Abstract
Animal movement and feeding studies shed light on ecological roles and can inform management strategies for fished species. However, the feeding and movement of nocturnal sea cucumbers have been rarely studied. We determined the movement dynamics, feeding ecology and sheltering behaviour of the nocturnal Stichopus cf. monotuberculatus (dragonfish) at One Tree Reef and Heron Island Reef on the southern Great Barrier Reef. The short-term movement of individual sea cucumbers were tracked, and sediment defecation rates were measured. Displacement rates averaged 3–33 cm h−1 across the four sites, with the sea cucumbers moving more slowly approaching sunrise and within patches of organically rich sediment. The movement paths were moderately tortuous, with larger sea cucumbers moving along straighter paths. Rates of sediment turnover averaged 7.8 g h−1 and were greatest for larger individuals. The faecal casts were organically richer and composed of finer grains than the ambient surface sediments, implying that S. cf. monotuberculatus avoids consuming coarse sediments and feeds on nutritionally rich particles. The sea cucumbers sheltered under hard reef substrata and moved non-directionally on the seascape, with a few individuals homing back to their original refuges. The affinity to reef substrata and short nightly net displacements of S. cf. monotuberculatus have implications for the spatial management of this and other similar species. Larger individuals occupied deeper refuges, implying that projected declines in substratum rugosity on coral reefs due to climate change could impact the size structure of sea cucumber populations, with implications for fisheries.
Similar content being viewed by others
Introduction
Animal movement and feeding are key ecological processes that influence the structure and dynamics of ecosystems by providing links to trophic and nutrient-flux hierarchies (Polis et al. 1997). Studies of animal movement and feeding ecology provide insight into their ecological roles (e.g., Navarro et al. 2017; Kristensen et al. 2021) and are important for harvested species to inform conservation and fisheries management (Pittman and McAlpine 2003). Despite their prominence in benthic ecosystems globally, the feeding ecology and movement patterns of many commercially exploited sea cucumbers (holothuroids) are not well understood (Eriksson et al. 2013; Navarro et al. 2014; Wolfe and Byrne 2017).
Deposit-feeding sea cucumbers are ubiquitous on coral reefs, where their sediment bioturbation and feeding behaviours have significant effects on physio-chemical processes (Purcell et al. 2016a). In these oligotrophic systems, holothuroids play a key role in recycling nutrients (Massin 1982; Uthicke 2001b; Purcell et al. 2016a). Deposit-feeding by sea cucumbers also influences local seawater carbonate chemistry, potentially mitigating the effects of anthropogenic ocean acidification at local scales (Schneider et al. 2011, 2013; Wolfe et al. 2018). Further, through their feeding activities, holothuroids facilitate the regeneration and remineralisation of surface sediments (Purcell et al. 2016a). With some species able to turnover vast quantities of sediment, sea cucumbers play a vital role in maintaining sediment and thereby ecosystem health (Purcell et al. 2016a; Wolfe and Byrne 2017; Williamson et al. 2021).
The overexploitation of sea cucumbers for the luxury dried food commodity ‘bêche-de-mer’ and the production of pharmaceuticals is a major threat to benthic marine communities (Purcell et al. 2013, 2016a; Wolfe and Byrne 2022). Holothuroids are particularly vulnerable to overfishing due to their life-history traits, ease of collection (Uthicke et al. 2004; Purcell et al. 2010) and high market value (Purcell et al. 2014b). The removal of holothuroids from coral reefs has been linked to the overgrowth of algal mats (Moriarty et al. 1985; Uthicke 1999), sediment anoxia (Lee et al. 2017) and the susceptibility of hard corals to heat-activated pathogens (Grayson et al. 2022). For many species, management strategies are poorly informed due to a lack of fundamental species-specific biological data (Uthicke et al. 2004; Purcell et al. 2013).
Spatial management measures, such as no-take zones and rotational-fishing zone systems, are used to conserve relatively sedentary animals, such as holothuroids (Hastings and Botsford 2003; Uthicke 2004). Spatial protection should ideally be guided by an understanding of species mobility and the likelihood that animals will ‘spillover’ across reserve boundaries (Grüss et al. 2011; Hernandez‐Lamb et al. 2012). Despite being regarded as sedentary, recent studies show that some sea cucumbers are highly mobile at hourly timescales (e.g., Hammond et al. 2020; Gray et al. 2022). In addition, some species exhibit site fidelity and do not displace far in the long-term (Conand 1991; Purcell et al. 2016b; Purcell et al. 2023a; Hammond and Purcell 2023). Due to the difficulties of tagging sea cucumbers (see Purcell et al. 2016b), the movement dynamics of many bêche-de-mer species are unknown (Eriksson et al. 2013; Wolfe and Byrne 2017).
Most animals do not move randomly and instead establish a home range where they undertake routine activities, such as foraging and resting (Quinn and Brodeur 1991). Within this home range, animals often move between distinct resting and feeding sites (e.g., Tuya et al. 2004). A behavioural pattern observed for several sea cucumber species is a diel cycle of diurnal sheltering and nocturnal feeding (Hammond 1982; Mercier et al. 1999). Most sea cucumber movement is associated with foraging, and many species modify their movement to feed selectively on organically rich deposits (Da Silva et al. 1986; Mercier et al. 1999; Navarro et al. 2013). Some holothuroids also feed selectively for grain size (Moriarty 1982; Uthicke 1999), while others show no preference (Uthicke and Karez 1999).
Among marine species, larger individuals tend to displace further than smaller ones (Quinn and Brodeur 1991; Allen et al. 2018), although there are exceptions (e.g., Purcell et al. 2023a). Movement behaviour can also vary among conspecifics, with some individuals exhibiting long-term site fidelity while others disperse large distances (e.g., Purcell et al. 2016b; Hammond and Purcell 2023). Refuge quality might influence movement patterns of species with diel migration between shelters and foraging grounds (Hammond 1982; Graham and Battaglene 2004).
Refuges, or shelters, are structural microhabitats individuals utilise to decrease their exposure to predators, sunlight, and unfavourable environmental conditions (Forsythe et al. 2003; Ménard et al. 2008). Marine species often occupy shelters that closely correspond to their size (Forsythe et al. 2003; Booth and Ayers 2005). Such relationships are often related to the variable protective capacity of refuges across body sizes (Eggleston et al. 1990). For some marine invertebrates, the quality and abundance of available shelters may influence population size and structure (Eggleston et al. 1990; Beck 1995). With reef substratum rugosity projected to decline due to the impacts of climate change (Rogers et al. 2014), understanding refuge selection can provide insight into the challenges facing species in a changing ocean (e.g., Fontoura et al. 2020).
The Stichopodidae is a diverse holothuroid family, with several species commercially exploited in wild fisheries and aquaculture (Byrne et al. 2010; Woo et al. 2019; Cheng et al. 2021). Across the Indo-Pacific the Stichopus cf. monotuberculatus species complex is harvested with the identity of the Pacific species uncertain, due to the inability to compare with specimens from the type locality in the Western Indian Ocean (Mauritius) (see Byrne et al. 2010). Stichopus cf. monotuberculatus is often misidentified in fishery data as the morphologically similar S. horrens and both are commonly known as dragonfish (Eriksson et al. 2007; Byrne et al. 2010). Hence, S. cf. monotuberculatus is probably more exploited than reported. Several recent studies have emerged on the potential for commercial mariculture and sea ranching of this species. One found high growth rates of the sea cucumbers on artificial reefs, with animals feeding on sediment detritus containing food sources such as microbes, algal debris and phytoplankton (Xu et al. 2022). In tanks, this species was more active at higher seawater temperatures and tends to move down-current in simulated flow experiments (Chen et al. 2022). Further, a recent study determined the morphometric relationships of S. cf. monotuberculatus to generate biomass estimates for fisheries stock assessments (Gray et al. 2023). Apart from it’s nocturnal nature and being found sheltering in reef infrastructure during the day (Purcell et al. 2023), the ecology of this species in natural habitats is practically undocumented. This cryptic behavioural pattern has likely contributed to our limited ecological knowledge of this species and other nocturnal coral reef holothuroids.
Here, we investigated the short-term movement patterns and sediment turnover of S. cf. monotuberculatus. Our objectives were to: (1) determine trends in the displacement and directionality of individuals movement paths, (2) quantify sediment turnover and whether the species feeds selectively with respect to grain size and organic content, and (3) investigate sheltering behaviour, and whether S. cf. monotuberculatus occupies certain refuge types. The variable and distinctive colour patterns among individual S. cf. monotuberculatus provided an opportunity to use photographic records to validate individuals with temporally repeated observations. Fieldwork was undertaken at two reefs in the southern Great Barrier Reef, Australia, at localities where the species occurs in high densities. Our findings provide essential data on the movement dynamics of S. cf. monotuberculatus to inform spatial management measures, while shedding light on the species’ ecological roles. These data broaden our understanding of the movement and feeding ecology of sea cucumbers and furnish a rare view of movement patterns in nocturnal reef invertebrates.
Materials and methods
Study sites
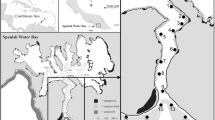
Fieldwork was undertaken at One Tree Reef (23.51° S, 152.09° E; OTR) and Heron Island Reef (23.44° S, 151.92° E; HIR) in the Capricorn Bunker Group, southern Great Barrier Reef in April–May 2022 (Fig. 1a). The study sites at OTR are located in the Great Barrier Reef Marine Park Authority Scientific Research Zone, and in the Green Zone (Marine National Park) at HIR. Both locations, One Tree Reef and Heron Island Reef, have a near-continuous rim around the reef that ponds seawater at low tide. Coral communities are diverse and there are extensive areas of soft sediments interspersed among the hard reef structures at both locations (Eriksson et al. 2013; Wolfe and Byrne 2017). Mean daily seawater temperatures in 2022 ranged 19.7–28.2 °C and diel seawater temperatures were similar between locations, ranging 23.4–26.2 °C (AIMS 2023).
Sites with sufficient populations of S. cf. monotuberculatus were identified by reconnaissance on the nights prior to monitoring. At OTR, two sites were identified in the lagoon, along the edge of a small patch reef at the channel entrance to the One Tree Island Research Station (Fig. 1b). Both sites are shallow (< 1 m deep at low tide) and are separated by approximately 30 m. At HIR, the southern (Site 1, ≤ 1.5 m corrected to zero tidal datum) and northern (Site 2, ≤ 1.7 m) sides of the HIR boat channel served as respective sites (Fig. 1c). These sites are approximately 50 m from each other and are bordered by concrete walls, which extend into the reef flat.
Field data collection
The S. cf. monotuberculatus were located on snorkel using dive torches by searching sand, reef, and rubble habitat within the study areas. Due to their high densities at the study sites, individuals were selected for monitoring based on their distinct identifiable colour patterns (Fig. 2). Once an individual was identified, the time of each initial observation, body colour and markings were recorded. Where possible, the refuges that individuals emerged from were identified and marked with a weight and float. Simultaneously, a numbered peg with flagging tape was placed 1 cm behind the anus of each individual. Every 2–3 h from sunset to sunrise, we located the sea cucumbers and recorded their linear displacement (± 1 cm) from the peg to the posterior end of the sea cucumber with a measuring tape and compass bearing (± 5° degrees from north) with a wrist-mounted dive compass. The times of all observations were recorded before the pegs were placed behind the animals. This process occurred at each observation period, making it relatively easy to locate the individuals.
The highly variable and individually distinctive colour patterns of Stichopus cf. monotuberculatus. Examples of individuals examined in the present study with various colour patterns, used to aid in verification of individuals over the observation periods. These photographs were all taken at night during the study. Photos: SW Purcell
To ensure that the same individuals were tracked throughout the night, we photographed each individual at the start and end of the night, and later confirmed their identity by inspection of the photographs. We referred to these initial photographs throughout the night, as needed. During the second observation period (~ 23:15–02:00), we measured each individual’s body length and width (at midpoint; ± 0.5 cm) in situ with a ruler. Care was taken not to touch or disturb the sea cucumbers as this could influence movement rates (see Pan et al. 2015).
During the third observation period (~ 02:00), all faecal casts between the individuals and their respective pegs were gently scooped into labelled 70-ml vials using a tablespoon. The time that the samples were taken was recorded to later calculate defecation rates, which serve as a proxy for sediment reworking (Hammond 1982; Uthicke 1999). Faecal casts were only collected when we could be sure they were from the monitored individual (i.e., casts were not collected when multiple sea cucumbers paths crossed). Surface sediment samples (~ 30 ml) were also collected from the surrounding sediment 30–40 cm either side of each individual for which faecal casts were collected. The upper ~ 3 mm of sediments were sampled as above, approximating the depth to which sea cucumbers feed (Yingst 1982).
To determine if S. cf. monotuberculatus exhibited preferences for particular types of refuges, we recorded into broad benthic categories the type of refuge where animals had emerged at the start of the evening or, when that was not possible (HIR Site 2), at the end of the night. To examine selectivity for shelter size, we measured the refuge entrance width (± 0.5 cm; at the base of the refuge), refuge height at midpoint, and depth (i.e., how far laterally into the reef structure could a ruler be inserted). Animals were considered to be refuging if they were observed atleast partially sheltering within/under substrate at the time of encounter.
Sediment processing
In the laboratory, the seawater with each sample of faecal cast and ambient surface sediments was decanted on the day following collection and samples were rinsed with tap water three times to remove the majority of salts (3-h settling time between rinses; Purcell 1996). Samples were dried in an oven at 60 °C to constant weight before being standardised through a 2000-µm sieve (Tebbett et al. 2022) and weighed to the nearest 0.001 g using an electronic analytical balance. The dry weight of faecal cast samples was used as a proxy for the amount of sediment reworked (Hammond 1982). A standard bleaching procedure (see Tebbett et al. 2022) with minor modification was used to quantify the percentage of organic and inorganic material in the samples. Hydrogen peroxide (30%) was added slowly to the samples over ten days until no new bubbles formed (Schumacher 2002). The fluid was decanted from the sample vials, and the samples were then dried and weighed as before to determine the inorganic particulate mass. The grain size composition of samples was quantified by dry sieving through a sieve stack (1000, 500, 250, 125, 63-µm) for 2 min (Purcell 1996, 2000). The sediment retained in each fraction was weighed to calculate the percentage grain size composition.
Mathematical procedures
Sea cucumber displacement rates (cm h−1) and turning angles (°) for each observation period were derived from the raw movement data. Displacement rates were calculated by dividing linear displacement (cm) between successive movement steps by the time (hours) within the given observation period. Turning angles were calculated by subtracting the compass bearing for the previous observation period from the bearing for the subsequent observation period. Movement steps were assumed to follow a straight path, likely underestimating displacement and tortuosity (Turchin et al. 1991; Estevez and Christman 2006). The sea cucumbers movement paths were plotted using trigonometry in SigmaPlot V14, and individuals’ net (Euclidian) displacements throughout the 12-h monitoring periods were calculated. The mean cosine and sine of turn angles furnished further metrics used in other movement studies.
Angular dispersion (AD) was used as a proxy for the tortuosity of sea cucumbers movement paths, i.e., the degree to which a movement path differs from a straight line (Batschelet 1981; Fisher 1995). This parameter ranges from 0 (high tortuosity) to 1 (straight line movement; Fisher 1995; Miller et al. 2011) and was calculated for individual movement paths, as per the below equation:
where \(n\) is the number of moves (i.e., trajectories) in the movement path, and the constants \(C\) and \(S\) are the sum of all respective cosines and sines of turning angles.
The geometric mean grain size and sorting of the faecal cast and surrounding surface sediment samples were determined from the percentage grain size composition using GRADISTAT V8.0 (Blott and Pye 2001). All unspecified sediments (< 63 µm) were assumed to be > 1 µm.
Statistical analyses
The average hourly displacement rate (cm h−1) and tortuosity (AD) data were analysed by a two-factor mixed-model permutational analysis of covariance (PERMANCOVA) in PRIMER v7 based on Euclidean distances (similarity) (Anderson et al. 2008). The two reef systems, One Tree Reef and Heron Island Reef, are separated by 7 km and were considered ‘Locations’, with the two sites within each Location about 30–50 m apart. Location (OTR and HIR) was treated as a fixed factor; site was considered as a random factor, nested within the Location; and sea cucumber body length (cm) was the covariate. Individual sea cucumbers were considered as replicates (n = 41). A one-way PERMANCOVA analysed defecation rates of the sea cucumbers from the three study sites with the same design structure (n = 18). This second analysis did not include the data from HIR Site 2, as only a single faecal cast sample could be collected from this site. Datasets were Z-score normalised prior to analyses (Anderson et al. 2008) and PERMANCOVAs were tested under the reduced model using 9999 permutations, with Type I sequential sum-of-squares. The assumption of homogeneity of variance was met for all datasets.
To compare the granulometric characteristics of faecal casts (n = 19) and surface sediments (n = 19), data from all sites were combined. Granulometric parameters (% organic matter, mean grain size and sorting) and percentage grain size fractions (> 1000 & > 500; > 250 & > 125; > 63 & < 63 μm) were compared using separate Paired Samples t-Tests using IBM SPSS statistics v.28 software (IBM Corp 2021). Granulometric parameters were log10 transformed, and percentage grain size fractions were arcsine transformed prior to analyses to improve normality. Wilk–Shapiro tests confirmed that each dataset followed a normal distribution. For all tests, α = 0.05.
To determine whether sediment organic content influenced movement and sediment turnover, separate nonlinear regression analyses were used to test the potential relationships between sediment organic content (% dry wt.) and (a) displacement rate (cm h−1) and (b) defecation rates (g h−1). Although the PERMANCOVA analyses applied the covariate linearly, body size may have influenced response variables in a curvilinear fashion that warranted further examination. Hence, nonlinear regressions were also run for the following variable pairs: sea cucumber body length (cm) and (a) average displacement rate, (b) tortuosity (AD), (c) defecation rates, and (d) refuge dimensions (width, height, and depth; cm). All analyses were undertaken using DataFit v9 software (Oakdale Engineering 2009). Data from the third observation period were used for all equations concerning surface sediment organic content. For all relationships, the goodness of fit was compared across the 298 equation forms available in the software, and the 2-parameter models with the highest r-squared value were identified in order to avoid overfitting with polynomial models. Only statistically significant relationships (P ≤ 0.05) are graphed.
Results
Displacement
Displacement rates of individual Stichopus cf. monotuberculatus over the 12-h monitoring periods were variable, ranging from 7 to 46 cm h−1. Across all sites, movement rates averaged 22 cm h−1 (± 1.3 SE, n = 41; Fig. 3a), and these did not vary between Locations (F1,36 = 0.857, P = 0.631; Table S1) but did differ at the scale of sites within Locations (F2,36 = 3.963, P = 0.028). Since site was a random factor, the sampling design presents no grounds for post-hoc testing here, although the differences are apparent in Fig. 3a.
a Average displacement rates (cm h−1 ± SE) of Stichopus cf. monotuberculatus across all observation periods at One Tree Reef (OTR) Sites 1 and 2, and Heron Island Reef (HIR) Sites 1 and 2. b Average displacement rates (± SE) throughout observation periods. Data are plotted at the midpoint of observation periods; purple shading represents night-time and yellow represents daytime the following morning. c Proportion of sea cucumbers not sheltering. Data are plotted at the midpoint of observation periods. n = 10 at OTR Site 1; n = 9 at OTR Site 2; n = 11 at HIR Site 1 and 2
The diurnal trends in displacement rates varied among individuals, sites, and observation periods (Fig. 3b). Nonetheless, at OTR Site 2 and HIR Site 2, the sea cucumbers displaced less from 04:30 to 06:45 relative to all other observation periods. These lower displacement rates coincided with our observations of individuals beginning to shelter before sunrise (Fig. 3c). Comparatively, at OTR Site 1 and HIR Site 1, displacement rates were somewhat variable across all observation periods, despite the sea cucumbers refuging at similar times.
Larger S. cf. monotuberculatus tended to displace more compared to smaller individuals, but this relationship was non-significant (r2 = 0.06, F1,36 = 2.12, P = 0.15; Table S2). Further, the sea cucumbers moved faster where the organic content in the sediment was lowest (r2 = 0.53, F1,17 = 18.94, P < 0.001; Fig. S1, Table S3).
Movement paths and tortuosity
The length of moves and turning angles of S. cf. monotuberculatus movement paths were variable among individuals (Fig. S2). Across the study sites, individuals displaced an average net distance of 178.5 cm (± 29.5 SE, n = 36) from their origin points. After emerging from their refuges, the sea cucumbers were observed feeding on sandy areas in close proximity to patches of live coral, rubble, and boulders. Stichopus cf. monotuberculatus appeared to forage with low directional persistence, regularly reversing their direction of travel before sheltering in reef structures in the early morning.
The sea cucumbers movement paths were moderately tortuous (Fig. 4a), with the direction of travel seeming unrelated to previous movement steps. The tortuosity of movement paths did not differ between OTR and HIR (F1,34 = 0.03, P = 0.838; Table S4) but differed among sites within Locations (F2,34 = 5.35, P = 0.01). Smaller sea cucumbers exhibited more tortuous movement paths than larger individuals (r2 = 0.10, F1,37 = 4.15, P = 0.049; Fig. 4b; Table S5).
a Average tortuosity (AD ± SE) of Stichopus cf. monotuberculatus movement paths at One Tree Reef (OTR) Sites 1 and 2, and Heron Island Reef (HIR) Sites 1 and 2. Only paths with ≥ 3 movement steps are included. n = 10 at OTR Site 1, HIR Site 1 and 2; n = 9 at OTR Site 2. b Nonlinear relationship between body length \(x\) (cm) and tortuosity \(y\) (AD) for S. cf. monotuberculatus at the four study sites; n = 39. Black solid line is the fitted equation \(y = 1.255 - 2.486/{\text{ln}}\left( x \right)\); blue dashed lines are 95% CIs
Feeding and sediment turnover
Sediment defecation rates of S. cf. monotuberculatus across all sites averaged 7.8 g h−1 (± 1 SE, n = 19), ranging from 1.2 to 16.1 g h−1 and were similar among sites (F2,14 = 0.271, P = 0.763; Fig. 5a; Table S6). Relative to their body size, larger sea cucumbers defecated greater volumes of sediment than smaller ones (r2 = 0.33, F1,16 = 7.74, P = 0.013; Fig. 5b; Table S7). The availability of organic matter in the surrounding surface sediments had little effect on defecation rates (r2 = 0.09, F1,17 = 1.75, P = 0.204).
a Average defecation rate (g h−1 ± SE) of Stichopus cf. monotuberculatus at One Tree Reef (OTR) Sites 1 and 2, and Heron Island Reef (HIR) Sites 1 and 2. n = 6 at OTR Sites 1 and 2 and HIR Site 1; n = 1 at HIR Site 2. b nonlinear relationship between body length \(x\) (cm) and defecation rate \(y\) (g h−1) at the four sites (n = 19). Black solid line is the fitted equation \(y = 2.711 + 0.002x^{2.5}\); blue dashed lines are 95% CIs
The faecal cast samples contained a significantly greater proportion of very fine sand and silt (t18 = 3.99, P < 0.001) and coarse sand (t18 = 4.16, P < 0.001) than what was available in the surrounding surface sediments (Fig. 6; Table S8). There was no significant difference in the proportion of medium and fine sand between the faecal casts and the ambient (t18 = 0.99, P = 0.337). Defecated sediments had a finer mean grain size (t18 = 5.22, P < 0.001) and were more well-sorted (t18 = 2.42, P = 0.026) relative to the ambient surface sediments. The faecal cast samples had a significantly higher organic content than the samples of surrounding sediments (t18 = 5.32, P < 0.001).
Refuging and homing behaviours
The dimensions (height, width, and depth) and microhabitat types of refuges that S. cf. monotuberculatus occupied varied among the study sites, largely due to differences in local habitat structure (Table S9; Fig. S3). At OTR, most of the sea cucumbers emerged from and returned to shelters under live Isopora sp. colonies, where they were present in the habitat. At HIR, Isopora sp. was not common, and most of the S. cf. monotuberculatus sheltered under coral rubble and in rocky crevices. Individuals that homed back to their shelters (i.e., exhibited refuge fidelity) tended to occupy relatively narrower live coral shelters compared to those that did not ‘home’.
Larger S. cf. monotuberculatus tended to occupy deeper refuges (i.e., lateral intrusion into reef structures) relative to smaller individuals (r2 = 0.23, F1,21 = 6.23, P = 0.021; Fig. 7; Table S10). However, there was no relationship between animal body length and refuge height (r2 = 0.03, F1,21 = 0.64, P = 0.43) or body width and refuge width (r2 = 0.02, F1,21 = 0.4, P = 0.53).
Discussion
In one of the few studies of a nocturnal sea cucumber (see Hammond 1982; Navarro et al. 2013), we successfully tracked the movement and determined sediment turnover by individual Stichopus cf. monotuberculatus over short temporal scales. Such studies are rare for nocturnal species due to the challenges of working intermittently throughout the night. The variable and distinctive colour patterns of S. cf. monotuberculatus proved to be a useful trait for tracking individuals over short temporal scales and could also be applied to other nocturnal holothuroids (e.g., Stichopus horrens). Our study shows that S. cf. monotuberculatus exhibits a distinct diel pattern of diurnal sheltering and nocturnal foraging. Our observations of nocturnal activity in natural habitats concur with studies in captivity (Chen et al. 2022) and on artificial reefs (Xu et al. 2022), implying that future population assessments of S. cf. monotuberculatus would best be undertaken approximately one hour after sunset when most of the animals have emerged. The movement and feeding data presented here help to characterise the functional role of S. cf. monotuberculatus. This is also the first study to collect data on the refuge sizes and types occupied by sea cucumbers, providing valuable insight into their sheltering behaviours.
Movement and foraging patterns
Our tracking data indicate that S. cf. monotuberculatus displaces relatively small net distances, undertaking short diel foraging migrations around nearby reef shelters. Hence, movement is likely constrained by the spatial arrangement of refuges, with individuals unlikely to disperse large distances over unbounded habitats. The average hourly displacement rate determined (22 cm h−1) is comparable to that for S. chloronotus (24 cm h−1; Uthicke 2001b), which has a similar body size. Our data suggest that S. cf. monotuberculatus populations may be relatively well protected by spatial management measures that are sized similarly to those implemented for other small- to medium-bodied holothuroids. Determining whether the species exhibits long-term site fidelity would have important implications for gauging the potential spillover of this and other similar species into adjacent fishing grounds (see Grüss et al. 2011). However, assessing the movement patterns of S. cf. monotuberculatus over longer periods is difficult due to asexual reproduction through fission (Uthicke 2001a).
There was no obvious peak in movement rates of the S. cf. monotuberculatus over the night. This finding contrasts with other nocturnal holothuroids, such as Actinopyga mauritiana, which tend to displace further in the middle of the night (Graham and Battaglene 2004), and Holothuria sanctori that ‘rush’ before sunrise in search of refuges (Navarro et al. 2013). As for the closely related cryptic holothuroid Stichopus horrens (Palomar-Abesamis et al. 2018), the underlying cue for emergence in S. cf. monotuberculatus is likely to be light intensity. Thus, the activity patterns of these species are likely to vary seasonally and geographically.
While there is a general trend among marine species for larger individuals to be more mobile than smaller ones (Quinn and Brodeur 1991; Allen et al. 2018), movement rates of S. cf. monotuberculatus were comparable across body sizes. We posit that increased body size of S. cf. monotuberculatus enhances mobility by allowing larger individuals to displace directly over reef features, resulting in more linear movement paths. This coincides with our observations of large individuals climbing across the top of corals and boulders. Typically, smaller animals are more impeded by rugose substrata and require more tortuous paths to navigate their surroundings (Prevedello et al. 2010; Mancinelli and Pasquali 2016). The relationship between tortuosity and body size suggests that the more linear movement of larger individuals might translate to weaker fidelity to specific refuges and better chances of accessing foraging areas further from reef structures. More linear paths of larger individuals might also increase their chances of spilling over from within marine reserves into adjacent fishing grounds, although movement distances in this study were still rather short.
Feeding and ecological roles
The average hourly sediment reworking rate of S. cf. monotuberculatus (7.8 g h−1) is comparable to that of other stichopodids, such as S. chloronotus (11.5 g h−1; Uthicke 1999) and Isostichopus badionotus (2.8–4.9 g h−1; Hammond 1982); but lower than for larger-bodied species, such as S. herrmanni at the same locality (11.7–28.6 g h−1; Wolfe and Byrne 2017) and Thelenota anax (34 g h−1; Hammond et al. 2020). It appeared that S. cf. monotuberculatus exhibited a 10-h daily feeding cycle. We estimate that each individual reworks on average 28.5 kg of surface sediment each year, although this turnover rate is likely moderated by seasonal variations in feeding activity (see Wolfe and Byrne 2017). As shown for other sea cucumbers that occur at high densities on unfished reefs (Uthicke 2001a; Williamson et al. 2021; Grayson et al. 2022), deposit-feeding by S. cf. monotuberculatus is likely to strongly influence the sediment biome in coral reef ecosystems.
Based on the model which explains defecation rates as a function of S. cf. monotuberculatus body size, we can predict that an individual 15 cm long processes 4.5 g sediment h−1, while one 30 cm long processes 12.6 g h−1. This exponential relationship suggests that removing large individuals through fishing may disproportionately impact ecosystem function. Further, this means that larger animals would be more efficient at remediating waste from integrated aquaculture due to the greater rates of sediment turnover.
While compensatory feeding on patches of lower organic content is reported for some holothuroids (e.g., Yingst 1982; Hammond et al. 2020), sediment organic content did not noticeably influence sediment turnover of S. cf. monotuberculatus. The variation in sediment processing rates among the reef sites highlights the need for further studies to identify factors influencing sediment turnover by holothuroids, as this will have implications for sediment health and benthic productivity, and for predictions about their influence in aquaculture settings.
Stichopus cf. monotuberculatus selectively ingests finer-grained particles (< 63–250 μm) and avoids ingesting coarser grains (≥ 500 μm), as found for other deposit-feeding sea cucumbers (review by Pierrat et al. 2022). This feeding selectivity may be influenced by the higher availability of organic content of smaller sediment size fractions (Jones and Jordan 1979). This finding highlights the need to supply fine sediments for pond culture and to select sites with fine granulometry for sea ranching. Moreover, by partially digesting and reconcentrating organic matter into faecal casts, S. cf. monotuberculatus is likely to provide an enriched substrate for bacterial remineralisation, as shown for other holothuroids (MacTavish et al. 2012; Purcell et al. 2016a).
Refuging and homing behaviours
Despite utilising a broad range of refuge types, our data suggest that S. cf. monotuberculatus occupied shelters non-randomly, with larger individuals inhabiting deeper refuges. Similar size relationships have been established for various crustaceans (e.g., Forsythe et al. 2003; Booth and Ayers 2005) and are related to the variable protective capacity of refuges across body sizes (Eggleston et al. 1990). With the projected decrease in reef substratum rugosity resulting from anthropogenically driven climate change (Rogers et al. 2014), our data suggest that reduced availability of suitably sized refuges might impact population size and structure of refuging sea cucumbers, such as S. cf. monotuberculatus.
Understanding the spatial distribution and demography of sea cucumber populations and the factors that influence their use of refuges is important for stock assessment and management (Félix et al. 2021; Azevedo e Silva et al. 2023). Homing S. cf. monotuberculatus (at OTR Site 2) tended to occupy relatively narrower shelters, suggesting shelter ‘quality’ may have influenced refuge fidelity. Hammond (1982) observed a similar behaviour for Holothuria thomasi, suggesting that shelters with close access to large sand patches were preferred. These observations could be incorporated into sea ranching of sea cucumbers, where provision of artificial structures with narrow openings are used to promote site fidelity, with shelter depth tailored to the size of animals being grown (Hu et al. 2021; Tian et al. 2023).
Conclusion
Here, we have shown that S. cf. monotuberculatus turns over similar volumes of sediments to that determined for diurnal sea cucumbers. Thus, often overlooked nocturnal sea cucumbers may fulfil similar ecological roles to diurnally active species. Overharvest of S. cf. monotuberculatus and other nocturnal holothuroids would lead to diminished sediment turnover on coral reef systems. Overall, the sea cucumbers displaced short net distances, with movement largely confined to foraging around reef substrata. Individuals of this species are unlikely to disperse large distances over open sandy habitats, and hence, spatial protection measures may be an effective tool for conserving S. cf. monotuberculatus populations. Stichopus cf. monotuberculatus appears to utilise available hard substrate for shelter, with the size of individuals governing refuge selection within the reef structures. This correlation between body size and reef refuge depth provides insight into factors that drive patterns of holothuroid sheltering. Finally, projected declines in reef structural complexity have implications for sea cucumber populations and their fisheries in a changing ocean.
Data availability
The datasets generated and analysed in this study are available from the corresponding author on reasonable request.
References
AIMS (2023) Research Data Platform: Temperature Loggers. https://www.aims.gov.au/data accessed 30 July 2023
Allen RM, Metaxas A, Snelgrove PV (2018) Applying movement ecology to marine animals with complex life cycles. Annu Rev Mar Sci 10:19–42. https://doi.org/10.1146/-annurev-marine-121916-063134
Anderson M, Gorley RN, Clarke RK (2008) Permanova+ for primer: Guide to software and statistical methods. Primer-E Limited
Azevedo e Silva F, Brito AC, Pombo A, Simões T, Marques TA, Rocha C, ... & Félix PM (2023) Spatiotemporal distribution patterns of the sea cucumber Holothuria arguinensis on a rocky-reef coast (northeast Atlantic). Estuaries Coast 46:1035–1045. https://doi.org/10.1007/s12237-023-01201-1
Batschelet E (1981) Circular statistics in biology. Academic Press, New York, USA
Beck MW (1995) Size-specific shelter limitation in stone crabs: a test of the demographic bottleneck hypothesis. Ecology 76:968–980. https://doi.org/10.2307/1939360
Blott SJ, Pye K (2001) GRADISTAT: a grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf Proc Land 26:1237–1248. https://doi.org/10.1002/esp.261
Booth JD, Ayers D (2005) Characterising shelter preferences in captive juvenile Jasus edwardsii (Palinuridae). New Zeal J Mar Fresh 39:373–382. https://doi.org/10.1080/00288330.2005.9517318
Byrne M, Rowe F, Uthicke S (2010) Molecular taxonomy, phylogeny and evolution in the family Stichopodidae (Aspidochirotida: Holothuroidea) based on COI and 16S mitochondrial DNA. Mol Phylogenet Evol 56:1068–1081. https://doi.org/10.1016/-j.ympev.2010.04.013
Chen M, Sun S, Xu Q, Gao F, Wang H, Wang A (2022) Influence of water temperature and flow velocity on locomotion behavior in tropical commercially important sea cucumber Stichopus monotuberculatus. Front Mar Sci 9:931430. https://doi.org/10.3389/fmars.2022.931430
Cheng C, Wu F, Ren C, Jiang X, Zhang X, Li X, Luo P, Hu C, Chen T (2021) Aquaculture of the tropical sea cucumber, Stichopus monotuberculatus: Induced spawning, detailed records of gonadal and embryonic development, and improvements in larval breeding by digestive enzyme supply in diet. Aquaculture 540:736690. https://doi.org/10.1016/j.aquaculture.2021.736690
Conand C (1991) Long-term movements and mortality of some tropical sea-cucumbers monitored by tagging and recapture. In: Yanagisawa, Yasumasu, Oguro, Suzuki and Motokawa (eds) Biology of Echinodermata. Balkema, Rotterdam, pp 169–175
Da Silva J, Cameron JL, Fankboner PV (1986) Movement and orientation patterns in the commercial sea cucumber Parastichopus californicus (Stimpson) (Holothuroidea: Aspidochirotida). Mar Freshw Behav Phy 12:133–147. https://doi.org/10.1080/10236248609378640
Eggleston DB, Lipcius RN, Miller DL, Coba-Cetina L (1990) Shelter scaling regulates survival of juvenile Caribbean spiny lobster Panulirus argus. Mar Ecol Prog Ser 62:79–88. https://doi.org/10.3354/meps062079
Eriksson H, Friedman K, Solofa A, Mulipola A (2007) A pilot study to investigate the survival of Stichopus horrens after viscera harvest in Samoa. SPC Beche-De-Mer Inf Bull 26:2–4
Eriksson H, Thorne BV, Byrne M (2013) Population metrics in protected commercial sea cucumber populations (curryfish: Stichopus herrmanni) on One Tree Reef, Great Barrier Reef. Mar Ecol Prog Ser 473:225–234. https://doi.org/10.3354/meps10054
Estevez I, Christman MC (2006) Analysis of the movement and use of space of animals in confinement: The effect of sampling effort. Appl Anim Behav Sci 97:221–240. https://doi.org/10.1016/j.applanim.2005.01.013
Félix PM, Pombo A, AzevedoeSilva F, Simões T, Marques TA, Melo R, Rocha C, Sousa J, Venâncio E, Costa JL (2021) Modelling the distribution of a commercial NE-Atlantic sea cucumber, Holothuria mammata: demographic and abundance spatio-temporal patterns. Front Mar Sci 8:675330. https://doi.org/10.3389/fmars.2021.675330
Fisher NI (1995) Statistical Analysis of Circular Data. Cambridge University Press, Cambridge, UK
Fontoura L, Zawada KJ, D’agata S, Álvarez-Noriega M, Baird AH, Boutros N, Dornelas M, Luiz OJ, Madin JS, Maina JM (2020) Climate-driven shift in coral morphological structure predicts decline of juvenile reef fishes. Glob Change Biol 26:557–567. https://doi.org/10.1111/gcb.14911
Forsythe PS, Wyatt DS, Switzer PV (2003) Effects of experience and body size on refuge choice in the crayfish Orconectes immunis. J Freshwater Ecol 18:305–313. https://doi.org/10.1080/02705060.2003.9664497
Graham JC, Battaglene SC (2004) Periodic movement and sheltering behaviour of Actinopyga mauritiana (Holothuroidea: Aspidochirotidae) in Solomon Islands. SPC Beche-De-Mer Inf Bull 19:23–31
Gray BCT, Calvert LA, Purcell SW (2022) Short-term movement dynamics of the world’s largest sea cucumbers (genus Thelenota). Mar Ecol 43:e12705. https://doi.org/10.1111/maec.12705
Gray BC, Byrne M, Clements M, Foo SA, Purcell SW (2023) Length-weight relationship for the dragonfish, Stichopus cf. monotuberculatus (Holothuroidea). Fish Res 268:106851
Grayson N, Clements CS, Towner AA, Beatty DS, Hay ME (2022) Did the historic overharvesting of sea cucumbers make coral more susceptible to pathogens? Coral Reefs 41:447–453. https://doi.org/10.1007/s00338-022-02227-w
Grüss A, Kaplan DM, Guénette S, Roberts CM, Botsford LW (2011) Consequences of adult and juvenile movement for marine protected areas. Biol Conserv 144:692–702. https://doi.org/10.1016/j.biocon.2010.12.015
Hammond AR, Meyers L, Purcell SW (2020) Not so sluggish: movement and sediment turnover of the world’s heaviest holothuroid, Thelenota anax. Mar Biol 167:1–9. https://doi.org/10.1007/s00227-020-3671-5
Hammond AR, Purcell SW (2023) Limited long-term movement and slow growth of the sea cucumber Pearsonothuria graeffei. Mar Ecol Prog Ser 704:1–14. https://doi.org/10.3354/meps14240
Hammond L (1982) Patterns of feeding and activity in deposit-feeding holothurians and echinoids (Echinodermata) from a shallow back-reef lagoon, Discovery Bay, Jamaica. Bull Mar Sci 32:549–571
Hastings A, Botsford LW (2003) Comparing designs of marine reserves for fisheries and for biodiversity. Ecol Appl 13:65–70. https://doi.org/10.1890/1051-0761
Hernandez-Lamb J, Dibello A, Lewis S, Mackin G, Kirby K, Acosta C (2012) Modelling the effects of reserve size and fishing mortality for Caribbean queen conch Strombus gigas. Aquat Conserv 22:721–730. https://doi.org/10.1002/aqc.2271
Hu F, Ding P, Yu Y, Wen B, Cui Z, Yang M, Chi X, Sun J, Luo J, Sun Z (2021) Effects of artificial reefs on selectivity and behaviors of the sea cucumber Apostichopus japonicas: New insights into the pond culture. Aquac Rep 21:100842. https://doi.org/10.1016/j.aqrep.2021.100842
IBM Corp (2021) IBM SPSS Statistics for Windows, Version 28.0. In: Corp I (ed), Armonk, NY
Jones G, Jordan M (1979) The distribution of organic material and trace metals in sediments from the River Liffey Estuary, Dublin. Estuar Coast Mar Sci 8:37–47. https://doi.org/10.1016/0302-3524(79)90104-X
Kristensen ML, Olsen EM, Moland E, Knutsen H, Grønkjær P, Koed A, Källo K, Aarestrup K (2021) Disparate movement behavior and feeding ecology in sympatric ecotypes of Atlantic cod. Ecol Evol 11:11477–11490. https://doi.org/10.1002/ece3.7939
Lee S, Ferse SC, Ford A, Wild C, Mangubhai S (2017) Effect of sea cucumber density on the health of reef-flat sediments Fiji’s Sea Cucumber Fishery: Advances in Science for Improved Management. Wildlife Conservation Society. Report No. 01/17, pp 54–61
MacTavish T, Stenton-Dozey J, Vopel K, Savage C (2012) Deposit-feeding sea cucumbers enhance mineralization and nutrient cycling in organically-enriched coastal sediments. PLoS ONE 7:e50031. https://doi.org/10.1371/journal.pone.0050031
Mancinelli G, Pasquali V (2016) Body size-related constraints on the movement behaviour of the arctic notostracan Lepidurus arcticus (Pallas, 1973) under laboratory conditions. Rend Lincei-Sci Fis 27:207–215. https://doi.org/10.1007/s12210-016-0512-z
Massin C (1982) Effects of feeding on the environment: Holothuroidea. In Jangoux and Lawrence (eds) Echinoderm Nutrition. AA Balkema, Rotterdam, pp 493–497
Ménard A, Turgeon K, Kramer DL (2008) Selection of diurnal refuges by the nocturnal squirrelfish, Holocentrus rufus. Environ Biol Fish 82:59–70. https://doi.org/10.1007/s10641-007-9253-2
Mercier A, Battaglene SC, Hamel J-F (1999) Daily burrowing cycle and feeding activity of juvenile sea cucumbers Holothuria scabra in response to environmental factors. J Exp Mar Biol Ecol 239:125–156. https://doi.org/10.1016/S0022-0981(99)00034-9
Miller C, Christman MC, Estevez I (2011) Movement in a confined space: Estimating path tortuosity. Appl Anim Behav Sci 135:13–23. https://doi.org/10.1016/j.applanim.2011.09.002
Moriarty D (1982) Feeding of Holothuria atra and Stichopus chloronotus on bacteria, organic carbon and organic nitrogen in sediments of the Great Barrier Reef. Mar Freshw Res 33:255–263. https://doi.org/10.1071/MF9820255
Moriarty D, Pollard P, Hunt W, Moriarty C, Wassenberg T (1985) Productivity of bacteria and microalgae and the effect of grazing by holothurians in sediments on a coral reef flat. Mar Biol 85:293–300. https://doi.org/10.1007/BF00393250
Navarro J, Sáez-Liante R, Albo-Puigserver M, Coll M, Palomera I (2017) Feeding strategies and ecological roles of three predatory pelagic fish in the western Mediterranean Sea. D Sea Res II: Top Stu Oceano 140:9–17. https://doi.org/10.1016/j.dsr2.2016.06.009
Navarro P, García-Sanz S, Barrio J, Tuya F (2013) Feeding and movement patterns of the sea cucumber Holothuria sanctori. Mar Biol 160:2957–2966. https://doi.org/10.1007/s00227-013-2286-5
Navarro PG, García-Sanz S, Tuya F (2014) Contrasting displacement of the sea cucumber Holothuria arguinensis between adjacent nearshore habitats. J Exp Mar Biol Ecol 453:123–130. https://doi.org/10.1016/j.jembe.2014.01.008
Oakdale Engineering (2009) Datafit Oakdale Engineering, Oakdale PA, USA
Palomar-Abesamis N, Juinio-Meñez MA, Slater MJ (2018) Effects of light and microhabitat on activity pattern and behaviour of wild and hatchery-reared juveniles of Stichopus cf. horrens. J Mar Biol Assoc UK 98:1703–1713. https://doi.org/10.1017/S0025315417000972
Pan Y, Zhang L, Lin C, Sun J, Kan R, Yang H (2015) Influence of flow velocity on motor behavior of sea cucumber Apostichopus japonicus. Physiol Behav 144:52–59. https://doi.org/10.1016/j.physbeh.2015.02.046
Pierrat J, Bédier A, Eeckhaut I, Magalon H, Frouin P (2022) Sophistication in a seemingly simple creature: a review of wild holothurian nutrition in marine ecosystems. Biol Rev 97:273–298. https://doi.org/10.1111/brv.12799
Pittman S, McAlpine C (2003) Movements of marine fish and decapod crustaceans: process, theory and application. Adv Mar Biol 44:205–294. https://doi.org/10.1016/s0065-2881(03)44004-2
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Syst:289–316
Prevedello JA, Forero-Medina G, Vieira MV (2010) Movement behaviour within and beyond perceptual ranges in three small mammals: effects of matrix type and body mass. J Anim Ecol 79:1315–1323. https://doi.org/10.1111/j.1365-2656.2010.01736.x
Purcell SW (1996) A direct method for assessing sediment load in epilithic algal communities. Coral Reefs 15:211–213. https://doi.org/10.1007/BF01787453
Purcell SW (2000) Association of epilithic algae with sediment distribution on a windward reef in the northern Great Barrier Reef, Australia. Bull Mar Sci 66:199–214
Purcell SW, Conand C, Uthicke S, Byrne M (2016a) Ecological roles of exploited sea cucumbers. Oceanogr Mar Biol 54:367–386. https://doi.org/10.1201/9781315368597-8
Purcell SW, Lovatelli A, González-Wangüemert M, Solís-Marín FA, Samyn Y, Conand C (2023a) Commercially important sea cucumbers of the world, 2nd Edition. FAO Species Catalogue for Fishery Purposes 6, FAO, Rome, Italy
Purcell SW, Lovatelli A, Vasconcellos M, Ye Y (2010) Managing sea cucumber fisheries with an ecosystem approach. FAO Fisheries and Aquaculture Technical Paper No 520, FAO, Rome, Italy
Purcell SW, Mercier A, Conand C, Hamel JF, Toral-Granda MV, Lovatelli A, Uthicke S (2013) Sea cucumber fisheries: global analysis of stocks, management measures and drivers of overfishing. Fish Fish 14:34–59. https://doi.org/10.1111/j.1467-2979.2011.00443.x
Purcell SW, Piddocke TP, Dalton SJ, Wang YG (2016b) Movement and growth of the coral reef holothuroids Bohadschia argus and Thelenota ananas. Mar Ecol Prog Ser 551:201–214. https://doi.org/10.3354/meps11720
Purcell SW, Polidoro BA, Hamel J-F, Gamboa RU, Mercier A (2014b) The cost of being valuable: predictors of extinction risk in marine invertebrates exploited as luxury seafood. P Roy Soc B-Biol Sci 281:20133296. https://doi.org/10.1098/rspb.2013.3296
Purcell SW, Rallings SL, Hammond AR (in press) Long-term home ranging in the large sea cucumber, Holothuria fuscopunctata. Coral Reefs
Quinn TP, Brodeur RD (1991) Intra-specific variations in the movement patterns of marine animals. Am Zool 31:231–241. https://doi.org/10.1093/icb/31.1.231
Rogers A, Blanchard JL, Mumby PJ (2014) Vulnerability of coral reef fisheries to a loss of structural complexity. Curr Biol 24:1000–1005. https://doi.org/10.1016/j.cub.2014.03.026
Schneider K, Silverman J, Kravitz B, Rivlin T, Schneider-Mor A, Barbosa S, Byrne M, Caldeira K (2013) Inorganic carbon turnover caused by digestion of carbonate sands and metabolic activity of holothurians. Estuar Coast Shelf S 133:217–223. https://doi.org/10.1016/j.ecss.2013.08.029
Schneider K, Silverman J, Woolsey E, Eriksson H, Byrne M, Caldeira K (2011) Potential influence of sea cucumbers on coral reef CaCO3 budget: A case study at One Tree Reef. J Geophys Res Biogeo 116. https://doi.org/10.1029/2011JG001755
Schumacher BA (2002) Methods for the determination of total organic carbon (TOC) in soils and sediments. United States Environmental Protection Agency, Las Vegas
Tebbett SB, Sgarlatta MP, Pessarrodona A, Vergés A, Wernberg T, Bellwood DR (2022) How to quantify algal turf sediments and particulates on tropical and temperate reefs: An overview. Mar Environ Res:105673. https://doi.org/10.1016/j.marenvres.2022.105673
Tian R, Hu F, Wu G, Wang H, Ding J, Chang Y, Zhao C (2023) An effective approach to improving fitness-related behavior and digestive ability of small sea cucumbers Apostichopus japonicus at high temperature: New insights into seed production. Aquaculture 562:738755. https://doi.org/10.1016/j.aquaculture.2022.738755
Turchin P, Odendaal F, Rausher M (1991) Quantifying insect movement in the field. Environ Entmol 20:955–963. https://doi.org/10.1093/ee/20.4.955
Tuya F, Martin J, Luque A (2004) Patterns of nocturnal movement of the long-spined sea urchin Diadema antillarum (Philippi) in Gran Canaria (the Canary Islands, central East Atlantic Ocean). Helgoland Mar Res 58:26–31. https://doi.org/10.1007/s10152-003-0164-0
Uthicke S (1999) Sediment bioturbation and impact of feeding activity of Holothuria (Halodeima) atra and Stichopus chloronotus, two sediment feeding holothurians, at Lizard Island, Great Barrier Reef. Bull Mar Sci 64:129–141
Uthicke S (2001a) Interactions between sediment-feeders and microalgae on coral reefs: grazing losses versus production enhancement. Mar Ecol Prog Ser 210:125–138. https://doi.org/10.3354/meps210125
Uthicke S (2001b) Nutrient regeneration by abundant coral reef holothurians. J Exp Mar Biol Ecol 265:153–170. https://doi.org/10.1016/S0022-0981(01)00329-X
Uthicke S (2004) Overfishing of holothurians: lessons from the Great Barrier Reef. In: Advances in Sea Cucumber Aquaculture and Management, FAO, Rome, Italy, pp 163–172
Uthicke S, Karez R (1999) Sediment patch selectivity in tropical sea cucumbers (Holothurioidea: Aspidochirotida) analysed with multiple choice experiments. J Exp Mar Biol Ecol 236:69–87. https://doi.org/10.1016/S0022-0981(98)00190-7
Uthicke S, Welch D, Benzie J (2004) Slow growth and lack of recovery in overfished holothurians on the Great Barrier Reef: evidence from DNA fingerprints and repeated large-scale surveys. Cons Biol 18:1395–1404. https://doi.org/10.1111/j.1523-1739.2004.00309.x
Williamson JE, Duce S, Joyce KE, Raoult V (2021) Putting sea cucumbers on the map: projected holothurian bioturbation rates on a coral reef scale. Coral Reefs 40:559–569. https://doi.org/10.1007/s00338-021-02057-2
Wolfe K, Byrne M (2017) Biology and ecology of the vulnerable holothuroid, Stichopus herrmanni, on a high-latitude coral reef on the Great Barrier Reef. Coral Reefs 36:1143–1156. https://doi.org/10.1007/s00338-017-1606-5
Wolfe K, Byrne M (2022) Overview of the Great Barrier Reef sea cucumber fishery with focus on vulnerable and endangered species. Biol Cons 266:109451. https://doi.org/10.1016/j.biocon.2022.109451
Wolfe K, Vidal-Ramirez F, Dove S, Deaker D, Byrne M (2018) Altered sediment biota and lagoon habitat carbonate dynamics due to sea cucumber bioturbation in a high-pCO2 environment. Glob Change Biol 24:465–480. https://doi.org/10.1111/gcb.13826
Woo SP, Kajihara H, Byrne M, Fujita T (2019) Description and phylogenetic relationships of a new genus of sea cucumbers from Australia, with two new combinations (Holothuroidea, Stichopodidae). Mar Biodivers 49:2499–2518. https://doi.org/10.1007/s12526-019-00974-8
Xu Q, Wu P, Huang D, Xiao Y, Wang X, Xia J, Ma W, Gao F, Wang A (2022) Sea ranching feasibility of the hatchery-reared tropical sea cucumber Stichopus monotuberculatus in an inshore coral reef island area in South China Sea (Sanya, China). Front Mar Sci 9:918158. https://doi.org/10.3389/fmars.2022.918158
Yingst JY (1982) Factors influencing rates of sediment ingestion by Parastichopus parvimensis (Clark), an epibenthic deposit-feeding holothurian. Est Coast Shelf S 14:119–134. https://doi.org/10.1016/S0302-3524(82)80040-6
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This work was partially funded by a student research grant from Southern Cross University. We thank Heinrich Breuer, Shawna Foo, and Dione Deaker for assisting with fieldwork. The authors thank the two anonymous reviewers for their time and insightful comments that helped to improve the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was partially supported by a student research grant from Southern Cross University. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception, design and fieldwork. Data analysis were performed by BCTG and SWP. The first draft of the manuscript was written by BCTG and all authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no relevant financial or non-financial interests to disclose.
Ethical approval
This is an observation study. Southern Cross University has confirmed that no ethics approval is required. Permission to undertake research was granted under GBRMPA permit no. G22/46746.1.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gray, B.C.T., Byrne, M., Clements, M. et al. Movement dynamics, sediment turnover and sheltering behaviours of the nocturnal coral reef sea cucumber, Stichopus cf. monotuberculatus. Coral Reefs 42, 1329–1341 (2023). https://doi.org/10.1007/s00338-023-02433-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02433-0