Abstract
Ocean acidification (OA) is a major threat to marine calcifying organisms. This manuscript gives an overview of the physiological effects of acidification on reef-building corals from a cellular to population scale. In addition, we present the first review of the indirect effects resulting from altered species interactions. We find that the direct effects of acidification are more consistently negative at larger spatial scales, suggesting an accumulation of sub-lethal physiological effects can result in notable changes at a population and an ecosystem level. We identify that the indirect effects of acidification also have the potential to contribute to declines in coral cover under future acidified conditions. Of particular concern for reef persistence are declines in the abundance of crustose coralline algae which can result in loss of stable substrate and settlement cues for corals, potentially compounding the direct negative effects on coral recruitment rates. In addition, an increase in the abundance of bioeroders and bioerosive capacity may compound declines in calcification and result in a shift towards net dissolution. There are significant knowledge gaps around many indirect effects, including changes in herbivory and associated coral–macroalgal interactions, and changes in habitat provision of corals to fish, invertebrates and plankton, and the impact of changes to these interactions for both individual corals and reef biodiversity as structural complexity declines. This research highlights the potential of indirect effects to contribute to alterations in reef ecosystem functions and processes. Such knowledge will be critical for scaling-up the impacts of OA from individual corals to reef ecosystems and for understanding the effects of OA on reef-dependent human societies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species interactions occur in every ecosystem and can be pivotal in ecosystem functioning and services (Jordano 2016). Nevertheless, much of the research that identifies how environmental change affects biological communities is based on the direct physiological and behavioural changes of individuals. While understanding the effects on individuals is important, biological communities can also be indirectly affected by environmental change. This occurs when direct effects on one or more species alter the extent or outcome of interactions between species. Such indirect effects, which affect both individuals and ecosystem functions and processes, can manifest over a far greater scale than that of direct effects (e.g. Connell et al. 2013; Alva-Basurto and Arias-González 2014) with small changes to an interaction resulting in substantial impacts (Mumby 2017). Furthermore, ecological interactions can be more vulnerable to environmental change than the biology of individuals (Jordano 2016). For instance, a recent review of extinctions as a result of climate change concluded that nearly 60% of both extinctions and declines in abundance were the result of a lost interaction rather than physiological tolerances of individuals being surpassed (Cahill et al. 2013). Consequently, understanding how ecosystems are likely to change under future climate scenarios requires understanding alterations in the extent and outcome of ecological interactions.
Ocean acidification (OA) is one component of the ongoing global environmental change resulting from increases in atmospheric carbon dioxide concentrations (Gattuso et al. 2015). These increases are buffered by oceanic absorption of carbon dioxide which result in falling oceanic pH (Zeebe 2012; IPCC 2014). This uptake of atmospheric carbon dioxide has already caused a 0.1 unit decline in oceanic pH since the industrial revolution and is likely to cause up to an additional 0.3–0.32 unit decline by the end of the century (IPCC 2014; Gattuso et al. 2015). Changes in pH also result in a change in the concentrations of carbonate and bicarbonate ions in seawater which can affect fundamental organism physiology, such as calcification (Erez et al. 2011). As a result, OA is widely recognised to be a pervasive threat to marine biodiversity (Garrard et al. 2013; Harvey et al. 2013).
While the effects of OA are ubiquitous, they are of particular concern for calcifying organisms such as scleractinian corals, which use carbonate ions to build their skeleton (Hofmann et al. 2010). This potential for OA to reduce calcification in corals has identified coral reefs as one of the most vulnerable ecosystems to OA (National Research Council 2010). The change in calcification and a suite of other physiological effects on corals have the potential to result in indirect effects through altered interactions with other reef organisms. This paper provides a short overview of the direct physiological effects alongside a detailed review of the indirect effects of OA on shallow water tropical and temperate corals, to gain new insights about how corals and coral communities may be affected under future acidified conditions. Specifically we aim to: (1) review the direct, physiological effects of OA on reef building corals across different levels of biological organisation; (2) identify the indirect effects of OA through changes to ecological interactions and provide evidence of how these manifest at an ecosystem level; (3) identify priority areas for future research on indirect effects. While we acknowledge the importance of symbionts and microbial communities in influencing coral responses to environmental change, this review focuses on the responses of, and impacts on, the coral host.
Methods
We completed a literature search on ISI Web of Science for studies that identified the direct and indirect effects of ocean acidification on corals (completed October 2021). We focused primarily on tropical shallow water corals with some temperate corals included to capture research from naturally acidified field sites in temperate regions. Deep water corals were excluded from this review on the basis that other variables such as temperature and light that also change with depth would confound comparisons of OA effects.
To review the literature on the physiological effects of acidification on corals, papers were selected using search terms for ocean acidification (‘ocean acidification’, ‘pCO2’, ‘pH’, ‘carbon dioxide’), ‘coral’ and a direct, physiological metric. These metrics were the eight broad headings presented here (Fig. 1): ‘tissue biomass’ OR ‘lipid’ OR ‘protein’; ‘calcification’; ‘photosynthesis’; ‘survival’ OR ‘mortality’; ‘growth’ OR ‘skeletal density’ OR ‘porosity’; ‘reproduction’; ‘abundance’; and ‘species richness’ OR ‘species diversity’ OR ‘community composition’. Where possible, all relevant studies found in the search were included with the exception of calcification and photosynthesis due to the vast body of literature on these metrics which have already been the subject of several reviews and meta-analyses (e.g. Erez et al. 2011; Kornder et al. 2018). Therefore, for these metrics, we focused on papers published in the last 10 years. To ensure there was no bias in the data from this particular selection of publications, additional observations of calcification under OA were recorded from studies focusing on another metric where calcification was also measured. Observations were excluded if they considered variations in other environmental variables (e.g. temperature, nutrients, light), although if possible, we included comparisons between ‘ambient’/‘control’ conditions with the acidified treatment/s.
A summary of the physiological effects of ocean acidification from a cell to community scale. Pie charts summarise the proportion of observations taken from the literature showing a significant decrease (green), no significant change (blue) or a significant increase (purple) in response to elevated pCO2. From within any study, only sequential, non-overlapping pCO2 step comparisons are presented. Number within each pie chart denotes the number of observations for that metric. N = 902 observations from 93 studies
For every study selected, the statistical outcomes of comparisons between acidification treatments for each metric were recorded as ‘significant increase’, ‘significant decrease’, or ‘no significant change’. Effects which were not statistically analysed were excluded. Many studies used pCO2 treatments or field sites with acidification levels well beyond that expected in the near future (i.e., up to 5000 µatm). To ensure the trends in significance were not driven by these extreme conditions we compared the full dataset to two subsets of the data where we excluded observations involving pCO2 1) > 1000 µatm; and 2) > 2000 µatm. As there were no consistent differences in the proportions of observations reporting positive, neutral or negative effects of OA among these different datasets, the results presented here include the full range of pCO2 treatments (N = 902 observations from 93 studies) (Fig. 1). To account for the often non-linear physiological responses of coral to acidification (e.g. Bove et al. 2019), we included only sequential, non-overlapping comparisons between pCO2 treatments (e.g. low pCO2 to mid, mid to high).
Studies on indirect effects were similarly identified using search terms for ocean acidification (‘ocean acidification’, ‘pCO2’, ‘pH’, ‘carbon dioxide’), ‘coral’ and an indirect effect. The effects considered here were selected using the terms: ‘compet*’; ‘predat*’ OR ‘coralliv*’; ‘crustose coralline algae’ OR ‘CCA’, ‘bioero*’; ‘habitat provision’ then ‘habitat’ AND ‘structural complexity’ and; ‘disease’). An overarching search also included the term ‘indirect’ with the acidification and coral terms. However, as indirect effects are rarely labelled as such, it was necessary to include other broader search methods. Additional searches were therefore conducted around these topics for studies on other marine taxa (e.g. macroalgae and bioeroders) to gain broader understanding of potential non-specified indirect effects of OA. Lastly, the reference section of papers which included relevant information were reviewed for additional studies. All relevant studies found in the search are included; however, due to the limited recognition of indirect effects, we acknowledge that some relevant studies that did not explicitly refer to indirect effects would not have been captured in our search.
Finally, we review the effects of acidification on the abundance and diversity of corals at naturally acidified reef sites (Table 2), as well as evidence of any indirect effects at such sites (Table 3). Observations of coral abundance and diversity were only taken from one study per site to avoid over-representation of a limited set of intensively studied locations within the data set. We focus on the effect of pCO2 as a ‘catch-all’ measure of OA effects. However, we note that other measures of water chemistry, such as aragonite saturation (Ω) and the relative abundance of carbonate (CO32−) and bicarbonate (HCO3−) ions, are correlated with pCO2 and that OA effects encompass changes in all of these variables.
Direct effects of OA on coral
Corals employ a range of biological responses to counteract the adverse effects of acidification. These stress responses can manifest at multiple levels of biological organisation ranging from molecular or cellular processes to whole organism responses (Edmunds et al. 2016). The cumulative effect of these physiological responses can translate into effects on populations by altering key demographic rates. This includes population growth through reproduction, recruitment, and colony growth, and population shrinkage through mortality. Ultimately, these demographic parameters then affect coral communities through changes in coral cover, and altered community composition and species diversity.
At the molecular and cellular level, corals have a variety of processes that allow them to survive and grow under acidified conditions. While mechanisms such as the ability to internally regulate pH (McCulloch et al. 2012, 2017) allow corals to adjust to acidic conditions, they are thought to be energetically costly (Allison et al. 2018). However, increased pCO2 does not always result in depletion of energy reserves (measured via tissue biomass; e.g. Strahl et al. 2016) (Fig. 1). This suggests that at least some coral species can maintain growth under OA via regulating pH at the site of calcification (see: Comeau et al. 2022).
At the ‘whole colony’ level, both calcification and photosynthesis can be affected by ocean acidification. For photosynthesis, effects of OA are highly variable among studies (Fig. 1). The majority of observations considered here showed no significant changes in the rates or efficiency of photosynthesis under OA (e.g. Bedwell-Ivers et al. 2017; Bahr et al. 2018), nor in the density of symbionts or the chlorophyll within each symbiont (e.g. Bedwell-Ivers et al. 2017; Rivest et al. 2017). Changes in photosynthetic acquisition of energy under OA are therefore unlikely to be the limiting factor for coral persistence in the future.
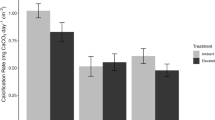
The effect of acidification on calcification is the most widely recognised threat to corals and there is a substantial body of research showing declines in calcification (Erez et al. 2011; Chan and Connolly 2013; Kornder et al. 2018), as well as some evidence of increased dissolution (e.g. Fine and Tchernov 2007; Kline et al. 2019). However, calcification responses are highly variable, and both species and colony specific (e.g. Sekizawa et al. 2017; Bahr et al. 2018), with the overall majority of observations showing no significant effect of acidification (e.g. Carbonne et al. 2021) (Fig. 1). Furthermore, corals continue to calcify and grow in naturally acidified reefs (e.g. Inoue et al. 2013; Camp et al. 2017) (Fig. 1) under conditions far more acidic than the theoretical limits for calcification and net carbonate accretion (560 ppm atmospheric CO2, Silverman et al. 2009; 2.3 to 3.5 Ω aragonite, Kline et al. 2019) (Table 2).
At the population level, negative effects of OA are more apparent (Fig. 1) suggesting that small changes in multiple aspects of physiology might accumulate to comprise a significant impact when summed across all individuals in the population. There is some evidence of a shift in growth strategies under acidification, with some corals maintaining linear extension by building less dense, more porous, skeletons (Mollica et al. 2018; Teixidó et al. 2020). This strategy is unlikely to drive changes in coral cover under acidification; however, there may be significant indirect effects through increased vulnerability to breakages in weaker skeletons (Hennige et al. 2015). In contrast, other colonies maintain skeletal density/porosity at the expense of linear extension (Enochs et al. 2015a), which can reduce lifetime reproductive because slower growth means corals are more likely to die before reaching the largest, most fecund, colony sizes.
For reproduction metrics, the effect of acidification is again variable among studies. Overall, the early stages of reproduction (production of gametes, fertilisation, and larval development, growth and survival) appear to largely be unaffected by acidification (e.g. Rivest et al. 2018; Pitts et al. 2020). Conversely, the later stages of reproduction (settlement, metamorphosis, recruit growth and survival) show more consistent negative effects (e.g. Jiang et al. 2018; Yuan et al. 2018), suggesting that recruits are more vulnerable to acidification than gametes or larvae. This is of concern because even a moderate (20%) decline in recruitment was estimated to reduce coral cover by 15% over 7 year period (Evensen et al. 2021). However, declines in recruitment are estimated to potentially rise as high as 52% by mid-century conditions (~ 560 µatm), and 73–91% by end of century conditions (~ 800 µatm) (Albright et al. 2010; Fabricius et al. 2017). Such reductions are therefore likely to present a significant threat to the persistence of corals and coral communities under acidified conditions. Finally, despite the described physiological effects, increased pCO2 does not consistently reduce adult colony survival, under acute experimental or naturally acidified conditions. Rather, OA appears to result in sub-lethal effects, defined here as “physiological effects that reduce coral fitness (e.g., cause slower growth or lower reproductive output) but do not result in mortality”. The accumulation of such sub-lethal effects on growth and reproduction may suppress population growth rates in the future, contributing to changes in abundance, species richness and/or diversity, and community composition at an ecosystem level.
Overall, we find the direct effects of acidification on corals are highly variable. This variation may result from differences in experimental methodology or pCO2 levels; however, it is also likely to represent the significant variation both between individuals, species and sites. In total across all scales 52% of observations included here found no significant difference with acidification (Fig. 1). Interestingly however, it appears that consistently negative effects are more often seen at a population level (26% of 274 observations), rather than cell (10% of 80 observations) or colony level (16% of 388 observations). This suggests that while the acidification has largely sub-lethal physiological effects on individuals, coral populations and communities may still be altered. We therefore stress the importance of researching the effects of acidification on coral populations and communities in addition to effects on individual coral colonies.
Indirect effects of OA on corals
Indirect effects are defined here to be a change in an ecological interaction between species. This may occur when a physiological or behavioural change affects either the extent or outcome of an interaction with another species or taxon. Indirect effects can occur as a result of physiological changes to one or both of the interacting taxa, changes to the interaction itself or changes in environment in which the interaction occurs and, consequently predicting indirect effects is challenging, and requires knowledge of both the stressor and all individuals involved (Connell et al. 2011; Sunday et al. 2016). In the following section we review the indirect effects of OA for reef-building corals, including interactions between corals, and between corals and other taxa (see Table 1).
Competition
For sessile benthic organisms, competition is primarily for resources such as space and light (Lang and Chornesky 1990) and helps to structure communities and determine species distributions (e.g. Connell et al. 2004). OA may affect competition dynamics through; (1) a change in the frequency of competition as coral cover changes, resulting in either intensification or competitive release (Hofmann et al. 2010); (2) a change in the identity of competitors as species composition changes; (3) an altered outcome of an existing competitive interaction, resulting from differences in the magnitude to which different species are affected by OA; (4) an altered outcome of an existing competitive interaction due to changes in availability of resources, or; (5) novel competitive interactions as a result of shifting distributions (e.g. Alexander et al. 2016).
Competition between hard corals
To date, there has been limited investigation of the impact of ocean acidification on competition between hard corals (Table 1). However, given the physiological and population-level effects outlined above, we may expect a number of possible changes. Firstly, competitive interactions only occur when colonies are in close proximity (Edmunds et al. 2016) therefore, the occurrence of coral–coral interactions is likely to be lower under acidified conditions if coral cover is reduced. However, lower coral cover does not necessarily correspond to fewer competitive interactions if the remaining colonies are spatially aggregated due microhabitat requirements, or if only a small proportion of free space is actually colonisable. There are currently no studies that demonstrate how the prevalence of competition changes as a result of OA.
Secondly, if there are changes in species richness under OA, different intensity of intra- compared with inter-specific competition may occur in the future (Table 1). To date, three studies have examined changes to inter- and intra-specific coral competition under high pCO2 all of which report that while interspecific competition significantly suppressed growth, the addition of high pCO2 conditions caused no additional suppression under that kind of competition (Evensen et al. 2015; Evensen and Edmunds 2016; Horwitz et al. 2017; Table 1). However, these studies show different trends for intraspecific competition. One study found no additional suppressive effect of OA above that of intraspecific competition on growth (Evensen et al. 2015), another reported significant declines in growth in five out of the six species (Horwitz et al. 2017). One potential explanation for this difference is the experimental duration, with additional suppressive effects only seen in the longest duration study (1 year; Horwitz et al. 2017). Nevertheless, the scarcity of research on this topic means that it remains unclear whether and how release from interspecific competition will affect coral communities on reefs in the future.
Finally, the physiological cost of competition under OA could be higher in acidified conditions (Table 1) if costs of maintaining growth reduce the ability of corals to invest energy in winning competitive interactions. The single study that has investigated this to date found that corals (Galaxea fascicularis) responded more rapidly to competition under acidified conditions but that net tissue necrosis was the same between treatments (Evensen and Edmunds 2018). While the effect of competition on growth suggests there is a greater physiological cost of competition under OA, the lack of impact on competitive ability potentially indicates a change in allocation of resources to maintain competitive ability. However, while Evensen and Edmunds (2018) focused on mesenterial filaments, these are only one of a number of competitive mechanisms corals use, and the effect of OA on other competitive methods has not been considered as yet.
How the effects of competition under acidification scale up to an ecosystem level is largely unknown. Early evidence of the effect of competition at an ecosystem level found that recovery on coral reefs under the combination of OA and competition reduced recovery an additional 14% compared to OA alone (Evensen et al. 2021). However, competition was found to be less important than coral growth in recovery. Therefore, to understand how OA-induced changes to coral–coral interactions play out at an ecosystem level requires additional research on the extent to which corals compete, their competitive ability or aggression, and the impacts of competition on colony growth and survival.
Competition between hard and soft corals
In general, soft corals appear to be more resistant to high pCO2 than hard corals (Gabay et al. 2014) suggesting that soft corals could win more competitive interactions with hard corals in the future. Under recent historical ambient conditions, adult soft corals were often the subordinate competitors (Dai 1990), although chemical defences used by soft corals have been shown to affect hard corals through inhibiting recruitment (Atrigenio and Alino 1996) and growth, as well as causing significant tissue necrosis and mortality (Sammarco et al. 1983). However, ambient oceanic pCO2 has increased dramatically since these studies were published and it is not known whether these interactions still occur or result in the same outcome under today’s or future pCO2 conditions. Only one study has experimentally tested the physiological effects of competition between hard and soft corals under high pCO2 conditions. This study found that the presence of a soft coral competitor had a negative effect on photosynthesis but not growth of the hard coral (Porites cylindrica), and negligible changes in competitive ability in response to changing pCO2 conditions (Brien et al. 2016; Table 1). In contrast, the cytotoxicity of the soft coral Sarcophyton spp. declined with increasing pCO2 (Januar et al. 2016) potentially indicating a reduced capacity for competition. However, this decline coincided with a decrease in overall coral cover and may therefore represent a decreased need to compete. Similarly, at all three naturally acidified sites that reported abundances for both soft and hard corals, soft corals increased in abundance from ambient to mid pCO2 before declining again under high pCO2 (Inoue et al. 2013; Januar et al. 2017; Agostini et al. 2018). However, this result could reflect opportunistic colonisation of space as hard coral abundance declined rather than altered competitive ability. Studies that directly quantify how the prevalence and outcomes of competition change between corals at natural CO2 seeps compared to control sites will help to resolve knowledge gaps about how OA will affect reef ecosystems in the future.
Competition between hard corals and macroalgae
Competition on reefs also occurs between corals and other sessile benthic organisms such as macroalgae. Calcifying macroalgae (e.g. Halimeda) can decline in abundance and diversity under OA in a similar way to corals (Johnson et al. 2014; Zunino et al. 2017). While fleshy macroalgal species (hereafter, macroalgae) can also suffer negative effects of OA on their physiology, diversity or abundance (e.g. Page et al. 2021), overall the observed effects on this taxon are predominantly positive (Connell et al. 2013; Kroeker et al. 2013; Johnson et al. 2014; Zunino et al. 2017).
Coral–algal interactions are commonplace, especially in temperate environments, and under ambient conditions are not a significant threat to corals (Vieira 2020). However, under acidification a disparity between the predominantly negative physiological effect on corals and the predominantly positive effect on macroalgae has the potential to shift this balance, affecting coral–algal competition in two ways. Increases in macroalgae abundance (Connell et al. 2013) are likely to increase the frequency of coral–algal interactions. This increase of interactions has not been not been explicitly tested under acidification but has been shown under ambient conditions where macroalgae increased in abundance due to fishing pressure removing herbivorous fish (Bonaldo and Hay 2014). Furthermore, there is some evidence that coral–algal interactions are more detrimental to corals under high pCO2 (Table 1). For example, direct contact between corals and macroalgae under high pCO2 conditions results in significantly greater and faster coral mortality than under ambient conditions, in both adult colonies (Del Monaco et al. 2017) and coral larvae (Campbell et al. 2017). Furthermore, acidification is anticipated to result in smaller colony sizes (e.g. Teixidó et al. 2020) which have been shown to suffer greater mortality than larger corals when interacting with macroalgae under ambient conditions (Ferrari et al. 2012). The mechanisms behind these effects include enhanced macroalgal allelopathy (Del Monaco et al. 2017) and/or elevated production of DOC (dissolved organic carbon) which encourages microbial activity with negative consequences for coral (Diaz‐Pulido and Barrón 2020).
To date, the balance of evidence suggests that algae can often outcompete corals under acidification. At a colony level this may result in an increased likelihood of coral overgrowth (Diaz-Pulido et al. 2011); however, there are also wider ecosystem level effects. For example, changes in coral–algal interactions under acidified conditions in favour of macroalgae can create a density-dependent negative feedback loop where increased macroalgal abundance results in more coral mortality which creates more space for macroalgal colonisation. This can result in ecosystem phase shifts from coral to macroalgal dominated states (Diaz-Pulido et al. 2011), which has been seen in at least one naturally acidified site (Enochs et al. 2015b). However, the maintenance of coral dominance at some naturally acidified sites, despite increased abundance of macroalgae (e.g. Fabricius et al. 2011) suggests that the (chronic) effect of acidification on coral–macroalgal interactions is not sufficient, on its own, to cause this phase shift. There may be a number of explanations for this. Firstly, competition can have negative effects for macroalgae as well as corals (Diaz-Pulido et al. 2011), and the presence of coral–algal competition does not always have additional suppressive effect on growth (Page et al. 2021) or tissue loss from allelopathy (2 out of 3 species; Del Monaco et al. 2017) above that of acidification alone. Secondly, the effect and outcome of coral–algal competition differs between species (e.g. Del Monaco et al. 2017; Vieira 2020) suggesting phase shifts towards macroalgae are dependent on the macroalgal community composition. Thirdly, other environmental factors such as temperature may play a role. For example, coral–algal interactions vary temporally due to seasonal changes in temperature (Brown et al. 2019). Finally, additional indirect effects, such as decreasing herbivory (see section below), can determine the likelihood of a phase shift (Enochs et al. 2015b). Therefore, we suggest that while altered coral–algae competitive interactions have significant implications for coral communities, broader knowledge of species-specific tolerances to OA for both corals and algae, and better knowledge of the mechanisms and costs of competitive interactions, are required before the outcomes of such encounters can be accurately predicted in the future.
Corallivory
Under ambient conditions (~ 390–420 µatm pCO2, based on the approximate global average pCO2 concentrations from 2010 to 2022), corallivory (predation) of corals can be a chronic stressor to coral communities (Cole et al. 2011), potentially affecting species abundance and diversity (e.g. Littler et al. 1989; Lenihan et al. 2011). Changes in the rate of corallivory are likely based on demonstrated changes in fish behaviour under OA (Ferrari et al. 2011; Munday et al. 2014). Moreover, the potential effect of acidification on fish physiology, such as bone strength/mineralisation (Di Santo 2019; Mirasole et al. 2021) and skeletal deformities (Pimentel et al. 2014), may alter the impact of corallivory. However, to the best of our knowledge, only two studies have considered how corallivory changes as a result of ocean acidification (Table 1). One study found that very high pCO2 (1608 µatm) conditions did not affect the rate of coral consumption, or further reduce coral survival, following predation from Crown-of-Thorns starfish (COTs, Acanthaster plancii; Kamya et al. 2018). A second study showed that injuries made on small corals to simulate bite scars of corallivorous parrotfish healed at the same rate under ambient (420 µatm) and high (1050 µatm) pCO2 conditions (Edmunds and Yarid 2017), suggesting acidification does not affect recovery from predation. However, potential changes in rates of predation by different corallivores remains a significant knowledge gap. While as yet untested, acidification could also result in secondary impacts of predation. For example, bite scars from parrotfish are larger on low-density substrate than on high-density substrate (Bruggemann et al. 1994) and, therefore, if coral skeletal density declines as a result of acidified conditions (Tambutté et al. 2015; Mollica et al. 2018) the potential size and impact of predation scars might increase.
Corallivory is an important driver of changes in coral cover. Models of future coral cover on the Great Barrier Reef found managing predation by COTs to be one of the most effective strategies in delaying declines in coral cover (Condie et al. 2021). However, the cumulative impacts of changes to corallivory on coral populations and communities under OA are, as yet, unknown. Despite this, we suggest that predation pressure on individual coral colonies could increase under OA due to potential decreases in coral abundance (Enochs et al. 2015b) reducing food supply, a shift in community composition to a species less preferred for predation (e.g., Porites spp.; Fabricius et al. 2011), but little to no change in corallivorous fish community (Munday et al. 2014). Detailed investigation of both the rates and the impact of corallivory will be important to reveal the implications of changes in predation pressure for corals and coral communities in the future.
Herbivory
Herbivory has been shown to be a critical function in the resilience of coral reefs (Hughes et al. 2007). Under ambient conditions, a loss of herbivory has been noted as significant factor in the occurrence of a number of coral to macroalgal phase shifts (Vieira 2020). Fortunately, under acidified conditions, the abundance of herbivores is expected to be equal or greater than ambient conditions, due to the increased availability of food and a simplification of coral reef food webs (Kroeker et al. 2011; Alva-Basurto and Arias-González 2014; Vizzini et al. 2017; Harvey et al. 2019). Therefore herbivory is likely to continue being a major benthic structuring process under acidification (Baggini et al. 2015). However, where the herbivores are themselves negatively affected by acidification (e.g. sea urchins; Hall-Spencer et al. 2008) or the algal community becomes less palatable to herbivores (e.g. Harvey et al. 2019), this can reduce the top-down control of macroalgae and result in shifts in the benthic community. These effects are ‘secondary’ indirect effects where the impact on corals occurs via the impact of herbivores on the macroalgal community. In addition, herbivory can also affect corals directly. For example, under acidified conditions coral recruits had significantly higher post-settlement mortality and a higher size-escape threshold from grazing herbivorous fish (Doropoulos et al. 2012). Although we were unable to find any studies explicitly testing the secondary effects of herbivory on coral communities, we suggest changes in herbivory may be a significant factor in moderating the impacts of acidification on corals, and highlight the need for additional research on this topic.
Crustose coralline algae (CCA)
CCA, like corals, are calcifying organisms and are therefore likely to be negatively affected by acidification. For example, increased pCO2 on CCA has been shown to cause declines in recruitment (Ordoñez et al. 2017), competitive ability (Kuffner et al. 2008; Crook et al. 2016), growth (Johnson et al. 2014), calcification and recovery following injury (Manning et al. 2019). Ultimately, these changes result in up to 70 to 90% declines in CCA abundance (Kuffner et al. 2008; Fabricius et al. 2015; Vogel et al. 2016), however, these effects are species specific with some species persisting while others suffer reduced abundance or are lost completely (e.g. Peña et al. 2021). Therefore, acidification is also likely to cause shifts in community composition of CCA on reefs.
CCA play a number of vitally important roles for corals. CCA cement and stabilise the reef substratum (Guinotte and Fabry 2008) which provides stable and suitable habitat for juvenile corals to settle on. However, reef pavement (solid carbonate substratum covered with CCA, turf algae and other sessile invertebrates), has been found to have dissolution rates 86% higher in acidified compared to ambient conditions. Similarly, reefs with low cementation rates under natural acidification suffered an almost complete loss of reef framework following a disturbance, with very low rates of coral recovery (Manzello et al. 2008). Furthermore, like corals, CCA are likely susceptible to increased bioerosion under OA. In addition to reduced abundance, this may contribute to a decrease in reef cementation in the future, reducing the available substrate for settlement of coral recruits.
CCA are often used as a suitable settlement substrate, with declines in CCA abundance therefore reducing available settlement space. For example, during an in situ experiment at naturally acidified reefs in Papua New Guinea, 81% of coral recruits settled on CCA, despite it only accounting for 12% of substrate (Fabricius et al. 2017). This will therefore compound the effects of reduced reef cementation, further reducing the availability of suitable space for larval settlement. Finally, CCA act as an important settlement cue for coral larvae (Heyward and Negri 1999) and increased pCO2 can disrupt the response of corals to CCA (Table 1). For example, when CCA were pre-exposed to high pCO2, larval settlement declined between 54 to 87% (Doropoulos and Diaz-Pulido 2013; Webster et al. 2013). In addition, a loss of affinity has been shown between coral larvae and the most favourable CCA species (Titanoderma sp.) which may further reduce settlement (Doropoulos et al. 2012). To exacerbate this effect, CCA species are differentially affected by increased pCO2 (see review: Hofmann and Bischof 2014) resulting in altered community compositions, with preferred settlement species such as Titanoderma sp. thought to be less tolerant of acidified conditions (Fabricius et al. 2015). These effects suggest loss of CCA, or changes to the community composition of CCA, is a major pathway of indirect effects of OA on coral communities.
Habitat provision
Corals are ecosystem engineers that build three-dimensional structures that provide habitat, shelter and food for many other reef organisms. This structural complexity they create affects species richness and abundance of many reef organisms, and is a driver of reef ecosystem functioning (Graham and Nash 2013). Consequently, degradation of structural complexity from acidification (Fabricius et al. 2011; Manzello et al. 2014; Enochs et al. 2015b) can have significant implications for a wide variety of taxa and the functioning of acidified reefs. Here we consider the interactions between corals with fish, invertebrates and zooplankton.
Under ambient conditions, fish–coral interactions are often mutually beneficial. For example, associations with fish have been shown to benefit corals through enhanced colony growth (Chase et al. 2014) and photosynthesis (Garcia-Herrera et al. 2017), reduced sedimentation and sediment-induced partial mortality (Chase et al. 2018), and protection from predation (Chase et al. 2014). Furthermore, provision of habitat and shelter for herbivorous fish also benefit corals through maintenance of algal populations. For instance, herbivory pressure was > 7 times higher in high-complexity areas compared to low-complexity areas (Santano et al. 2021). Moreover, declines in structural complexity at naturally acidified sites can drive a shift in fish community composition (Cattano et al. 2020), and altered abundances of some fish species at acidified compared with ambient areas (Munday et al. 2014). To the best of our knowledge, the effect of ocean acidification on interactions between corals and fish have not yet been investigated, and therefore, how changes in fish abundance or community composition indirectly affect corals ability to persist under acidified conditions remains unknown.
Many invertebrates use coral for habitat, with almost 1,000 known coral–invertebrate associations (Stella et al. 2011), although this number is likely to have increased over the last decade. This includes species which live in the branches and crevices coral provide, and boring organisms who reside within the coral tissue/skeleton (Stella et al. 2011). Overall, invertebrate communities appear to be negatively affected by acidification and the associated loss in structural complexity (Fabricius et al. 2014). The implications of changes in invertebrate communities or coral–invert interactions depend on the interacting taxa. For example, populations of the herbivorous urchin Diadema savignyi, increased dramatically at the acidified site in Papua New Guinea (PNG; Fabricius et al. 2014) which could benefit corals through increased herbivory. In contrast, increased abundance of vermitid gastropods which bore into large Porites colonies (Fabricius et al. 2014), may negatively affect corals through weakening their structure. Finally, the mutualistic relationship between the coral host (Pocillopora verrucosa), and two ectosymbionts Trapezia spp. crabs and Alpheus, spp. shrimp reduced the impact of acidification on calcification in the host coral (Doo et al. 2018; Table 1). However, it is not clear whether this is because the ectosymbionts reduced the sensitivity of the coral to acidification or provided a resource or service that prevented the reduction in calcification seen in the corals without ectosymbionts. Due to high diversity of coral-associated invertebrates and the range of responses in interactions to acidification, it is hard to predict the potential changes in interactions and what the outcomes of those may be for corals. Furthermore, additional changes in the physical (water flow and temperature) and chemical (e.g. dissolved nutrients and oxygen) conditions of the ocean as a result of acidification and climate change could further exacerbate changes in interactions between the organisms involved in these symbioses.
Demersal zooplankton use coral to shelter from predation during the day (Alldredge and King 1977). Similarly to fish, declines in structural complexity under acidified conditions have also been shown to result in reduced biomass and abundance of zooplankton (Smith et al. 2016; Allen et al. 2017). However, zooplankton are an important heterotrophic food source for corals (Houlbrèque and Ferrier‐Pagès 2009) and declines in their abundance has the potential to impact corals, through altered availability of food. The extent of this impact is likely to be species specific, depending on the reliance of each species on heterotrophic food sources. Moreover, some species are capable of mitigating the effects of acidification by increasing heterotrophic energy contributions (Towle et al. 2015). A decline in zooplankton may therefore reduce the resilience of some corals to acidification.
Overall, we suggest that the changes in habitat interactions reviewed here are likely to have only sub-lethal consequences for corals. However, they highlight the possibility of multiple small shifts in the functioning of coral reef ecosystems to accumulate, which may ultimately contribute to a shift in communities greater than that expected from the physiological stressors. This may be particularly be the case where changes affect mutually beneficial relationships, creating the possibility of a negative feedback loop. In this case, fewer beneficial habitat associations reduce physiological coral health and/or survival, which further affects habitat associations. Such effects are extremely hard to predict and with the current paucity of data on the indirect effects on corals of changes to habitat associations, this remains a significant knowledge gap.
Bioerosion
Bioeroders are a diverse range of organisms including bacteria, algae, sponges, molluscs and fish, which cause erosion through grazing of, attachment to, or embedding in, the coral skeleton (Wisshak et al. 2012; Glynn and Manzello 2015; Schönberg et al. 2017). There is significant evidence from naturally acidified reefs that bioeroder abundance increases under high pCO2 (Crook et al. 2013; Enochs et al. 2016b) (Table 1). This may be the result of reduced competition for space between settling bioeroders such as sponges (Schönberg and Ortiz 2008) or an increase in dead coral habitat which supports a high number of invertebrates (Tribollet and Golubic 2011; Valentino 2014). Increased bioeroder abundance may have additional indirect effects on coral populations by reducing the available space for settlement. However, increased bioeroder abundance is not found for all bioeroding species (e.g. grazers; Schönberg et al. 2017) or where other factors like habitat are limiting (Valentino 2014).
The bioerosive capacity of many species is increased under high pCO2 (Table 1). Chemical bioerosion is less physiologically costly under high pCO2 (Wisshak et al. 2012) which may increase the bioerosive capacity of boring species. For example, it is estimated that rates of chemical bioerosion by an excavating sponge (Cliona caribbaea) could double by the end of the century (Webb et al. 2017). In addition, taxa using mechanical bioerosion may have increased bioerosive capacity where coral skeletons are weaker, less dense/more porous. While corals with lower density were more likely to have evidence of bioerosion than high density corals (Barkley et al. 2015), this correlation has not been explicitly tested under acidification. Furthermore, under ambient conditions, skeletal density does not always result in increased bioerosion. For example, the highest rates of bioerosion were found in the coral genus with the highest skeletal density (Pavona; Cosain-Díaz et al. 2021). These contrasting findings may be explained in part by variable rates of bioerosion between morphologies, with the most dense coral also being the morphology with the highest rates of bioerosion (massive; Cosain-Díaz et al. 2021). However, where density does affect bioerosive capacity, we are likely to underestimate the net effect of OA on coral growth by considering the direct effects in isolation. Finally, bioeroders may have increased biomass and faster growth (Fang et al. 2013; Uthicke et al. 2016), ultimately allowing an individual to have a greater rate of bioerosion or lifetime erosive capacity. The combination of increased bioeroder abundance and bioerosion capacity is expected to result in a large increase in total bioerosion under high pCO2. By the end of this century, these increases in bioerosion from sponges alone are estimated to be 7% under RCP 4.5, 16% under RCP6 and 31% under RCP8.5 compared to ambient conditions (393 µatm; Wisshak et al. 2012; IPCC 2014). Similarly, total volume removed by bioerosion under Ω aragonite saturation < 2 (comparable to RCP8.5) may increase up to 78% compared to ambient aragonite (Ω aragonite > 3.5; Crook et al. 2013). Such increases in bioerosion rates have been shown to result in significant declines in net calcification (Stubler et al. 2014) and even a switch from net calcification to net dissolution (Enochs et al. 2016a). Therefore, in conjunction with the predicted changes in net accretion, bioerosion could be a significant contributor to coral cover declines in the future.
Coral disease
Coral disease results from interactions between the host corals and the pathogen causing the disease. Worldwide, the prevalence of coral diseases has increased in recent decades due to a number of factors, such as rising water temperatures (Tracy et al. 2020), and decreasing water quality including nutrient enrichment (Vega-Thurber et al. 2014), plastic pollution (Lamb et al. 2018) and metal pollution (Tracy et al. 2020). However, the effect of acidification on coral diseases is largely unknown and remains a significant knowledge gap (Vega Thurber et al. 2020). To date, only one study has empirically shown an impact of acidification on disease dynamics, which found that low pH (7.7) decreased the progression of black bank disease on Orbicella faveolata under experimental conditions (Muller et al. 2017). This suggests that future acidified conditions may be less favourable to black band disease and result in decreased prevalence and/or progression. However, coral diseases are caused by a range of pathogens including marine and terrestrial bacteria, cyanobacteria and viruses (Sokolow 2009; Vega Thurber et al. 2020), meaning the results from Muller et al. (2017) cannot easily be extrapolated to diseases caused by other pathogens.
Despite limited information on coral disease under acidification, there are some indications that pathogen abundance and virulence, and coral susceptibility may change (Table 1). Increased abundances of disease-associated bacteria within the coral microbiome have been found experimentally (Meron et al. 2011; Grottoli et al. 2018; Table 1; Vega Thurber et al. 2020), and at the seep site in PNG (Morrow et al. 2015), however, this did not result in an increase in infections. Furthermore, microbial pathogens were absent entirely at the seep site in Ishia, Italy (Meron et al. 2012). In addition, there is some evidence that pathogen virulence may be affected by acidification, potentially due to pathogen pH tolerance limits. For example, growth in the bacterium Aurantimonas coralicida, which causes white plague type II, was strongly affected by pH with a clear lower limit beyond which no growth occurred (Remily and Richardson 2006). Finally, corals have shown decreased immunity under acidification, which may result in an increase in susceptibility (see review: Traylor-Knowles and Connelly 2017). Regardless of this potential decrease in virulence, we note that reduced growth and calcification in corals may reduce the ability of corals to recover if infection does occur and this could heighten the impacts of diseases on coral populations. Additional research is required on the prevalence of disease at naturally acidified sites, as well as both pathogen abundance and virulence, as well as coral susceptibility and recovery, to understand the effect of acidification on coral–pathogen interactions.
Coral communities under acidified conditions
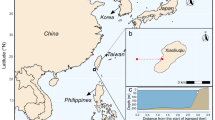
Ultimately, the coral communities we see in the future will be the result of the combined impacts of both the direct and the indirect effects on corals. With consistent variability in findings between species, location and experimental studies, as well as the paucity of data on many indirect effects, our ability to accurately scale up our current knowledge to an ecosystem scale is limited. However, globally, there are a number of locations with naturally low pH/high pCO2 conditions as a result of volcanic gas seeps, hydrographic processes and localised low pH submarine springs. The natural gradients of pCO2 around these sites reveal how the direct and indirect effects of OA that are reviewed above could manifest at an ecosystem level. We review the evidence of indirect effects and the result of both direct and indirect effects on ecosystem level metrics, at ten naturally acidified coral communities or coral reefs (Fig. 2, Table 3). Seven of these sites occur around volcanic vents, where gas containing a high percentage of CO2 seeps through the seafloor and acidifies the surrounding waters. Of these seven, three occur in tropical coral reef ecosystems, in North Sulawesi and Maluku Provinces (Indonesia), Dobu, Esa’Ala and Upa-Upasina (PNG), and Maug (Commonwealth of Northern Mariana Islands [CNMI]), and one on a sub-tropical reef in Iwotorishima (Japan). The remaining three vents are in temperate rocky shore environments where corals occur, in Shikine (Japan), and Panarea and Ishia (both Italy). Carbonate chemistry at vent sites is variable depending on the composition of the vent gases; however, pCO2 conditions close to the vent can be high and well in excess of what is predicted under end of century conditions, particularly in temperate regions (e.g. 5066 µatm in Ishia, Italy; Hall-Spencer et al. 2008). The remaining three sites occur as a result of three different processes. In Rock Island Bays in Palau, acidification occurs in tropical lagoonal reefs where there is restricted circulation of water in semi-enclosed bays (Golbuu et al. 2016). Acidification here is less extreme (~ 600 µatm) than at some of the vent sites. In Puerto Morelos (Mesoamerica) submarine springs (or ‘Ojos’) discharge low-pH, low aragonite saturation groundwater, through natural localised springs in close proximity to the tropical Mesoamerican Barrier Reef. This results in high levels of acidity (pH 7.05, pCO2 data is not stated) as well as reduced salinity and total alkalinity (Crook et al. 2012). Finally, seasonal upwelling results in mild acidification (588 µatm) at the southern-most reefs in the subtropical Galapagos Islands (Eastern Tropical Pacific [ETP]), as well as exposure to cool waters and elevated nutrient levels. Reefs further north are less affected by the upwelling and experience more ambient ocean conditions (Manzello et al. 2014).
The location of the ten natural acidified sites with associated corals which are reviewed here, ordered by smallest to largest decrease in coral cover. 1- Rock Island Bays, Palau; 2- North Sulawesi and Maluku Provinces, Indonesia; 3- Dobu, Esa’Ala and Upa-Upasina, Papua New Guinea (PNG); 4- Puerto Morelos, Mesoamerica; 5- Maug, Commonwealth of Northern Mariana Islands (CNMI); 6- Iwotorishima, Japan; 7- Galapagos, Eastern Tropical Pacific (ETP); 8- Shikine, Japan; 9- Panarea, Italy; 10- Ishia, Italy
A number of other acidified sites have also been described in the literature, however, they are excluded here because they also experience elevated temperatures and/or deoxygenation (Bouraké, New Caledonia; Mangrove lagoons, northern Great Barrier Reef; Bahia Concepcion, California), or because there are no ecological records of coral communities at those sites, (Ambitle Island, PNG; Mayreau Gardens, Caribbean) or where the pCO2 gradient occurs and dissipates daily (Moorea, French Polynesia).
Changes in coral abundance (coral cover) with increasing pCO2
The effect of acidification on hard coral abundance, with the exception of Porites spp., is largely negative (Fig. 3). Seven of the ten naturally high pCO2 sites showed reduced hard coral cover compared with adjacent control sites (Fig. 3, Table 2). Nevertheless, the effect is not always negative, and there is limited evidence of a physiological ‘tipping point’ or threshold response to increasing pCO2. While the three temperate/sub-tropical coral communities, as well as the CNMI and Mesoamerican coral reefs, showed rapid declines in coral cover at ~ 500 µatm pCO2, coral cover at similar concentrations increased significantly at Palau (~ 31%), was maintained at seep locations in Indonesia (~ 4% increase) and PNG (~ 2% increase) and shifted from hard to soft coral cover at Iwotorishima (Fig. 3, Table 2). This suggests that effects of pCO2 on coral abundance are likely to be influenced by other indirect effects and/or environmental factors, and also by the species composition of coral present at each site (see below). Overall, whereas all of the temperate/sub-tropical coral communities showed a complete loss of corals between control and seep sites, only two of the seven warm water coral reefs showed a significant loss of coral cover across a similar pCO2 gradient (Inoue et al. 2013; Enochs et al. 2015b; Fig. 3). Therefore, the high light levels available in warm tropical waters, and the increases in metabolic rates that accompany moderate increases in water temperature, could allow certain species of corals (e.g. Porites) to maintain growth rates on tropical reefs despite changes in pCO2. In contrast, increasing pCO2 has major effects on coral growth under more marginal conditions.
The percentage cover of hard corals (top) and soft corals (bottom) along a pCO2 gradient at eight of the ten naturally acidified sites reviewed here. Coral cover data are not shown for two sites because of lack of stated pCO2 values (Mesoamerica, site 4) and coral cover (Ishia, site 10); however, both studies note significant to total loss of coral cover. Abundances for Iwotorishima (Japan) are approximate, based on broad percentage categories. Site numbers in legend correspond to Fig. 2
Differences in local species composition may also influence the impact of pCO2 on abundance. Among-species variation in sensitivity to acidification has been widely discussed (e.g. McCulloch et al. 2012; Agostini et al. 2021) with the premise that declines in abundance are driven in part by declines in less tolerant, ‘loser’ species. However, ‘winners’ and ‘losers’ are not consistent between sites. For example the abundance of Acropora spp. declined at many of the acidified sites (e.g. Palau) but increased ~ 65% under high pCO2 at the Indonesia seep site (Barkley et al. 2015; Januar et al. 2016). Despite the suggested resilience of soft corals to acidification, of the four sites that reported soft coral abundance, only two noted a significant increase in at seep sites with intermediate pCO2 conditions compared to the control sites. Furthermore, at these locations, soft coral cover subsequently declined at the highest pCO2 conditions surveyed (Fig. 3, Table 2).
Changes in species diversity, richness, and community composition with increasing pCO2
Coral species richness at the ten acidified sites reviewed here consistently declined with increased pCO2, likely driven by the loss of vulnerable species. Species richness has been reported (or can be inferred) at eight of the ten high pCO2 sites (Table 2), of which seven showed large declines in species richness, from − 31% to − 100%. The remaining site, Palau, had a 50% increase in species richness and a 10% increase in diversity (Shannon diversity index) between the least and most acidic site (Shamberger et al. 2014; Table 2). While this site provides optimistic evidence on the effect of acidification, there are a number of potential reasons for an increase in species richness. For example, the retention of pCO2 adapted larvae within the semi-enclosed lagoon, the duration and source of acidic exposure (hydrographic processes; Golbuu et al. 2016) which result in alterations to other water chemistry parameters such as low total alkalinity, and habitat differences between the control (outer reef) and acidified (lagoonal) sites reflecting differences in community composition.
A shift in coral community composition has been observed at every naturally acidified site studied to date compared to the control sites. This includes a shift from hard to soft coral (Iwotorishima, Japan), hard coral to macroalgae with corals still present (CNMI) or with corals absent (Shikine, Japan; Ishia, Italy), reduced richness of hard coral (PNG, Indonesia, Mesoamerica and ETP), and increased species richness of hard coral but a shift in dominant species (Palau; Table 2). This variety again shows the complexity of predicting the effect of acidification on coral communities but suggests that reefs are unlikely to retain their current composition in the future.
Evidence of indirect effects at naturally acidified locations
By reviewing studies of coral diversity and abundance at naturally acidified locations (Table 2), we identify some common indirect effects of OA that structure coral communities. The majority of sites (6 of 10) report an increase in macroalgal abundance as pCO2 increases. These studies support interpretation of an increase in competitive dominance by algae, and overgrowth of corals (Enochs et al. 2015b; Glynn et al. 2018) and of CCA (Kroeker et al. 2013; Crook et al. 2016; Table 3) under OA, which in some cases resulted in a complete phase shift to macroalgal abundance (Enochs et al. 2015b). In addition to changes in macroalgae, CCA consistently declines in abundance and diversity as pCO2 increases (Table 3). Evidence from the PNG vents indicates that these declines in CCA abundance were the strongest predictor of recruit density (Fabricius et al. 2017) suggesting a reduction in CCA may have major implications for the long-term persistence of corals at this site. Effects on recruitment have yet to be investigated at any other site. However, acidification also appears to reduce reef cementation in the ETP (Manzello et al. 2008) and at the Shikine vent in Japan where the thick carbonate crusts formed in part by CCA were notably thinner (Peña et al. 2021). This indicates a potential reduction in substrate stability in the future, with further implications for additional declines in recruitment.
Bioerosion tends to be higher at the acidified sites compared to nearby control sites (Table 3). This is due to an increase in either the abundance of bioeroders (Fabricius et al. 2011) or their bioerosive capacity (Enochs et al. 2016a) and potentially exacerbated by decreased coral skeletal density (Manzello et al. 2014; Barkley et al. 2015). The extent of this bioerosion is variable but in the site where corals appear to fare best under acidification (Palau), bioerosion was still 11-fold higher than the control reefs (Barkley et al. 2015). Furthermore, at a reef with lower coral cover in the ETP, bioerosion resulted in the complete loss of the reef framework following a major coral mortality event and prevented recovery back to reef status (Manzello et al. 2014). Consequently, indirect effects of bioerosion on the integrity of coral skeletons and the reef substratum are a significant factor for long term persistence of reefs under acidification.
Linked to changes in coral cover and bioerosion, almost every site (8 of 10) exhibited a decrease in structural complexity with acidification (Table 3). These changes have indirect effects on other organisms, for example, changes in abundance within the fish community from PNG were likely driven by changes in structure and habitat provision (Munday et al. 2014). However, the implications of such changes for corals have not been investigated. Without knowing whether the interactions with corals and fish and/or invertebrates had positive, negative or neutral effects on corals, it is hard to extrapolate what the impact of these changes might be. However, we stress that as the structural complexity of the reef plays a key role in ecosystem functions and processes, the indirect effects of loss of structural complexity on corals, and many other reef organisms, may be important.
Finally, the among-site differences in responses to changing pCO2 highlight that there are many potential internal and external factors that moderate indirect effects and therefore may shape how coral communities respond to OA. For example, the indirect effects reported here for PNG and CNMI are similar (increased macroalgal abundance, decreased CCA, increased bioerosion and a decrease in structural complexity) yet in PNG coral cover is maintained and in CNMI coral cover decreases (Table 2). This difference may result from both direct physiological effects (calcification declined at CNMI but not at PNG) and indirect effects (in PNG there was evidence of an increase in herbivorous urchin (Fabricius et al. 2014), while in the CNMI, the herbivores avoided predating the dominant algal species (Enochs et al. 2015b)). As highly complex environments, the number of potential external factors which may result in indirect effects is significant. For example, reefs are increasingly exposed to other anthropogenic stressors like rising water temperatures and decreasing water quality such as increased turbidity, pollution and/or eutrophication. Similarly, internal factors from within the coral holobiont, such as changes in both the microbial communities and zooxanthellae populations resulting from OA (Mason 2018; Biagi et al. 2020), may also act as a source of indirect effects on corals and coral communities. In conjunction with OA, these other factors may ultimately exacerbate the extent to which indirect effects affect corals and coral communities, either through compounding the effects highlighted here, or through additional indirect effects not yet considered. Clearly, potential feedbacks between changes in the physical environment as well as the diversity, abundance and behaviours of species in different coral reef trophic groups will shape coral reef futures.
The future of indirect effects research
In this review we have identified a range of indirect effects which have the potential to have significant impacts on coral and coral communities. Consequently, single-species studies that assess the effects of OA on coral physiology potentially underestimate the effects of OA on coral communities and limit our ability to extrapolate findings to an ecosystem scale. We therefore recommend that the indirect effects of acidification be more widely considered in acidification research.
Indirect effects research can be done using a number of research methods. Small-scale, short-term experiments are the basis of much ocean acidification research and an effective method to establish the impact on simple or paired interactions. Larger-scale, long-term aquaria or mesocosm experiments allow us to potentially include more ecosystem functions/processes such as competition and bioerosion. However, these experimental set-ups are limited in ecological complexity and therefore need to target specific species, interactions or processes. In-situ manipulations are becoming more feasible through the use of FOCE (Free Ocean Carbon Enrichment; Stark et al. 2019) and SCoRE-FOCE (Shallow Coral Reef Free Ocean Carbon Enrichment; Srednick et al. 2020) experimental flumes. In-situ flumes use natural communities and therefore have greater ecological complexity; however, they may be unable to account for interactions with more mobile taxa such as fish and can be challenging/expensive to run over ecologically meaningful timescales. FOCE experiments may therefore be most useful for research on benthic indirect effects such as bioerosion.
At present, studies using naturally acidified sites remain the only method where it is possible to consider large-scale community and ecosystem level effects, including multi-species interactions and effects on food-webs, using corals which have experienced lifetime exposure to acidification. However, these sites are not perfect analogues of future acidified conditions. For example, these sites often have connectivity to ambient reefs which allows new recruits to arrive from ambient reefs and movement of mobile organisms in and out of the acidified area (Munday et al. 2014). Physically these sites may also differ from future acidified reefs. For example, the source of acidity may also result in other changes to the water chemistry such as elevated levels of DIC (dissolved inorganic carbon; Crook et al. 2012) or other volcanic gases or elements (see review; Gonzalez-Delgado and Hernández 2018). Nevertheless, these sites are useful proxies to identify indirect effects of potential concern. We suggest that a combination of all research methods will allow us the greatest progression in our understanding of the indirect effects of acidification on corals.
Here we focused specifically on how indirect effects affect corals and coral communities but our review of the literature illuminates many other potential indirect effects via other taxa, and potentially complex feedbacks among taxa in different trophic groups. For example the increased competitive success of fleshy algae over CCA (Crook et al. 2016) which may or may not be mediated by herbivory. Interactions such as these equally have the potential to affect structure and function of our future coral reefs and also need to be investigated. Understanding ecosystem trajectories is particularly challenging when ecosystem functioning could change via a cascade of altered interactions, such as what might occur in response to an ecological tipping point or within a trophic chain (e.g. Ferreira et al. 2021). Clearly, however, the ecological impact of these indirect effects can be significant (Mumby 2017), and therefore identifying and understanding them is vitally important in our ability to scale up direct physiological effects of OA to an ecosystem level.
Future directions and knowledge gaps
Our review highlights multiple pathways of indirect effects of OA that have the potential to exacerbate the impact of the direct physiological effects on coral colonies (i.e. lower calcification, slower growth, decreased recruitment) and communities (reduced hard coral abundance and species richness). These direct effects, alone, can have flow-on effects to reef ecosystem services like food provisioning and coastal protection. Based on our synthesis of the literature, we propose that the indirect effects of greatest concern are that a decline in CCA, and the settlement cues and habitat they provide, is likely to drive a reduction in recruitment of corals and impede population replenishment after disturbances. We suggest that manipulative field experiments, such as controlled seeding of coral larvae at naturally acidified locations and monitoring of settlement success and post-settlement survival compared with nearby control locations, are needed to resolve existing data gaps. Moreover, declining CCA will lead to diminished levels of reef cementation which can cause coral mortality due to substratum instability (Madin et al. 2012). Detailed monitoring of coral survival after storms at naturally acidified locations compared with nearby control sites, accompanied by measurement of CCA abundance and substratum strength, will provide new insights about how CCA loss affects coral survival.
In addition to changes in CCA, increased bioerosion is likely to compound effects of decreased calcification and increase dissolution, to shift corals from net skeleton accretion towards net skeleton loss. While as yet there is limited evidence of a significant direct effect on coral abundance and diversity resulting from reduced structural complexity, a recent study indicates that coral biodiversity is highest at intermediate roughness (rugosity) of reef substrata (Torres-Pulliza et al. 2020). Field-based observations of the magnitude of bioerosion, and the diversity and abundance of bioeroding taxa, are required to link changes in bioerosion to differences in structural complexity on reefs, and to link these changes to coral diversity. A loss of structural complexity on reefs due to OA (via decreased cementation and increased erosion) is also likely to result in changes to reef functions more broadly. More complex reefs support higher diversity and abundance of fishes and therefore OA could affect the critical reef ecosystem services of food provision.
Our review highlights the need to extend from single species studies, to studies that explicitly characterise how species interactions change, and how trophic linkages are altered, as pCO2 increases. Key knowledge gaps in the literature include the effects of OA on: the prevalence and outcomes of competition between corals and other benthic organisms in the field; the prevalence and impact of predation by corallivores on corals, as well as effects on mutualistic interactions between corals and small fishes that live within their branches; the prevalence and virulence of pathogens that cause coral diseases; and the capacity for herbivorous fishes to reduce coral–macroalgal competition. We recommend that mesocosm experiments that manipulate seawater carbonate chemistry are used alongside field observations at naturally acidified sites to generate new knowledge of OA effects. Understanding how both the direct and indirect effects of OA impact the ecosystem engineers of coral reefs, and how these effects propagate throughout the entire reef foodweb, is critical to predicting coral reef futures as atmospheric and seawater pCO2 continue to increase.
References
Agostini S, Harvey BP, Wada S, Kon K, Milazzo M, Inaba K, Hall-Spencer JM (2018) Ocean acidification drives community shifts towards simplified non-calcified habitats in a subtropical− temperate transition zone. Sci Rep 8:11354
Agostini S, Houlbrèque F, Biscéré T, Harvey BP, Heitzman JM, Takimoto R, Yamazaki W, Milazzo M, Rodolfo-Metalpa R (2021) Greater mitochondrial energy production provides resistance to ocean acidification in “winning” hermatypic corals. Front Mar Sci 7:600836
Albright R, Mason B, Miller M, Langdon C (2010) Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata. Proc Natl Acad Sci USA 107:20400–20404
Alexander JM, Diez JM, Hart SP, Levine JM (2016) When climate reshuffles competitors: a call for experimental macroecology. Trends Ecol Evol 31:831–841
Alldredge A, King J (1977) Distribution, abundance, and substrate preferences of demersal reef zooplankton at Lizard Island Lagoon, Great Barrier Reef. Mar Biol 41:317–333
Allen R, Foggo A, Fabricius K, Balistreri A, Hall-Spencer JM (2017) Tropical CO2 seeps reveal the impact of ocean acidification on coral reef invertebrate recruitment. Mar Pollut Bull 124:607–613
Allison N, Cole C, Hintz C, Hintz K, Rae J, Finch A (2018) The effect of ocean acidification on tropical coral calcification: insights from calcification fluid DIC chemistry. Chem Geol 497:162–169
Alva-Basurto JC, Arias-González JE (2014) Modelling the effects of climate change on a Caribbean coral reef food web. Ecol Modell 289:1–14
Atrigenio M, Alino P (1996) Effects of the soft coral Xenia puertogalerae on the recruitment of scleractinian corals. J Exp Mar Biol Ecol 203:179–189
Baggini C, Issaris Y, Salomidi M, Hall-Spencer J (2015) Herbivore diversity improves benthic community resilience to ocean acidification. J Exp Mar Biol Ecol 469:98–104
Bahr KD, Rodgers KS, Jokiel PL (2018) Ocean warming drives decline in coral metabolism while acidification highlights species-specific responses. Mar Biol Res 14:924–935
Barkley HC, Cohen AL, Golbuu Y, Starczak VR, DeCarlo TM, Shamberger KE (2015) Changes in coral reef communities across a natural gradient in seawater pH. Sci Adv 1:e1500328
Bedwell-Ivers HE, Koch MS, Peach KE, Joles L, Dutra E, Manfrino C (2017) The role of in hospite zooxanthellae photophysiology and reef chemistry on elevated pCO2 effects in two branching Caribbean corals: Acropora cervicornis and Porites divaricata. ICES J Mar Sci 74:1103–1112
Biagi E, Caroselli E, Barone M, Pezzimenti M, Teixido N, Soverini M, Rampelli S, Turroni S, Gambi MC, Brigidi P, Goffredo S (2020) Patterns in microbiome composition differ with ocean acidification in anatomic compartments of the Mediterranean coral Astroides calycularis living at CO2 vents. Sci Total Environ 724:138048
Bonaldo RM, Hay ME (2014) Seaweed-coral interactions: variance in seaweed allelopathy, coral susceptibility, and potential effects on coral resilience. PLoS ONE 9:e85786
Bove CB, Ries JB, Davies SW, Westfield IT, Umbanhowar J, Castillo KD (2019) Common Caribbean corals exhibit highly variable responses to future acidification and warming. Proc R Soc Lond B Biol Sci 286:20182840
Brien HV, Watson SA, Hoogenboom MO (2016) Presence of competitors influences photosynthesis, but not growth, of the hard coral Porites cylindrica at elevated seawater CO2. ICES J Mar Sci 73:659–669
Brown KT, Bender-Champ D, Kenyon TM, Rémond C, Hoegh-Guldberg O, Dove S (2019) Temporal effects of ocean warming and acidification on coral–algal competition. Coral Reefs 38:297–309
Bruggemann JH, Van Oppen MJ, Breeman AM (1994) Foraging by the stoplight parrotfish Sparisoma viride. I. Food selection in different, socially determined habitats. Mar Ecol Prog Ser 106:41–55
Cahill AE, Aiello-Lammens ME, Fisher-Reid MC, Hua X, Karanewsky CJ, Ryu HY, Sbeglia GC, Spagnolo F, Waldron JB, Warsi O, Wiens JJ (2013) How does climate change cause extinction? Proc R Soc Lond B Biol Sci 280:20121890
Camp EF, Nitschke MR, Rodolfo-Metalpa R, Houlbreque F, Gardner SG, Smith DJ, Zampighi M, Suggett DJ (2017) Reef-building corals thrive within hot-acidified and deoxygenated waters. Sci Rep 7:2434
Campbell JE, Sneed JM, Johnston L, Paul VJ (2017) Effects of ocean acidification and contact with the brown alga Stypopodium zonale on the settlement and early survival of the coral Porites astreoides. Mar Ecol Prog Ser 577:67–77
Carbonne C, Teixidó N, Moore B, Mirasole A, Guttierez T, Gattuso JP, Comeau S (2021) Two temperate corals are tolerant to low pH regardless of previous exposure to natural CO2 vents. Limnol Oceanogr 66:1–16
Cattano C, Agostini S, Harvey BP, Wada S, Quattrocchi F, Turco G, Inaba K, Hall-Spencer JM, Milazzo M (2020) Changes in fish communities due to benthic habitat shifts under ocean acidification conditions. Sci Total Environ 725:138501
Chan N, Connolly SR (2013) Sensitivity of coral calcification to ocean acidification: a meta-analysis. Glob Change Biol 19:282–290
Chase T, Pratchett M, Frank G, Hoogenboom M (2018) Coral-dwelling fish moderate bleaching susceptibility of coral hosts. PLoS ONE 13:e0208545
Chase TJ, Pratchett MS, Walker SPW, Hoogenboom MO (2014) Small-scale environmental variation influences whether coral-dwelling fish promote or impede coral growth. Oecologia 176:1009–1022
Cole A, Lawton R, Pratchett M, Wilson S (2011) Chronic coral consumption by butterflyfishes. Coral Reefs 30:85–93
Comeau S, Cornwall CE, Shlesinger T, Hoogenboom M, Mana R, McCulloch MT, Rodolfo-Metalpa R (2022) pH variability at volcanic CO2 seeps regulates coral calcifying fluid chemistry. Glob Change Biol. https://doi.org/10.1111/gcb.16093
Condie SA, Anthony KR, Babcock RC, Baird ME, Beeden R, Fletcher CS, Gorton R, Harrison D, Hobday AJ, Plagányi ÉE, Westcott DA (2021) Large-scale interventions may delay decline of the Great Barrier Reef. R Soc Open Sci 8:201296
Connell JH, Hughes TP, Wallace CC, Tanner JE, Harms KE, Kerr AM (2004) A long-term study of competition and diversity of corals. Ecol Monogr 74:179–210
Connell SD, Russell BD, Irving AD (2011) Can strong consumer and producer effects be reconciled to better forecast ‘catastrophic’ phase-shifts in marine ecosystems? J Exp Mar Biol Ecol 400:296–301
Connell SD, Kroeker KJ, Fabricius KE, Kline DI, Russell BD (2013) The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Philos Trans R Soc Lond B Biol Sci 368:20120442
Cosain-Díaz JA, Tortolero-Langarica JDJA, Rodríguez-Troncoso AP, Bautista-Guerrero E, Antuna-Roman DM, Salazar-Silva P, Cupul-Magaña AL (2021) Internal bioerosion in massive corals associated with reef communities in the northeastern tropical Pacific: the effect of intrinsic and extrinsic factors. Cienc Mar 47:33–47
Crook E, Potts D, Rebolledo-Vieyra M, Hernandez L, Paytan A (2012) Calcifying coral abundance near low-pH springs: implications for future ocean acidification. Coral Reefs 31:239–245
Crook ED, Cohen AL, Rebolledo-Vieyra M, Hernandez L, Paytan A (2013) Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. Proc Natl Acad Sci USA 110:11044–11049
Crook ED, Kroeker KJ, Potts DC, Rebolledo-Vieyra M, Hernandez-Terrones LM, Paytan A (2016) Recruitment and succession in a tropical benthic community in response to in-situ ocean acidification. PLoS ONE 11:e0146707
Dai C-f (1990) Interspecific competition in Taiwanese corals with special reference to interactions between alcyonaceans and scleractinians. Mar Ecol Prog Ser 60:291–297
DeCarlo TM, Cohen AL, Barkley HC, Cobban Q, Young C, Shamberger KE, Brainard RE, Golbuu Y (2015) Coral macrobioerosion is accelerated by ocean acidification and nutrients. Geology 43:7–10
Del Monaco C, Hay ME, Gartrell P, Mumby PJ, Diaz-Pulido G (2017) Effects of ocean acidification on the potency of macroalgal allelopathy to a common coral. Sci Rep 7:41053
Di Santo V (2019) Ocean acidification and warming affect skeletal mineralization in a marine fish. Proc R Soc B 286:20182187
Diaz-Pulido G, Gouezo M, Tilbrook B, Dove S, Anthony KR (2011) High CO2 enhances the competitive strength of seaweeds over corals. Ecol Lett 14:156–162
Diaz-Pulido G, Barrón C (2020) CO2 enrichment stimulates dissolved organic carbon release in coral reef macroalgae. J Phycol 56:1039–1052
Doo SS, Carpenter RC, Edmunds PJ (2018) Obligate ectosymbionts increase the physiological resilience of a scleractinian coral to high temperature and elevated pCO2. Coral Reefs 37:997–1001
Doropoulos C, Diaz-Pulido G (2013) High CO2 reduces the settlement of a spawning coral on three common species of crustose coralline algae. Mar Ecol Prog Ser 475:93–99
Doropoulos C, Ward S, Diaz-Pulido G, Hoegh-Guldberg O, Mumby PJ (2012) Ocean acidification reduces coral recruitment by disrupting intimate larval-algal settlement interactions. Ecol Lett 15:338–346
Edmunds PJ, Yarid A (2017) The effects of ocean acidification on wound repair in the coral Porites spp. J Exp Mar Biol Ecol 486:98–104
Edmunds PJ, Comeau S, Lantz C, Andersson A, Briggs C, Cohen A, Gattuso J-P, Grady JM, Gross K, Johnson M, Muller EB, Ries JB, Tambutté S, Tambutté E, Venn A, Carpenter RC (2016) Integrating the effects of ocean acidification across functional scales on tropical coral reefs. Bioscience 66:350–362
Enochs IC, Manzello DP, Carlton RD, Graham DM, Ruzicka R, Colella MA (2015a) Ocean acidification enhances the bioerosion of a common coral reef sponge: implications for the persistence of the Florida Reef Tract. Bull Mar Sci 91:271–290
Enochs IC, Manzello DP, Kolodziej G, Noonan SH, Valentino L, Fabricius KE (2016a) Enhanced macroboring and depressed calcification drive net dissolution at high-CO2 coral reefs. Proc R Soc Lond B Biol Sci 283:20161742
Enochs IC, Manzello DP, Tribollet A, Valentino L, Kolodziej G, Donham EM, Fitchett MD, Carlton R, Price NN (2016b) Elevated colonization of microborers at a volcanically acidified coral reef. PLoS ONE 11:e0159818
Enochs IC, Manzello DP, Donham EM, Kolodziej G, Okano R, Johnston L, Young C, Iguel J, Edwards CB, Fox MD, Valentino L, Johnson S, Benavente D, Clark SJ, Carlton R, Burton T, Eynaud Y, Price NN (2015b) Shift from coral to macroalgae dominance on a volcanically acidified reef. Nat Clim Change 5:1083–1088
Erez J, Reynaud S, Silverman J, Schneider K, Allemand D (2011) Coral calcification under ocean acidification and global change. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 151–176
Evensen NR, Edmunds PJ (2016) Interactive effects of ocean acidification and neighboring corals on the growth of Pocillopora verrucosa. Mar Biol 163:1–11
Evensen NR, Edmunds PJ (2018) Effect of elevated pCO2 on competition between the scleractinian corals Galaxea fascicularis and Acropora hyacinthus. J Exp Mar Biol Ecol 500:12–17
Evensen NR, Edmunds PJ, Sakai K (2015) Effects of pCO2 on spatial competition between the corals Montipora aequituberculata and Porites lutea. Mar Ecol Prog Ser 541:123–134
Evensen NR, Bozec Y-M, Edmunds PJ, Mumby PJ (2021) Scaling the effects of ocean acidification on coral growth and coral–coral competition on coral community recovery. PeerJ 9:e11608
Fabricius K, Kluibenschedl A, Harrington L, Noonan S, De’ath G (2015) In situ changes of tropical crustose coralline algae along carbon dioxide gradients. Sci Rep 5:9537
Fabricius KE, De’ath G, Noonan S, Uthicke S (2014) Ecological effects of ocean acidification and habitat complexity on reef-associated macroinvertebrate communities. Proc R Soc Lond B Biol Sci 281:20132479
Fabricius KE, Noonan SHC, Abrego D, Harrington L, De’ath G (2017) Low recruitment due to altered settlement substrata as primary constraint for coral communities under ocean acidification. Proc R Soc Lond B Biol Sci 284:20171536
Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, De’ath G, Okazaki R, Muehllehner N, Glas MS, Lough JM (2011) Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat Clim Change 1:165–169
Fang JK, Mello-Athayde MA, Schönberg CH, Kline DI, Hoegh-Guldberg O, Dove S (2013) Sponge biomass and bioerosion rates increase under ocean warming and acidification. Glob Change Biol 19:3581–3591
Fantazzini P, Mengoli S, Pasquini L, Bortolotti V, Brizi L, Mariani M, Di Giosia M, Fermani S, Capaccioni B, Caroselli E, Prada F, Zaccanti F, Levy O, Dubinsky Z, Kaandorp JA, Konglerd P, JrU H, Dauphin Y, Cuif J-P, Weaver JC, Fabricius KE, Wagermaier W, Fratzl P, Falini G, Goffredo S (2015) Gains and losses of coral skeletal porosity changes with ocean acidification acclimation. Nat Commun 6:7785
Ferrari MC, McCormick MI, Munday PL, Meekan MG, Dixson DL, Lonnstedt Ö, Chivers DP (2011) Putting prey and predator into the CO2 equation–qualitative and quantitative effects of ocean acidification on predator–prey interactions. Ecol Lett 14:1143–1148
Ferrari R, Gonzalez-Rivero M, Mumby PJ (2012) Size matters in competition between corals and macroalgae. Mar Ecol Prog Ser 467:77–88
Ferreira CM, Connell SD, Goldenberg SU, Nagelkerken I (2021) Positive species interactions strengthen in a high-CO2 ocean. Proc R Soc Lond B Biol Sci 288:20210475
Fine M, Tchernov D (2007) Scleractinian coral species survive and recover from decalcification. Science 315:1811
Foo SA, Byrne M, Ricevuto E, Gambi MC (2018) The carbon dioxide vents of Ischia, Italy, a natural system to assess impacts of ocean acidification on marine ecosystems: an overview of research and comparisons with other vent systems. Oceanogr Mar Biol Annu Rev 56:237–310
Gabay Y, Fine M, Barkay Z, Benayahu Y (2014) Octocoral tissue provides protection from declining oceanic pH. PLoS ONE 9:e91553
Garcia-Herrera N, Ferse SC, Kunzmann A, Genin A (2017) Mutualistic damselfish induce higher photosynthetic rates in their host coral. J Exp Biol 220:1803–1811
Garrard SL, Hunter RC, Frommel AY, Lane AC, Phillips JC, Cooper R, Dineshram R, Cardini U, McCoy SJ, Arnberg M, Rodrigues Alves BG, Annane S, de Orte MR, Kumar A, Aguirre-Martínez GV, Maneja RH, Basallote MD, Ape F, Torstensson A, Bjoerk MM (2013) Biological impacts of ocean acidification: a postgraduate perspective on research priorities. Mar Biol 160:1789–1805
Gattuso J-P, Magnan A, Billé R, Cheung WW, Howes EL, Joos F, Allemand D, Bopp L, Cooley SR, Eakin CM, Hoegh-Guldberg O, Kelly R, Portner H, Rogers A, Baxter J, Laffoley D, Osborn D, Rankovic A, Rochette J, Sumaila U, Treyer S, Turley C (2015) Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 349:aac4722
Glynn PW, Manzello DP (2015) Bioerosion and coral reef growth: a dynamic balance. In: Birkeland C (ed) Coral reefs in the Anthropocene. Springer, pp 67–97
Glynn PW, Feingold JS, Baker A, Banks S, Baums IB, Cole J, Colgan MW, Fong P, Glynn PJ, Keith I, Manzello DP, Riegl B, Ruttenberg BI, Smith TB, Vera-Zambrano M (2018) State of corals and coral reefs of the Galápagos Islands (Ecuador): Past, present and future. Mar Pollut Bull 133:717–733
Goffredo S, Prada F, Caroselli E, Capaccioni B, Zaccanti F, Pasquini L, Fantazzini P, Fermani S, Reggi M, Levy O, Fabricius K, Dubinsky Z, Falini G (2014) Biomineralization control related to population density under ocean acidification. Nat Clim Change 4:593–597
Golbuu Y, Gouezo M, Kurihara H, Rehm L, Wolanski E (2016) Long-term isolation and local adaptation in Palau’s Nikko Bay help corals thrive in acidic waters. Coral Reefs 35:909–918
Gonzalez-Delgado S, Hernández JC (2018) The importance of natural acidified systems in the study of ocean acidification: what have we learned? Adv Mar Biol 80:57–99
Graham NAJ, Nash KL (2013) The importance of structural complexity in coral reef ecosystems. Coral Reefs 32:315–326
Grottoli AG, Dalcin Martins P, Wilkins MJ, Johnston MD, Warner ME, Cai W-J, Melman TF, Hoadley KD, Pettay DT, Levas S, Schoepf V (2018) Coral physiology and microbiome dynamics under combined warming and ocean acidification. PLoS ONE 13:e0191156
Guinotte JM, Fabry VJ (2008) Ocean acidification and its potential effects on marine ecosystems. Ann N Y Acad Sci 1134:320–342
Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia M-C (2008) Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454:96–99
Harvey BP, Gwynn-Jones D, Moore PJ (2013) Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol Evol 3:1016–1030
Harvey BP, Agostini S, Kon K, Wada S, Hall-Spencer JM (2019) Diatoms dominate and alter marine food-webs when CO2 rises. Diversity 11:242
Hennige S, Wicks L, Kamenos N, Perna G, Findlay H, Roberts J (2015) Hidden impacts of ocean acidification to live and dead coral framework. Proc R Soc Lond B Biol Sci 282:20150990
Heyward A, Negri A (1999) Natural inducers for coral larval metamorphosis. Coral Reefs 18:273–279
Hofmann GE, Barry JP, Edmunds PJ, Gates RD, Hutchins DA, Klinger T, Sewell MA (2010) The effect of ocean acidification on calcifying organisms in marine ecosystems: an organism-to-ecosystem perspective. Annu Rev Ecol Evol Syst 41:127–147
Hofmann LC, Bischof K (2014) Ocean acidification effects on calcifying macroalgae. Aquat Biol 22:261–279
Horwitz R, Hoogenboom MO, Fine M (2017) Spatial competition dynamics between reef corals under ocean acidification. Sci Rep 7:40288
Houlbrèque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biol Rev 84:1–17
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–365
Inoue S, Kayanne H, Yamamoto S, Kurihara H (2013) Spatial community shift from hard to soft corals in acidified water. Nat Clim Change 3:683–687
IPCC (2014) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. [Core Writing Team, R.K. Pachauri, L.A. Meyers (eds.)]. IPCC, Geneva, Switzerland.
Januar HI, Zamani NP, Soedarma D, Chasanah E (2016) Changes in soft coral Sarcophyton sp. abundance and cytotoxicity at volcanic CO2 seeps in Indonesia. AIMS Environ Sci 3:239–248
Januar HI, Zamani NP, Soedarma D, Chasanah E, Wright AD (2017) Tropical coral reef coral patterns in Indonesian shallow water areas close to underwater volcanic vents at Minahasa Seashore, and Mahengetang and Gunung Api Islands. Mar Ecol 38:e12415
Jiang L, Zhang F, Guo M-L, Guo Y-J, Zhang Y-Y, Zhou G-W, Cai L, Lian J-S, Qian P-Y, Huang H (2018) Increased temperature mitigates the effects of ocean acidification on the calcification of juvenile Pocillopora damicornis, but at a cost. Coral Reefs 37:71–79
Johnson MD, Price NN, Smith JE (2014) Contrasting effects of ocean acidification on tropical fleshy and calcareous algae. PeerJ 2:e411
Jordano P (2016) Chasing ecological interactions. PLoS Biol 14:e1002559
Kamya PZ, Byrne M, Mos B, Dworjanyn SA (2018) Enhanced performance of juvenile crown of thorns starfish in a warm-high CO2 ocean exacerbates poor growth and survival of their coral prey. Coral Reefs 37:751–762
Kline DI, Teneva L, Okamoto DK, Schneider K, Caldeira K, Miard T, Chai A, Marker M, Dunbar RB, Mitchell BG, Dove S, Hoegh-Guldberg O (2019) Living coral tissue slows skeletal dissolution related to ocean acidification. Nat Ecol Evol 3:1438–1444
Kornder NA, Riegl BM, Figueiredo J (2018) Thresholds and drivers of coral calcification responses to climate change. Glob Change Biol 24:5084–5095
Kroeker KJ, Micheli F, Gambi MC (2013) Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat Clim Change 3:156
Kroeker KJ, Micheli F, Gambi MC, Martz TR (2011) Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc Natl Acad Sci USA 108:14515–14520
Kuffner IB, Andersson AJ, Jokiel PL, Ku’ulei SR, Mackenzie FT (2008) Decreased abundance of crustose coralline algae due to ocean acidification. Nat Geosci 1:114–117
Lamb JB, Willis BL, Fiorenza EA, Couch CS, Howard R, Rader DN, True JD, Kelly LA, Ahmad A, Jompa J, Harvell CD (2018) Plastic waste associated with disease on coral reefs. Science 359(6374):460–462
Lang JC, Chornesky EA (1990) Competition between scleractinian reef corals—a review of mechanisms and effects. In: Dubinsky Z (ed) Ecosystems of the world: coral Reefs. Elsevier, Amsterdam, pp 209–252
Lenihan HS, Holbrook SJ, Schmitt RJ, Brooks AJ (2011) Influence of corallivory, competition, and habitat structure on coral community shifts. Ecology 92:1959–1971
Littler MM, Taylor PR, Littler DS (1989) Complex interactions in the control of coral zonation on a Caribbean reef flat. Oecologia 80:331–340
Madin JS, Hughes TP, Connolly SR (2012) Calcification, storm damage and population resilience of tabular corals under climate change. PLoS ONE 7:e46637
Manning JC, Carpenter RC, Miranda EA (2019) Ocean acidification reduces net calcification and wound healing in the tropical crustose coralline alga, Porolithon onkodes (Corallinales, Rhodophyta). J Exp Mar Biol Ecol 520:151225
Manzello DP, Kleypas JA, Budd DA, Eakin CM, Glynn PW, Langdon C (2008) Poorly cemented coral reefs of the Eastern Tropical Pacific: possible insights into reef development in a high-CO2 world. Proc Natl Acad Sci 105:10450–10455
Manzello DP, Enochs IC, Bruckner A, Renaud PG, Kolodziej G, Budd DA, Carlton R, Glynn PW (2014) Galápagos coral reef persistence after ENSO warming across an acidification gradient. Geophys Res Lett 41:9001–9008
Marcelino VR, Morrow KM, van Oppen MJ, Bourne DG, Verbruggen H (2017) Diversity and stability of coral endolithic microbial communities at a naturally high pCO2 reef. Mol Ecol 26:5344–5357
Mason RA (2018) Decline in symbiont densities of tropical and subtropical scleractinian corals under ocean acidification. Coral Reefs 37:945–953
McCulloch M, Falter J, Trotter J, Montagna P (2012) Coral resilience to ocean acidification and global warming through pH up-regulation. Nat Clim Change 2:623–627
McCulloch MT, D’Olivo JP, Falter J, Holcomb M, Trotter JA (2017) Coral calcification in a changing world and the interactive dynamics of pH and DIC upregulation. Nat Commun 8:1–8
Meron D, Rodolfo-Metalpa R, Cunning R, Baker AC, Fine M, Banin E (2012) Changes in coral microbial communities in response to a natural pH gradient. ISME J 6:1775–1785
Meron D, Atias E, Kruh LI, Elifantz H, Minz D, Fine M, Banin E (2011) The impact of reduced pH on the microbial community of the coral Acropora eurystoma. ISME J 5:51–60
Mirasole A, Scopelliti G, Tramati C, Signa G, Mazzola A, Vizzini S (2021) Evidences on alterations in skeleton composition and mineralization in a site-attached fish under naturally acidified conditions in a shallow CO2 vent. Sci Total Environ 761:143309
Mollica NR, Guo W, Cohen AL, Huang K-F, Foster GL, Donald HK, Solow AR (2018) Ocean acidification affects coral growth by reducing skeletal density. Proc Natl Acad Sci USA 115:1754–1759
Morrow KM, Bourne DG, Humphrey C, Botte ES, Laffy P, Zaneveld J, Uthicke S, Fabricius KE, Webster NS (2015) Natural volcanic CO2 seeps reveal future trajectories for host-microbial associations in corals and sponges. ISME J 9:894–908
Muller EM, Leporacci NM, Macartney KJ, Shea AG, Crane RE, Hall ER, Ritchie KB (2017) Low pH reduces the virulence of black band disease on Orbicella faveolata. PLoS ONE 12:e0178869
Mumby PJ (2017) Embracing a world of subtlety and nuance on coral reefs. Coral Reefs 36:1003–1011
Munday PL, Cheal AJ, Dixson DL, Rummer JL, Fabricius KE (2014) Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nat Clim Change 4:487–492
National Research Council (2010) Ocean acidification: a national strategy to meet the challenges of a changing ocean. The National Academies Press, Washington, DC
Olsen K, Paul VJ, Ross C (2015) Direct effects of elevated temperature, reduced pH, and the presence of macroalgae (Dictyota spp.) on larvae of the Caribbean coral Porites astreoides. Bull Mar Sci 91:255–270
Ordoñez A, Kennedy EV, Diaz-Pulido G (2017) Reduced spore germination explains sensitivity of reef-building algae to climate change stressors. PLoS ONE 12:e0189122
Page HN, Hewett C, Tompkins H, Hall ER (2021) Ocean acidification and direct interactions affect coral, macroalga, and sponge growth in the Florida Keys. J Mar Sci Eng 9:739
Peña V, Harvey BP, Agostini S, Porzio L, Milazzo M, Horta P, Le Gall L, Hall-Spencer JM (2021) Major loss of coralline algal diversity in response to ocean acidification. Glob Change Biol 27:4785–4798
Pimentel MS, Faleiro F, Dionísio G, Repolho T, Pousão-Ferreira P, Machado J, Rosa R (2014) Defective skeletogenesis and oversized otoliths in fish early stages in a changing ocean. J Exp Bio 217(12):2062–2070
Pitts KA, Campbell JE, Figueiredo J, Fogarty ND (2020) Ocean acidification partially mitigates the negative effects of warming on the recruitment of the coral, Orbicella faveolata. Coral Reefs 39:281–292
Porzio L, Buia MC, Hall-Spencer JM (2011) Effects of ocean acidification on macroalgal communities. J Exp Mar Biol Ecol 400:278–287
Porzio L, Garrard SL, Buia MC (2013) The effect of ocean acidification on early algal colonization stages at natural CO2 vents. Mar Biol 160:2247–2259
Remily ER, Richardson LL (2006) Ecological physiology of a coral pathogen and the coral reef environment. Microb Ecol 51:345–352
Rivest EB, Kelly MW, DeBiasse MB, Hofmann GE (2018) Host and symbionts in Pocillopora damicornis larvae display different transcriptomic responses to ocean acidification and warming. Front Mar Sci 5:186
Rivest EB, Chen CS, Fan TY, Li HH, Hofmann GE (2017) Lipid consumption in coral larvae differs among sites: a consideration of environmental history in a global ocean change scenario. Proc R Soc Lond B Biol Sci 284:20162825
Sammarco P, Coll J, La Barre S, Willis B (1983) Competitive strategies of soft corals (Coelenterata: Octocorallia): allelopathic effects on selected scleractinian corals. Coral Reefs 1:173–178
Santano J, Milton IA, Navarro B, Warren RM, Barber PH, Fong P, Fong CR (2021) Structural complexity shapes the behavior and abundance of a common herbivorous fish, increasing herbivory on a turf-dominated, fringing reef. J Exp Mar Biol Ecol 537:151515
Schönberg C, Ortiz J (2008) Is sponge bioerosion increasing? In: Proceedings of the 11th International Coral Reef Symposium. Vol 1, pp 520–523
Schönberg CHL, Fang JKH, Carreiro-Silva M, Tribollet A, Wisshak M (2017) Bioerosion: the other ocean acidification problem. ICES J Mar Sci 74:895–925
Sekizawa A, Uechi H, Iguchi A, Nakamura T, Kumagai NH, Suzuki A, Sakai K, Nojiri Y (2017) Intraspecific variations in responses to ocean acidification in two branching coral species. Mar Pollut Bull 122:282–287
Shamberger KE, Lentz SJ, Cohen AL (2018) Low and variable ecosystem calcification in a coral reef lagoon under natural acidification. Limnol Oceanogr 63:714–730
Shamberger KE, Cohen AL, Golbuu Y, McCorkle DC, Lentz SJ, Barkley HC (2014) Diverse coral communities in naturally acidified waters of a Western Pacific reef. Geophys Res Lett 41:499–504
Silverman J, Lazar B, Cao L, Caldeira K, Erez J (2009) Coral reefs may start dissolving when atmospheric CO2 doubles. Geophys Res Lett 36:L05606
Smith JN, De’ath G, Richter C, Cornils A, Hall-Spencer JM, Fabricius KE (2016) Ocean acidification reduces demersal zooplankton that reside in tropical coral reefs. Nat Clim Change 6:1124–1130
Sokolow S (2009) Effects of a changing climate on the dynamics of coral infectious disease: a review of the evidence. Dis Aquat Org 87:5–18
Srednick G, Bergman JL, Doo SS, Hawthorn M, Ferree J, Rojas R, Arias R, Edmunds PJ, Carpenter RC (2020) Shallow coral reef free ocean carbon enrichment: novel in situ flumes to manipulate pCO2 on shallow tropical coral reef communities. Limnol Oceanogr Methods 18:116–128
Stark JS, Peltzer ET, Kline DI, Queirós AM, Cox TE, Headley K, Barry J, Gazeau F, Runcie JW, Widdicombe S (2019) Free Ocean CO2 Enrichment (FOCE) experiments: Scientific and technical recommendations for future in situ ocean acidification projects. Prog Oceanogr 172:89–107
Stella JS, Pratchett MS, Hutchings P, Jones GP (2011) Coral-associated invertebrates: diversity, ecological importance and vulnerability to disturbance. Oceanogr Mar Biol Annu Rev 49:43–104
Strahl J, Francis D, Doyle J, Humphrey C, Fabricius K (2016) Biochemical responses to ocean acidification contrast between tropical corals with high and low abundances at volcanic carbon dioxide seeps. ICES J Mar Sci 73:897–909
Stubler AD, Furman BT, Peterson BJ (2014) Effects of pCO2 on the interaction between an excavating sponge, Cliona varians, and a hermatypic coral, Porites furcata. Mar Biol 161:1851–1859
Stubler AD, Furman BT, Peterson BJ (2015) Sponge erosion under acidification and warming scenarios: differential impacts on living and dead coral. Glob Change Biol 21:4006–4020
Sunday JM, Fabricius KE, Kroeker KJ, Anderson KM, Brown NE, Barry JP, Connell SD, Dupont S, Gaylord B, Hall-Spencer JM, Klinger T, Milazzo M, Munday PL, Russell BD, Sanford E, Thiyagarajan V, Vaughan MLH, Widdicombe S, Harley CDG (2016) Ocean acidification can mediate biodiversity shifts by changing biogenic habitat. Nat Clim Change 7:81–85
Tambutté E, Venn AA, Holcomb M, Segonds N, Techer N, Zoccola D, Allemand D, Tambutté S (2015) Morphological plasticity of the coral skeleton under CO2-driven seawater acidification. Nat Commun 6:7368
Teixidó N, Caroselli E, Alliouane S, Ceccarelli C, Comeau S, Gattuso JP, Fici P, Micheli F, Mirasole A, Monismith SG, Munari M, Palumbi SR, Sheets E, Urbini L, De Vittor C, Goffredo S, Gambi MC (2020) Ocean acidification causes variable trait-shifts in a coral species. Glob Change Biol 26:6813–6830
Torres-Pulliza D, Dornelas MA, Pizarro O, Bewley M, Blowes SA, Boutros N, Brambilla V, Chase TJ, Frank G, Friedman A, Hoogenboom MO, Williams S, Zawada KJA, Madin JS (2020) A geometric basis for surface habitat complexity and biodiversity. Nat Ecol Evol 4:1495–1501
Towle EK, Enochs IC, Langdon C (2015) Threatened Caribbean coral is able to mitigate the adverse effects of ocean acidification on calcification by increasing feeding rate. PLoS ONE 10:e0123394
Tracy AM, Pielmeier ML, Yoshioka RM, Heron SF, Harvell CD (2019) Increases and decreases in marine disease reports in an era of global change. Proc R Soc Lond B Biol Sci 286:20191718
Tracy AM, Weil E, Harvell CD (2020) Warming and pollutants interact to modulate octocoral immunity and shape disease outcomes. Ecol Appl 30:e02024
Traylor-Knowles N, Connelly MT (2017) What is currently known about the effects of climate change on the coral immune response. Curr Clim Change Rep 3:252–260
Tribollet A, Golubic S (2011) Reef bioerosion: agents and processes. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 435–449
Tribollet A, Grange J, Parra H, Rodolfo-Metalpa R, Carreiro-Silva M (2018) Limited carbonate dissolution by boring microflora at two volcanically acidified temperate sites: Ischia (Italy, Mediterranean Sea) and Faial (Azores, NE Atlantic Ocean). Global Biogeochem Cycles 32:78–91
Uthicke S, Ebert T, Liddy M, Johansson C, Fabricius KE, Lamare M (2016) Echinometra sea urchins acclimatized to elevated pCO2 at volcanic vents outperform those under present-day pCO2 conditions. Glob Change Biol 22:2451–2461
Valentino LM (2014) The effects of ocean acidification on bioerosion in the back reef of Moorea, French Polynesia. California State University, Northridge
Vega Thurber R, Willner-Hall D, Rodriguez-Mueller B, Desnues C, Edwards RA, Angly F, Dinsdale E, Kelly L, Rohwer F (2009) Metagenomic analysis of stressed coral holobionts. Environ Microbiol 11:2148–2163
Vega Thurber RL, Burkepile DE, Fuchs C, Shantz AA, McMinds R, Zaneveld JR (2014) Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob Change Biol 20:544–554
Vega Thurber R, Mydlarz LD, Brandt M, Harvell D, Weil E, Raymundo L, Willis BL, Langevin S, Tracy AM, Littman R, Kemp KM, Dawkins P, Prager KC, Garren M, Lamb J (2020) Deciphering coral disease dynamics: integrating host, microbiome, and the changing environment. Front Ecol Evol 8:575927
Vieira C (2020) Lobophora–coral interactions and phase shifts: summary of current knowledge and future directions. Aquat Ecol 54:1–20
Vizzini S, Martinez-Crego B, Andolina C, Massa-Gallucci A, Connell SD, Gambi MC (2017) Ocean acidification as a driver of community simplification via the collapse of higher-order and rise of lower-order consumers. Sci Rep 7:4018
Vogel N, Cantin N, Strahl J, Kaniewska P, Bay L, Wild C, Uthicke S (2016) Interactive effects of ocean acidification and warming on coral reef associated epilithic algal communities under past, present-day and future ocean conditions. Coral Reefs 35:715–728
Webb AE, van Heuven SM, de Bakker DM, van Duyl FC, Reichart G-J, de Nooijer LJ (2017) Combined effects of experimental acidification and eutrophication on reef sponge bioerosion rates. Front Mar Sci 4:311
Webster NS, Uthicke S, Botte ES, Flores F, Negri AP (2013) Ocean acidification reduces induction of coral settlement by crustose coralline algae. Glob Change Biol 19:303–315
Wisshak M, Schönberg CH, Form A, Freiwald A (2012) Ocean acidification accelerates reef bioerosion. PLoS ONE 7:e45124
Yuan X, Yuan T, Huang H, Jiang L, Zhou W, Liu S (2018) Elevated CO2 delays the early development of scleractinian coral Acropora gemmifera. Sci Rep 8:2787
Zeebe RE (2012) History of seawater carbonate chemistry, atmospheric CO2, and ocean acidification. Annu Rev Earth Planet Sci 40:141–165
Zunino S, Canu DM, Bandelj V, Solidoro C (2017) Effects of ocean acidification on benthic organisms in the Mediterranean Sea under realistic climatic scenarios: a meta-analysis. Reg Stud Mar Sci 10:86–96
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Morgan S. Pratchett
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hill, T.S., Hoogenboom, M.O. The indirect effects of ocean acidification on corals and coral communities. Coral Reefs 41, 1557–1583 (2022). https://doi.org/10.1007/s00338-022-02286-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02286-z