anti-Atlas, Morocco). Acta Geol Pol 48(1):43–106
Kiessling W (2009) Geologic and biologic controls on the evolution of reefs. Annu Rev Ecol Evol Syst 40:173–192
Król JJ, Jakubowicz M, Zapalski MK, Berkowski B (2018) Massive tabulates in competition for space: A case study from Aferdou el Mrakib (Middle Devonian, Anti-Atlas, Morocco). Palaeogeogr Palaeoclimatol Palaeoecol 497:105–116
Król JJ, Denayer J, Wolniewicz P, Zapalski MK (2021) Heliolitid corals and their competitors: a case study from the Wellin patch reefs, Middle Devonian, Belgium. Lethaia 54(4):540–557
Kühlmann DHH (1983) Composition and ecology of deep-water coral associations. Helgoländer Meeresuntersuchungen 36(2):183–204
Lecompte MJ (1933) Le genre Alveolites Lamarck dans le Dévonien moyen et supérieur de l’Ardenne. Musée R D’histoire Nat Belgique 55:1–49
Lecompte MJ (1939) Les tabulés du Dévonien moyen et supérieur du bord sud du bassin de Dinant. Musée Royal D’histoire Naturelle De Belgique 90:1–229
Lecompte MJ (1959) Certain data on the genesis and ecologic character of Frasnian reefs of the Ardennes. Int Geol Rev 1(7):1–23
Lesser MP, Slattery M, Leichter JJ (2009) Ecology of mesophotic coral reefs. J Exp Mar Biol Ecol 375(1–2):1–8
Lister AM (2021) Phenotypic plasticity in the fossil record. In: Pfennig DW (ed) Phenotypic plasticity & evolution. CRC Press, pp 267–297
Mabille C, Boulvain F (2007) Sedimentology and magnetic susceptibility of the Couvin formation (Eifelian, south western Belgium): carbonate platform initiation in a hostile world. Geol Belg 10(1–2):47–67
Majchrzyk A, Jakubowicz M, Berkowski B, Bongaerts P, Zapalski MK (2022) In the shadow of a giant reef: Palaeoecology of mesophotic coral communities from the Givetian of Anti-Atlas (Morocco). Palaeogeogr Palaeoclimatol Palaeoecol 602:111177
Muir P, Wallace C, Bridge TC, Bongaerts P (2015) Diverse staghorn coral fauna on the mesophotic reefs of north-east Australia. PLoS ONE 10(2):e0117933
Muko S, Kawasaki K, Sakai K, Takasu F, Shigesada N (2000) Morphological plasticity in the coral Porites silimaniani and its adaptative significance. Bull Mar Sci 66(1):225–239
Niko S (2003) Devonian coenitid tabulate corals from the Fukuji Formation, Gifu Prefecture. Bull Natl Sci Museum Series C Geol Palaeontol 29:19–24
Padilla-Gamiño JL, Hanson KM, Stat M, Gates RD (2012) Phenotypic plasticity of the coral Porites rus: Acclimatization responses to a turbid environment. J Exp Mar Biol Ecol 434–435:71–80
Pigliucci M (2005) Evolution of phenotypic plasticity: Where are we going now? Trends Ecol Evol 20(9):481–486
Racki G (1992) Evolution of the bank to reef complex in the Devonian of the Holy Cross Mountains. Acta Palaeontol Pol 37:87–182
Renema W (2019) Large benthic foraminifera in low-light environments. In: Loya Y, Puglise KA, Bridge T (eds) Mesophotic coral ecosystems. Springer, Cham, pp 553–561
Scotese CR (2001) Atlas of Earth history. PALEOMAP Project. University of Texas, Arlington
Scotese CR, Song H, Mills BJ, van der Meer DG (2021) Phanerozoic paleotemperatures: the earth’s changing climate during the last 540 million yrs. Earth Sci Rev 215:103503
Scrutton CT (1998) The Palaeozoic corals, II: structure, variation and palaeoecology. Proc Yorks Geol Soc 52(1):1–57
Segal B, Castro CB (2011) Coral community structure and sedimentation at different distances from the coast of the Abrolhos Bank, Brazil. Brazil J Oceanogr 59(2):119–129
Shaish L, Abelson A, Rinkevich B (2007) How plastic can phenotypic plasticity be? The branching coral Stylophora pistillata as a model system. PLoS ONE 2(7):1–9
Sorauf JE (2003) The function of dissepiments and marginaria in the Rugosa (Cnidaria, Zoantharia). Fossil Corals and Sponges, Proceedings of the 9th International Symposium on Fossil Cnidaria and Porifera, Graz, 11–9
Stasińska A (1959) Tabulata, Heliolitida et Chaetetida du devonien moyen des monts de Sainte-Croix. Acta Palaeontol Pol 3(3–4):161–282
Stearns S (1989) The evolutionary significance of phenotypic plasticity—phenotypic sources of variation among organisms can be described by developmental switches and reaction norms. Bioscience 39(7):436–445
Stumm EC (1964) Silurian and Devonian corals of the Falls of the Ohio. Geol Soc Am Mem 93:1–184
Talent JA (1988) Organic reef-building: episodes of extinction and symbiosis? Senckenb Lethaea 69:315–368
Tambutté E, Venn AA, Holcomb M, Segonds N, Techer N, Zoccola D, Allemand D, Tambutté S (2015) Morphological plasticity of the coral skeleton under CO2-driven seawater acidification. Nat Commun 6:1–9
Todd PA (2008) Morphological plasticity in scleractinian corals. Biol Rev 83:315–337
Torda G, Donelson JM, Aranda M, Barshis DJ, Bay L, Berumen ML, Bourne DG, Cantin N, Foret S, Matz M, Miller DJ, Moya A, Putnam HM, Ravasi T, Van Oppen MJH, Thurber RV, Vidal-Dupiol J, Voolstra CR, Watson SA, Munday PL (2017) Rapid adaptive responses to climate change in corals. Nat Clim Change 7(9):627–636
Tsien HH, Molintjoy EW, Bouckaert J, Bultynck P, Dricot E, Tsien HH, Mouravieff AN (1980) Devonian reefs in Belgium. Geobios 4:17–33
Veron JV (2000) Corals of the World. Australian Institute of Marine Science, Townsville
Voolstra CR, Suggett DJ, Peixoto RS, Parkinson JE, Quigley KM, Silveira CB, Sweet CB, Muller M, Barish EM, Bourne DJ, Aranda M (2021) Extending the natural adaptive capacity of coral holobionts. Nat Rev Earth Environ 2(11):747–762
Wood R (2001) Biodiversity and the history of reefs. Geol J 36(3–4):251–263
Zapalski MK (2012) Tabulate corals from the Givetian and Frasnian of the southern region of the Holy Cross Mountains (Poland). Spec Pap Palaeontol 87:1–100
Zapalski MK (2014) Evidence of photosymbiosis in Palaeozoic tabulate corals. Proc R Soc B: Biol Sci 281(1775):20132663
Zapalski MK, Berkowski B (2019) The Silurian mesophotic coral ecosystems: 430 million yrs of photosymbiosis. Coral Reefs 38(1):137–147
Zapalski MK, Hubert BL, Nicollin JP, Mistiaen B, Brice D (2007) The palaeobiodiversity of stromatoporoids, tabulates and brachiopods in the Devonian of the Ardennes–changes through time. Bull Soc Géol France 178(5):383–390
Zapalski MK, Wrzołek T, Skompski S, Berkowski B (2017a) Deep in shadows, deep in time: the oldest mesophotic coral ecosystems from the Devonian of the Holy Cross Mountains (Poland). Coral Reefs 36:847–860
Zapalski MK, Nowicki J, Jakubowicz M, Berkowski B (2017b) Tabulate corals across the Frasnian/Famennian boundary: architectural turnover and its possible relation to ancient photosymbiosis. Palaeogeogr Palaeoclimatol Palaeoecol 487:416–429
Zapalski MK, Baird AH, Bridge T, Jakubowicz M, Daniell J (2021) Unusual shallow water Devonian coral community from Queensland and its recent analogues from the inshore Great Barrier Reef. Coral Reefs 40(2):417–431
Zapalski MK, Król JJ, Halamski AT, Wrzołek T, Rakociński M, Baird AH (2022) Coralliths of tabulate corals from the Devonian of the Holy Cross Mountains (Poland). Palaeogeogr Palaeoclimatol Palaeoecol 585:110745
Zatoń M, Zapalski MK, Berkowski B, Wrzołek T (2018) Cryptic encrusting communities in a Middle Devonian mesophotic paleoenvironment of the Holy Cross Mountains, Poland. Palaeogeogr Palaeoclimatol Palaeoecolog 501:82–91
Zatoń M, Malec J, Wrzołek T, Kubiszyn B, Zapalski MK (2022) Episkeletobionts of large rugose corals from the Middle Devonian mesophotic palaeoenvironment recorded in the Pokrzywianka Beds (Holy Cross Mountains, Poland). Ann Soc Geol Pol 92:1–20
Zatoń M, Jakubowicz M, Król J, Zapalski MK, Słowiński J, Rakociński M, Berkowski B (2023) Tiny inhabitants of a large middle devonian reef of northern Gondwana: sclerobionts of the coral-stromatoporoid Aferdou el Mrakib buildup, eastern Anti-Atlas, Morocco, Palaeogeography, Palaeoclimatology. Palaeoecology 612:111392
Acknowledgements
This is a contribution to the research project 2020/37/N/ST10/00773 (AM) and 2018/29/B/ST10/00954 (MKZ), both funded by the National Science Centre of Poland. We are grateful to Annelise Folie, Julien Lalanne and Jolanta Kobylińska for substantial help in accessing the palaeontological specimens housed in the Institut royal des Sciences naturelles de Belgique and Institute of Palaeobiology of the Polish Academy of Sciences. We are indebted to Mr. Bogusław Waksmundzki (University of Warsaw) for drawing the reconstructions presented here and Tom Bridge (ARC Centre of Excellence for Coral Reef Studies, Townsville) for providing us with the photographs of modern coral assemblages. We wish to thank the representatives of the Ministère de l'Energie, des Mines, de l'Eau et de l'Environnement (Morocco), Ahmed Benlakhdim, Khalid El Hmidi and Aissam El Khlifi, for the work permit in Morocco and logistic advice. Zdzisław Bełka, Błażej Berkowski, Jan J. Król (all Adam Mickiewicz University), Michał Zatoń and Michał Rakociński (both University of Silesia) are cordially thanked for their kind assistance and valuable discussions in the field. The reviewer is gratefully acknowledged for constructive comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Majchrzyk, A., Jakubowicz, M., Bongaerts, P. et al. Different times, similar mechanism? Convergent patterns in light-induced phenotypic plasticity in Devonian and modern corals. Coral Reefs 42, 893–903 (2023). https://doi.org/10.1007/s00338-023-02394-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02394-4