Abstract
High-latitude reefs are suboptimal coral habitats, but such habitats are increasingly considered to be potential refugia from climate change for range-shifting coral reef species. Notably, tropical reef fish have been observed along the south-east coast of Australia, but their establishment on temperate rocky reefs is currently limited by winter minimum temperatures and other resource needs, such as structurally complex habitats typical of tropical reefs. Recent expansion of the branching subtropical coral Pocillopora aliciae in rocky reefs near Sydney (34° S) could diversify the architectural structure of temperate marine environments, thereby providing potential shelter for tropical reef taxa in warming seas. Here, we investigated whether future environmental conditions (i.e. temperature increase) can influence the dominance of the subtropical branching coral P. aliciae over the resident encrusting coral Plesiastrea versipora in coastal Sydney by characterising physiological (e.g. metabolic stability) and behavioural (e.g. interspecific competitive hierarchy) traits that contribute to their competitive fitness. Our results suggest that a metabolic response, mediated by sterol and lipid metabolic pathways and provision of antioxidants, allows P. aliciae to reduce cellular stress and withstand exposure to short-term increased temperature. Conversely, P. versipora was more susceptible to heat exposure with no metabolic mediation observed. While P. versipora displayed greater aggressive behaviour when in direct contact with P. aliciae under all temperature conditions, the superior physiological and metabolic flexibility under increased temperatures of P. aliciae suggests that this species will likely outperform P. versipora under future increased temperatures. Such contrasting responses to environmental change would facilitate shifts in coral community and functional composition that could support further tropicalisation of coastal New South Wales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Warming of the global oceans from climate change is causing marine species to extend their ranges poleward to stay within the bounds of their thermal optima (Cheung et al. 2012; Poloczanska et al. 2013; Vergés et al. 2014). Although high-latitude ecosystems represent suboptimal habitats for tropical reef-building corals (Order Scleractinia) as a result of lower aragonite saturation state and pronounced seasonality of temperature and light regimes (Sommer et al. 2018), they are increasingly recognised as potential refugia for tropical reef species impacted by climate change, including scleractinian corals (Beger et al. 2014; Camp et al. 2018a, b). Indeed, marine tropical species have already migrated into cooler, higher latitude areas in response to climate change (Poloczanska et al. 2016), like for instance tropical reef fish and coral species in Australia (Baird et al. 2012; Booth and Sear 2018). Over 150 have been observed along the temperate coast of Sydney, Australia (34° S) since 2002, although only 20 have overwintered for at least one year (Booth et al. 2018). While overwinter survival of tropical fish is closely linked with minimum temperatures, other factors (e.g. increased habitat structural complexity provided by corals) may further contribute to the establishment of tropical fish communities on temperate reefs (Booth and Sear 2018).
Coastal waters of south-east Australia support diverse temperate reef ecosystems, which host several species of scleractinian corals including Plesiastrea versipora, an encrusting cosmopolitan species found in relatively high abundance in coastal Sydney (Madsen et al. 2014) and further south (Madin et al. 2015). No architecturally complex corals existed in Sydney, where seawater temperature ranges between 18.8 and 24.7 °C (2012–2022 monthly average), until the subtropical endemic branching coral Pocillopora aliciae (Schmidt-Roach et al. 2013) was recorded in 2013 (Booth and Sear 2018) and has since increased in abundance, with over 70% cover in some densely populated patches (Sommer unpublished). Recent projections of P. aliciae population structure under recurrent thermal stress scenarios suggest that this species is vulnerable to heat stress at the core of its range in the Solitary Islands Marine Park, New South Wales, Australia (30.3° S, 153.143° E) (Lachs et al. 2021), where seawater temperature normally ranges between 16.6 and 27.5 °C (Malcolm et al. 2011). Furthermore, the establishment of P. aliciae populations at higher latitudes appears to rely on larval supply from the subtropical populations, which renders the potential of temperate reefs as biodiversity refugia less likely (Cant et al. 2021). Indeed, recent work highlights the role of ocean currents in mediating poleward range extension of corals in eastern Australia, with lack of tropicalisation of coral assemblages in Solitary Islands Marine Park (30.3° S) between 1990 and 2014 linked to low larval connectivity with coral populations on the southern Great Barrier Reef (Mizerek et al. 2021). Nevertheless, patches of P. aliciae colonies (that can exceed 30 cm in diameter) are being increasingly observed in coastal Sydney (33.8° S, 151.305° E) and are predicted to further establish where strengthening of the East Australian Current is expected to accelerate tropicalisation of this habitat (Booth and Sear 2018). Development of a 3D reef structure attributed to the growth of branching corals could lead to an increase in benthic habitat complexity and, in turn, facilitate tropicalisation of reef fish communities along the Sydney coast.
A suite of abiotic and biotic factors has been proposed to limit the range extension of corals into high-latitude regions, many of which require further examination (Abrego et al. 2021). For a given coral species to expand its geographic range, environmental conditions at a new location must fall within temperature, light, aragonite saturation, dissolved oxygen, salinity, and nutrient availability tolerance limits (Yamano et al. 2011), and coral larvae must find the right type of symbionts and settlement cues (Abrego et al. 2021). In addition, the coral must be capable of surviving predation and interspecific competition for space within the native biological community (Preston et al. 2008). In tropical waters, the capacity of corals to thrive into less-optimal environments is usually associated with a shift in metabolic balance of the host coral and/or the algal endosymbionts (Family Symbiodiniaceae) to enhance stress tolerance (Camp et al. 2020; Roach et al. 2021). Such metabolic shifts can be associated with a fitness cost, such as a slower coral growth rate (Camp et al. 2017). An example of such metabolic shifts is presented by the adaptation of Astrangia poculata to locations with different thermal regimes, which is mediated by metabolic and physiological adjustments from the host associated with genetic population differentiation driven by adaptive loci (Aichelman et al. 2019; Aichelman and Barshis 2020). Aggressive interactions between coral species, such as the protrusion of mesenteric filaments to digest neighbouring colonies and the use of sweeper tentacles, may play a key role shaping the architecture and composition of certain reef communities (Einat and Nanette 2006), and environmental changes have been shown to alter the competitive hierarchy of coral-coral interactions (Johnston et al. 2020). Whether similar metabolic and competition hierarchy responses hold true for (sub)tropical coral taxa expanding into temperate habitats remains unexplored, but such an assessment will benefit model predictions of changing population dynamics and subtropical reef structure under future climate scenarios.
An example of the mechanisms enabling the persistence of corals at high latitude is provided by the Sydney population of P. versipora, which can subsist despite its isolation from other populations through high survival of reproductive stages and sporadic recruitment peaks (Precoda et al. 2018). Also, high-latitude corals can exhibit different susceptibility to thermal stress posed by climate change, as evidenced by distinct physiological responses not necessarily coupled with Symbiodiniaceae composition (Goyen et al. 2019). However, little is known about specific physiological and behavioural adaptations that allow coral species from lower latitudes to expand into, and persist in, high-latitude reef habitats, as well as the impacts of the colonisation upon the native temperate coral community. While a previous study has shown that P. versipora undergoes heat stress and bleaching at 26 °C in Sydney (Goyen et al. 2019), the upper thermotolerance limit of the invasive P. aliciae remains untested. Therefore, it is uncertain which species will have a competitive advantage under future seawater warming at this location. To address this knowledge gap, we characterised and compared the physiological and metabolic responses of the subtropical P. aliciae colonising Sydney, and of the native P. versipora, under current and predicted sea surface temperature scenarios. We discuss our results in the context of the potential ecological implications of tropicalisation of temperate reef ecosystems.
Material and methods
Coral collection
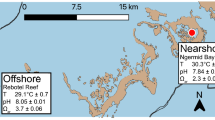
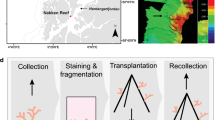
In October 2019, ten fragments of each P. aliciae (~ 5 cm length) and P. versipora (~ 25 cm2) were collected from separate coral colonies offshore of Manly Head and Cobblers Beach, Sydney, Australia, respectively (Fig. S1). Samples of P. aliciae were collected using pliers and those of P. versipora with hammer and chisel. Coral fragments were transported in aerated seawater back to aquaria facilities at the University of Technology Sydney (UTS). Aquaria setup and coral growth conditions are described in Method S1.
Heat exposure experiment
An experiment was conducted to determine the physiological responses of P. aliciae and P. versipora under ambient versus elevated temperature regimes. The experiment was designed following recommendations from the Coral Bleaching Research Coordination Network for short-term exposure to heat (Grottoli et al. 2021). The experimental setup is detailed in Method S2 and included five coral fragments of each species per treatment randomly arranged in four tanks (two for control and two for treatment). After a ten-day acclimation period at 20 ± 1 °C (similar to in situ temperature), the temperature was increased in the treatment tanks, while the remaining physicochemical properties (monitored daily; Method S2) were maintained at similar levels to controls (Table S1 and Fig. S2), with the exception of dissolved oxygen (DO) that exhibited a decline with increasing temperature due to the inverse relationship between temperature and DO solubility (Fig. S3). Corals in the treatment tanks were exposed to a temperature ramping of 1 °C daily over a five-day period up to a sustained temperature of 26 °C, i.e. the upper tolerance limit of P. versipora in Sydney (Goyen et al. 2019), and + 3 °C of the average maximum monthly mean (MMM) for Sydney in October for the past 5 years calculated using the historical Sea Surface Temperatures data (available online from the Australian Government Bureau of Meteorology) for a further six days to assess acute responses to heat. The surface seawater temperature for the south-eastern coast of Australia is predicted to increase up to 3 °C above the MMM temperature (to ~ 27˚C in Sydney) between 2080 and 2100 (Bull et al. 2020). All corals were sampled for metabolic profiling and quantification of bleaching response at the end of the experiment.
Photobiology
A Diving-PAM fluorometer (Walz, Effeltrich, Germany) was used to monitor coral photosynthetic performance via daily measurements of the Photosystem II (PSII) maximum quantum yield (Fv/Fm) as described previously (Goyen et al. 2019). After verifying no significant tank effect in photophysiology (F = 2.98, p > 0.12), non-normal data were analysed using the Kruskal–Wallis test (p < 0.05, SPSS v. 20, IBM Corp.). Briefly, measurements were performed on each coral fragment within the first two hours of the photoperiod, at a fixed distance of 1 mm from the coral’s surface (measuring light intensity 4, saturation pulse intensity 4, saturating width 0.6 s, damping 2, gain 1). Closed system respirometry was used to determine the ratio of gross photosynthesis to dark respiration (P:R) 1–2 days prior to sampling for metabolic profiling for 3 replicates per species and treatment, as described in Method S3. Effect of species and temperature treatment upon P:R was tested using a two-way ANOVA after confirming assumptions of normality and homoscedasticity using Levene’s and Shapiro–Wilk tests, respectively; evaluation of P:R data was performed using IBM SPSS Statistics v26.
Competition experiments
Parallel experiments were used to determine whether the range-expanding P. aliciae showed a behavioural advantage over the local Sydney species P. versipora to compete for space under different temperature scenarios. Distinct coral fragments from each species (in addition to the 10 fragments per species for the heat exposure experiment) were used. A fragment of P. aliciae was placed next to one of P. versipora, ensuring surfaces of both coral fragments were in physical contact and 24 h footage (and photographs at start and end) were taken using a Dino-Lite AM4115T digital microscope and the software DinoXcope v1.25 (dinolite.us). This was repeated at three separate temperatures. The first two replicates were performed after two weeks of acclimation in the experimental setup (Videos S1 and S2). Another two replicates, one for control (Video S3) and one for treatment (Video S4), were carried out just before the temperature ramp. The last two replicates, one for control (Video S5) and one for treatment (Video S6), were performed during the sustained exposure to heat (D3 and D4). We considered the recorded aggressive behaviour, in which one coral fragment ejects its mesenterial filaments to digest the tissue of the other, as the criterion to determine outcompetition.
Intracellular metabolite extraction and data analysis
Sample processing protocols for coral and symbiont fraction separation and intracellular metabolites extraction were adapted from Matthews et al. (2020); a summary can be found in Method S4. Concentrated samples were derivatised and analysed as per Matthews et al. (2018), and details are provided in Method S5. Unfortunately, one P. aliciae and one P. versipora control host samples were lost during GC–MS analysis due to an instrument malfunction. Relative metabolite concentrations of both the host and symbiont fractions between species and treatments were evaluated by principal component analyses (PCA). In addition, a permutational multivariate analysis of variance (PERMANOVA) was conducted using PRIMER v6.0 (PRIMER-E Ltd, UK) for both host and symbiont fractions on a Bray–Curtis dissimilarity matrix comprising a two-factor design (species and treatment), type III sum of squares, and 9,999 permutations under a reduced model. Significance Analysis of Microarrays (and Metabolites) [SAM; (Xia and Wishart 2011)] is considered robust for detecting differences between treatments with unequal small sample sizes and unequal variances in metabolomic datasets (Tusher et al. 2001; Yang et al. 2006). SAM was performed to identify metabolites in the coral host and symbiont fractions that varied according to temperature treatment within and between species. SAM significant metabolites were determined based on a False Discovery Rate (FDR)-corrected significance value (padj < 0.05).
Quantification of bleaching response
Symbiont cell densities were quantified from 500 µL aliquots of host slurry (Method S4) using an Improved Neubauer haemocytometer (Boeco, Germany), with 10 replicate cell counts per sample to a confidence interval of below 10%. Cell density was normalised to host protein content (Smart et al. 2010). Non-normal data were analysed using the Kruskal–Wallis test (p < 0.05, SPSS v. 20, IBM Corp.).
Symbiont chlorophyll a (Chl a) content was quantified after N, N-dimethylformamide (DMF) extraction in darkness over 48 h at 4 °C. Extracts were centrifuged (16,000 g for 5 min) and triplicate 200 µL aliquots were analysed in 96-well plates (UVStar, Greiner Bio-One GmbH, Germany) at 646.8, 663.8 and 750 nm, using a microplate reader (Enspire 2300, Perkin-Elmer, Waltham, MA, USA). Chl a concentration was determined after optical path-length correction (0.555 cm) using the equations of Porra et al. (1989). Data were analysed using a one-way ANOVA (p < 0.05, SPSS v. 20, IBM Corp).
Symbiodiniaceae composition identification
Symbiont DNA was extracted and the Internal Transcribed Spacer 2 region (ITS2) amplified as per Ros et al. (2021) only from control samples of both coral species (Method S6). Samples were processed at the Australian Genome Research Facility (AGRF; Melbourne, Australia) using two-stage amplicon generation and ITS2 sequencing on the Illumina MiSeq platform using a 600-cycle kit. To determine symbiont type per sample, demultiplexed read pairs were analysed using SymPortal (Hume et al. 2019).
Results
Thermal stress indicators
Various photophysiological and biomass parameters of the endosymbiotic Symbiodiniaceae were measured as a proxy for photosynthetic capacity of control versus heat-exposed P. aliciae and P. versipora (Table S2). As expected, symbiont cell density (Fig. 1a) was significantly reduced in P. versipora under increased temperatures (two-sided t-test, t = 3.232, p = 0.012), but did not differ significantly between control and heat conditions in P. aliciae (two-sided t-test, t = 1.336, p = 0.229). While changes in maximum quantum yield of PSII (Fv/Fm) were minimal at the last day of the experiment (D6) between conditions in P. aliciae (Tukey HSD, p = 0.995), it significantly decreased in colonies of P. versipora under increased temperatures (Tukey HSD, p < 0.002; Fig. 1c). Fv/Fm did not differ significantly between control condition of each species (Tukey HSD, p = 0.998). Chl a concentration per symbiont cell (Fig. 1b) was not significantly affected in response to increased temperatures in P. aliciae or P. versipora (two-sided t-test, t = − 0.170 and p = 0.869, and t = − 0.323 and p = 0.755, respectively).
Photophysiological and biomass measurements of Symbiodiniaceae symbionts. Symbiont cell density (cells mL−1) (a) and chlorophyll a content (µg cell−1 mL−1) (b) per species and condition. An asterisk (*) represents significant (p ≤ 0.05) difference in the within-species comparison based on a Kruskal–Wallis test. (c) Daily maximum quantum yield of PSII (Fv/Fm) is shown as mean ± SEM of both coral species in the control and high-temperature (treatment) conditions (n = 5) for the five days of temperature ramping (R1-5) and the six days of the experiment (D1-6). (d) Gross photosynthesis to dark respiration ratio (P:R) per species and condition (n = 3). The mean for each instance is shown as a square with the corresponding condition colour. Grey lines connecting the condition means highlight the difference between species
The ratio of gross photosynthesis to dark respiration (P:R) was measured as a proxy of coral holobiont functional performance (Fig. 1d). While the average P:R was smaller for the heat condition (26˚C) in both species, the difference between conditions was more prominent in P. aliciae (average of 2.45 at 20 °C and 1.99 at 26 °C) than in P. versipora (average of 2.44 at 20 °C and 2.24 at 26 °C), driven by both an increase in P and a decrease in R (Fig. S4). Even so, there were no significant effects (two-way ANOVA) on P:R between treatments (p = 0.15) or species (p = 0.57), or for the interaction between treatment and species (p = 0.53), possibly due to the small sample sizes.
Host metabolite profiles
Non-targeted GC–MS analysis identified 54 metabolites in P. aliciae and P. versipora host tissues, including carbohydrates, amino acids, fatty acids, lipogenesis intermediates and organic acids (Table S3). PERMANOVA revealed no significance between the treatments when coral species were pooled, or for the species × treatment interaction (p = 0.77; Table S4). PCA of the metabolite profiles revealed a slight separation between control and heat-treated P. aliciae samples along PC1 (42.6%; Fig. 2a). Within-species analysis (via SAM) revealed this was driven by a significant increase in relative abundance (SAM, padj < 0.05) of three metabolites under increased temperatures (Fig. 2b): campesterol (a sterol), stearic acid [C18:0] (a saturated fatty acid) and oleic acid [C18:1] (an unsaturated fatty acid). No metabolites significantly decreased in relative abundance for P. aliciae under heat. Meanwhile, no separation was revealed in the PCA between the P. versipora metabolite profiles from the two temperature conditions (Fig. 2c). Furthermore, SAM revealed no metabolites significantly differed between control and heat-treated samples of P. versipora (SAM, padj > 0.05; Fig. 2d). As such, P. aliciae appears to be undergoing a metabolic shift, while P. versipora is not.
Metabolite responses of coral hosts to heat. Top panels show PCA (a) and SAM (b) plots of the Pocillopora aliciae metabolite profiles under control conditions and heat. Bottom panels show the corresponding PCA (c) and SAM (d) plots for the Plesiastrea versipora hosts under both experimental conditions. Significant metabolites identified by SAM in (d) are shown according to the legend at the bottom
The metabolite profiles of coral host tissues were significantly different between P. aliciae and P. versipora species under both control and heat-treated conditions (PERMANOVA p = 0.0001; Fig. 3 and Table S4). Under control conditions, PCA (Fig. 3a) revealed separation between the two coral species along PC1 (45.4%). Six metabolites differed significantly between species (SAM, padj < 0.05; Fig. 3b): fucosterol (a sterol) was more abundant in P. aliciae tissues, while cholesterol, ornithine, arginine, oleic acid [C18:1] and sucrose were more abundant in P. versipora tissues. Similarly, under raised heat PCA revealed a separation between P. aliciae and P. versipora metabolite profiles along PC1 (61.5%; Fig. 3c), driven by a higher relative abundance of fucosterol, inositol-1-phosphate and citric acid in P. aliciae (SAM, padj < 0.05; Fig. 3d). In P. versipora, most metabolites abundant under control conditions were no longer significantly different between the two species under heat, except for cholesterol, sucrose, and fructose (SAM, padj < 0.01).
Metabolite profile differences between host tissues of the two coral species. Top panels show PCA (a) and SAM (b) plots of the metabolite profiles from the host tissues of the two coral species under control conditions. Bottom panels show the corresponding PCA (c) and SAM (d) plots for the coral hosts under the heat treatment. Significant metabolites identified by SAM are shown in colour in (b) and (d) according to the legend at the bottom
Symbiont metabolite profiles
Symbiodiniaceae composition analysis (Fig. S5) revealed that Cladocopium sp. C75h (profile C75h-C75i-C75g) was the predominant symbiont in all five P. aliciae control samples, whereas the dominating symbiont in all P. versipora control samples was Breviolum sp. B18 (profile B18-B18a). Non-targeted GC–MS analysis identified 37 metabolites in P. aliciae and P. versipora symbionts, including carbohydrates, amino acids, fatty acids, lipogenesis intermediates and organic acids (Table S5). PERMANOVA (Table S4) revealed that symbiont metabolite profiles differed significantly between species (p = 0.0001), treatments (p = 0.034) and the interaction between species and treatment (p = 0.007). PCA of the symbiont metabolite profiles of P. aliciae under control and increased temperature exhibits a shift along both PC1 (57.6%) and PC2 (21.2%; Fig. 4a), which is driven by the significant increase of two metabolites (galactosylgycerol and myristic acid [C14:0]) in P. aliciae symbionts as response to raised temperatures (SAM, padj < 0.01; Fig. 4b). Meanwhile, no differences were found in the symbiont metabolite profiles of P. versipora between temperature treatments (SAM; Fig. 4c and d).
Metabolite responses of symbionts to heat. Panels in the top show PCA (a) and SAM (b) plots of the Pocillopora aliciae symbionts metabolite profiles under control conditions and heat. Panels in the bottom show the corresponding PCA (c) and SAM (d) plots for the Plesiastrea versipora symbionts under both experimental conditions. Significant metabolites identified by SAM in (d) are shown in colour according to the legend at the bottom
PCA revealed a separation along both axes (PC1 47.2%; PC2 29.1%) between the symbiont metabolite profiles of the two coral species under control conditions (Fig. 5a), which is consistent with the distinct dominant symbionts in each species (Breviolum sp. B18 in P. versipora and Cladocopium sp. C15h in P. aliciae; Fig. S5). Nine metabolites significantly differed in relative abundance between the two coral species under control conditions (SAM, padj < 0.05; Fig. 5b): three sterols (ß-sitosterol, campesterol and fucosterol) were more abundant in P. aliciae symbionts, while mannitol, glucuronic acid, pyroglutamic acid, and the carbohydrates mannose, glucose, and arabinose, were more abundant in P. versipora symbionts. Substantial separation along PC1 was found between the symbiont metabolite profiles of the two coral species under heat exposure (Fig. 5c), of which 12 metabolites significantly differed (padj < 0.05; Fig. 5d). Campesterol and fucosterol were still more abundant in P. aliciae symbionts, along with inositol-1-phosphate, myo-inositol, gentibiose, glycerol, and an unsaturated fatty acid [C18:2]. In P. versipora symbionts, mannose and arabinose remained more abundant, while trehalose, eicosanoic acid [C20:0] and malic acid were more abundant under heat, and not in control conditions, compared to P. aliciae symbiont metabolite pools.
Metabolite profile differences between symbionts of the two coral species. Top panels show PCA (a) and SAM (b) plots of the metabolite profiles from symbiont cells of the two coral species under control conditions. Bottom panels show the corresponding PCA (c) and SAM (d) plots for the symbionts under the heat treatment. Significant metabolites identified by SAM in (b) and (d) are shown in colour according to the legend at the bottom
Interspecific competition
Simulated interspecific competition for space between P. versipora and P. aliciae was recorded at three time points: (i) during the acclimation period (Videos S1 and S2), (ii) immediately prior to the temperature ramp (Videos S3 and S4), and (iii) during the heat exposure experiment (Videos S5 and S6 for control and treatment tanks, respectively). In all instances, P. versipora extruded mesenteric filaments that digested tissue of P. aliciae in close proximity, causing a wound that exposed its skeleton. However, the tissue of P. aliciae exhibited more activity initially, notably polyp movement and extension, in the trials performed during the acclimation period. Overall, the interaction between both coral species followed a regular pattern, without evident differences between control and heat conditions, in which P. versipora always outcompeted P. aliciae (Fig. S6): soft tissue pulsed inflation and mesenterial filament projection in P. versipora, followed by tissue retraction and loss in P. aliciae, secretion of mucus that forms a barrier between the two colonies, and finally retraction of mesenterial filaments by P. versipora. Although it is unclear which species secreted the mucus observed in the footage, it is likely a response from the immunocompromised tissues of P. aliciae (Bythell and Wild 2011).
Discussion
Unaltered photophysiology of P. aliciae under short-term heat exposure
We observed no significant changes in the photophysiology of P. aliciae under increased temperatures during this experiment. In contrast, P. versipora exhibited a typical bleaching response upon exposure to 26 °C, consisting of declines in both density and photochemical efficiency of algal symbionts, that has been reported previously (Goyen et al. 2019). This suggests that the subtropical endemic P. aliciae may be better adapted to acute seawater temperature increases than, and therefore holds a competitive advantage over, the native P. versipora in coastal New South Wales (NSW), Australia. Such findings contradict the conventional notion that branching corals exhibit greater thermosensitivity compared to encrusting (and massive) corals (Loya et al. 2001). Differential responses in photobiology between coral species are inherently linked to functional diversity between Symbiodiniaceae species (Suggett et al. 2017; Camp et al. 2020; Ros et al. 2021). P. versipora exhibits the broadest latitudinal range of any scleractinian coral (Burgess et al. 2009), possibly because it associates with distinct symbiotic partners at different latitudes. Specifically, at lower latitudes (e.g. Orpheus Island, Great Barrier Reef), P. versipora typically associates with symbionts in an undescribed species of the genus Cladocopium, whereas at higher latitudes, e.g. NSW, Breviolum are usually the dominant endosymbionts (Rodriguez-Lanetty et al. 2001), notably the same type found in this study (Breviolum sp. B18) (Goyen et al. 2019). It has been hypothesised that such a pattern is explained by the distinct physiological capabilities of the algal symbionts as a result of adaptation to different seawater temperatures, supporting the idea that flexible symbioses in corals can lead to extended phenotypes with adaptive potential (Parkinson and Baums 2014; Matthews et al. 2020). While the thermal stability of symbiosis that we report here for P. aliciae adult stages suggests a physiological capacity to facilitate further expansion into temperate reefs systems along the south-east Australian coast upon temperature increase, other studies have shown that juvenile stages of this species are susceptible to similar increases in seawater temperature (> 24 °C) at a lower latitude based on growth, survival and recruitment rates (Cant et al. 2021; Lachs et al. 2021). Surface seawater temperature for the south-eastern coast of Australia is predicted to increase up to 3 °C above the maximum monthly mean temperature (increased to ~ 27 °C at Sydney Harbour) between 2080 and 2100 according to the RCP8.5 scenario due to the intensification of the East Australian Current depending on local wind dynamics (Bull et al. 2020). Therefore, follow-up investigations regarding the effects of heat stress on recruitment for both species are essential to better predict future coral-community and reef-architecture dynamics of Sydney in the face of climate change.
Species-specific metabolic responses to raised temperatures
Within-species response
The metabolic shift in P. aliciae in response to heat treatment involved an increase in free campesterol, two saturated fatty acids and a monounsaturated fatty acid in the host (Fig. 2b). Campesterol is produced only by Symbiodiniaceae and then translocated to the coral host (Treignier et al. 2009; Crandall et al. 2016). While knowledge of sterol metabolic pathways in cnidarians is lacking (Carreón-Palau et al. 2020), a heat-stress response in coral species of Acropora has been hypothesised to involve plant signalling hormones synthesised from the steroid-derivative campesterol (Hillyer et al. 2017). Therefore, the increased abundance of campesterol in P. aliciae host tissues is consistent with a concerted metabolic response to heat between coral hosts and symbionts (Suggett and Smith 2020; Rädecker et al. 2021; Roach et al. 2021; Ros et al. 2021), which might be related to the higher photosynthetic rate observed under heat (Fig. S4).
Increased saturation of the cell lipid bilayer functions in thermal acclimation, directly and indirectly, by increasing stability and protecting it against damage by reactive oxygen species (Tchernov et al. 2004; Papina et al. 2007). In P. aliciae, we observed a significant increase in the abundance of pools of the saturated fatty acid stearic acid (C18:0) in the host tissues (as well as an increased abundance of monounsaturated oleic acid C18:1), and myristic acid (C14:0) in the symbiont cells (Figs. 2b and 4b). While our methods cannot distinguish whether these changes were associated with modifications to cell membranes, or other processes, increasing membrane saturation is energetically costly, and we also observed an increase in abundance of the lipogenesis intermediate galactosylglycerol in the P. aliciae symbionts (Fig. 4b). Previous studies propose that the increased abundance of lipid intermediates in response to heat stress and bleaching of other coral species is likely implicated with the alteration of anabolic and catabolic pathways caused by energetic constraints (Hillyer et al. 2017, 2018). The breakdown of lipid stores as an alternative source of energy appears to be a common mechanism in the heat-stress response of scleractinians when the availability of photosynthetically derived metabolites is diminished, and our results suggest a similar response in P. aliciae, as supported by a heat-induced reduction of the respiration rate (Fig. S4). Overall, our results indicate that P. aliciae is capable of coping with short-term heat by a metabolic shift that might help maintain energy availability and potentially changes to membrane saturation that support symbiosis stability, which implies an adaptive advantage for the forecasted tropicalisation scenario.
Despite the photophysiological impairment in P. versipora under raised temperatures, neither the host nor the symbiont metabolite profiles revealed any metabolic responses to stress or adjustment to compensate for the decreased photosynthetic capacity (Figs. 2d and 4d). The lack of metabolic response in P. versipora suggests that either: (1) sampling occurred prior to observable metabolic shifts, (2) the drop in Fv/Fm and symbiont cell density remained within a normal physiological range, or (3) there are limited/no metabolic mechanisms to adjust for short-term thermal stress, i.e. host and symbionts lack cellular mechanisms for a quick response to bleaching-inducing conditions (e.g. increased antioxidant production, increased mobilisation of stored energy when photosynthesis-derived metabolism is reduced). While the first scenario is plausible, recent evidence suggests that decreased metabolic exchange precedes bleaching (Rädecker et al. 2021), and we were able to detect a reduction in Fv/Fm (~ 0.05). Support for the second possibility is given by the small reduction in Fv/Fm too, which was also variable across replicates (Fig. 1c). It has been proposed previously that P. versipora can adjust its metabolism to temperature changes that range within normal values for its distribution (10–21 °C) (Howe and Marshall 2001, 2002). Indeed, bleaching and metabolic shifts have been reported in P. versipora at 26 °C (Goyen et al. 2019), but those observations were done following a natural heat wave in Sydney Harbour that lasted several months, in which exposure to heat was more prolonged than in our experiment. However, the third alternative suggests that P. versipora does not possess cellular mechanisms necessary to withstand higher temperatures at this latitude, which is also supported by recent findings (Goyen et al. 2019). More specifically, P. versipora symbionts might not be able to enhance photosynthesis to maintain the nutritional exchange with the host (Fig. S4), but this is not conclusive given our low sample size and variability across replicates. Together, these observations affirm the competitive advantage of P. aliciae over P. versipora during acute heat exposure (e.g. when heat waves cause the seawater temperature to exceed the MMM), and may explain the more recent establishment and growth of P. aliciae in Sydney given the recent heatwave-induced mass bleaching of P. versipora at this location (Goyen et al. 2019). However, it is important to note that fitness post-bleaching was not assessed for either coral species in this study, and therefore whether this competitive advantage holds true over longer timeframes requires further study–particularly given the documented quick and widespread recovery of P. versipora from the aforementioned bleaching event might represent a greater competitive advantage for this species.
Between-species response
Host and symbiont metabolism under control conditions and heat differed between P. aliciae and P. versipora (Fig. 6). Under control conditions, the P. aliciae holobiont exhibited generally more compounds related to sterol metabolism than that of P. versipora. While the role of sterols in corals remains unclear (Rosset et al. 2021), sterols and their derivatives function in regulating physiological membrane function, transmembrane signalling and immune responses, and in the cnidarian-dinoflagellate symbiosis. Genomic evidence suggests that the hosts rely on the symbionts for sterol supplies (Baumgarten et al. 2015), and sterol trafficking features in optimal host-symbiont pairings (Matthews et al. 2017). Thus, the relatively more active sterol metabolism in P. aliciae under control conditions may reflect interpartner signalling and homeostasis in this holobiont. Meanwhile, P. versipora showed more free simple sugars and intermediates of antioxidants and of the urea cycle (Figs. 4d and 6d). This might be explained by the symbiont species composition in P. versipora in this condition (Table 1) as nitrogen cycling within the coral-dinoflagellate symbiosis involves ammonium produced via the urea cycle (Wang and Douglas 1998; Grover et al. 2006). In turn, the increased activity of the urea cycle may enhance symbiont replication (Matthews et al. 2018). In addition, potential impacts of the host nutritional state and seasonal availability of inorganic nitrogen on nitrogen metabolism of P. versipora symbionts (Davy et al. 2006), which affect photosynthetic performance, need to be considered. The increased abundance of free carbohydrates could indicate an active turnover of energy derived from the tricarboxylic acid (TCA) cycle. The higher photosynthetic activity per host cell in P. versipora (Fig. 1) may also explain the higher levels of free carbohydrates and antioxidants in the host tissues of this coral.
Diagrammatic representation of the metabolic differences between Plesiastrea versipora and Pocillopora aliciae based on our analyses of response to heat within each species (continuous arrows) and on the interspecific differences observed in control and heat conditions (dashed arrows). TCA: tricarboxylic acid cycle, LB: lipid biosynthesis pathway
Under heat (Fig. 6b), the higher abundance of the C20:0 fatty acid in P. versipora symbionts versus P. aliciae symbionts might be related to the activation of eicosanoids; these intercellular signalling compounds have been previously implicated in the stress response of cnidarians, also mediated by lipid signalling (Lõhelaid et al. 2015; Matthews et al. 2017). Inositol signalling has been identified as a signalling pathway commonly associated with stress response in corals, including bleaching (Hillyer et al. 2017, 2018), and is thought to be involved in the maintenance of a functional coral-dinoflagellate symbiosis (Matthews et al. 2017, 2018). Accordingly, this signalling pathway appears to be important for P. aliciae to maintain symbiosis and cell homeostasis under raised temperatures, as supported by the excess of several intermediates of this pathway in both host (Fig. 4d) and symbiont (Fig. 6d). Abundance of free sterols and C18:2 in P. aliciae symbionts under heat suggests either breakdown or enhanced division of these cells, but the lack of significant changes in P. aliciae symbiont density between the two experimental conditions (Fig. 2b) makes it impossible to ascertain which process dominated.
The higher abundance of free sugars in algal symbionts after heat exposure, such as that observed in P. versipora symbionts, has been linked to the production and mobilisation of photosynthates in synchrony with enhanced respiratory reactions for the generation of energy in the thermally stressed host (Hillyer et al. 2017). However, this notion disagrees with the reduction of respiration rate in P. versipora after exposure to heat (Fig. S4).
Potential implications of P. versipora and P. aliciae competition on future reef structure
Although outcomes of competition between coral species vary in time and space (Einat and Nanette 2006), our competition experiments suggest that P. versipora is capable of outcompeting P. aliciae in the race for space when in close contact, even under acute heat exposure. Our results suggest that the aggressive behaviour of P. versipora can prevent the establishment of P. aliciae in close proximity (although no direct competition has yet been observed in coastal NSW); however, increasing temperatures could change these dynamics as P. versipora colonies are lost. The faster growth rates of branching corals like P. aliciae (Pinzón et al. 2014; Pratchett et al. 2015) could lead to shading effects that would limit sunlight access to slower-growing encrusting P. versipora colonies, contributing further to its decimation primarily driven by increasing seawater temperature.
Although P. aliciae and P. versipora co-occur in coastal habitats in New South Wales (Sommer et al. 2014), there is currently no noticeable competition between the two species (Sommer’s personal observation). In addition to competitive ability in close contact, the different life history traits and environmental tolerances of P. aliciae and P. versipora likely influence their distribution and abundance patterns in these marginal habitats (Sommer et al. 2017). For instance, brooding reproduction (Schmidt-Roach et al. 2013), rapid settlement and high self-recruitment of brooding species (Figueiredo et al. 2013), and fast growth rates (Harriott 1999) once established, make P. aliciae a good coloniser of new habitats and it is likely that P. aliciae will establish in areas currently occupied by P. versipora. Altogether, our study suggests that future conditions may contribute to the further proliferation of P. aliciae over P. versipora. This could lead to a change in the architecture of coastal ecosystems in Sydney, shifting from predominantly encrusting corals to branching species.
Coastal NSW reef ecosystem as a refuge for tropical reef species from climate change
Previous studies suggest that high-latitude marine environments of East Australia have limited potential as refugia for the biodiversity of the Great Barrier Reef from climate change because of natural instability, anthropogenic perturbances and massive bleaching events of native coral communities (Lybolt et al. 2011; Goyen et al. 2019; Kim et al. 2019). However, such studies are generally limited to corals at subtropical latitudes and do not consider the tropicalisation of temperate marine ecosystems and the poleward range expansion of corals to temperate latitudes in East Australia (> 30° S). Our data suggest that the subtropical P. aliciae colonising the Sydney seaboard (34° S) can cope better under increased temperature at this location, and hence also under the raising temperatures during the process of tropicalisation, than the native species P. versipora. Although the settlement of P. aliciae in NSW coastal waters currently depends largely on larval recruitment from lower-latitude populations (Cant et al. 2021), which are under severe threat due to heat stress (Kim et al. 2019; Lachs et al. 2021), this might change as this brooder population becomes more established. Therefore, it is essential to determine whether the temperate P. aliciae population will be able to subsist from local recruitment only to confidently assess the possibility of P. aliciae further proliferating and, perhaps, even displacing native coral species, like P. versipora, as this reef ecosystem undergoes tropicalisation. A drastic increase in architectural complexity in the coastal NSW reef ecosystem would likely facilitate colonisation and establishment of other tropical and subtropical reef species, as it already provides habitat for coral reef fish (Booth and Sear 2018; Booth et al. 2018; Figueira and Booth 2010). Still, a stricter multi-pronged assessment (Kavousi and Keppel 2017) remains to be done to ascertain whether coastal waters of NSW have the potential to act as a refuge for subtropical and tropical reef species from climate change.
The ecological services and economic revenue of the Sydney marine environment makes it a very valuable ecosystem (Banks et al. 2016). In addition to the global threat to coral reefs posed by climate change, urbanisation in coastal NSW represents a major continuous threat through, for example, increased stormwater drainage inflow (Varkey et al. 2018) and habitat modification (Mayer-Pinto et al. 2015). Although management strategies are already in place, we hope that the recognition of coastal NSW as a potential biodiversity refuge has a greater impact on its protection.
References
Abrego D, Howells EJ, Smith SDA, Madin JS, Sommer B, Schmidt-Roach S, Cumbo VR, Thomson DP, Rosser NL, Baird AH (2021) Factors limiting the range extension of corals into high-latitude reef regions. Diversity 13(12):632
Aichelman HE, Barshis DJ (2020) Adaptive divergence, neutral panmixia, and algal symbiont population structure in the temperate coral Astrangia poculata along the Mid-Atlantic United States. PeerJ 8:e10201
Aichelman HE, Zimmerman RC, Barshis DJ (2019) Adaptive signatures in thermal performance of the temperate coral Astrangia poculata. J Exp Bio 222(5):jep189225
Baird AH, Sommer B, Madin JS (2012) Pole-ward range expansion of Acropora spp. along the east coast of Australia. Coral Reefs 31:1063–1063
Banks J, Hedge LH, Hoisington C, Strain EM, Steinberg PD, Johnston EL (2016) Sydney harbour: beautiful, diverse, valuable and pressured. Reg Stud Mar Sci 8:353–361
Baumgarten S, Simakov O, Esherick LY, Liew YJ, Lehnert EM, Michell CT, Li Y, Hambleton EA, Guse A, Oates ME, Gough J, Weis VM, Aranda M, Pringle JR, Voolstra CR (2015) The genome of Aiptasia a sea anemone model for coral symbiosis. PNAS 112:11893–11898
Beger M, Sommer B, Harrison PL, Smith SDA, Pandolfi JM (2014) Conserving potential coral reef refuges at high latitudes. Diver Distrib 20:245–257
Booth DJ, Sear J (2018) Coral expansion in Sydney and associated coral-reef fishes. Coral Reefs 37:995
Booth DJ, Beretta GA, Brown L, Figueira WF (2018) Predicting success of range-expanding coral reef fish in temperate habitats using temperature-abundance relationships. Front Mar Sci. https://doi.org/10.3389/fmars.2018.00031
Bull CYS, Kiss AE, Gupta AS, Jourdain NC, Argüeso D, Di Luca A, Sérazin G (2020) Regional versus remote atmosphere-ocean drivers of the rapid projected intensification of the east Australian current. J Geophys Res-Oceans 125:e2019JC015889
Burgess SN, McCulloch MT, Mortimer GE, Ward TM (2009) Structure and growth rates of the high-latitude coral: Plesiastrea versipora. Coral Reefs 28:1005
Bythell JC, Wild C (2011) Biology and ecology of coral mucus release. J Expl Mar Bio Ecol 408:88–93
Camp EF, Kahlke T, Nitschke MR, Varkey D, Fisher NL, Fujise L, Goyen S, Hughes DJ, Lawson CA, Ros M, Woodcock S, Xiao K, Leggatt W, Suggett DJ (2020) Revealing changes in the microbiome of Symbiodiniaceae under thermal stress. Environ Microbiol. https://doi.org/10.1111/1462-2920.14935
Camp EF, Nitschke MR, Rodolfo-Metalpa R, Houlbreque F, Gardner SG, Smith DJ, Zampighi M, Suggett DJ (2017) Reef-building corals thrive within hot-acidified and deoxygenated waters. Sci Rep 7:2434
Camp EF, Schoepf V, Suggett DJ (2018a) How can “super corals” facilitate global coral reef survival under rapid environmental and climatic change? Glob Chang Bio 24:2755–2757
Camp EF, Schoepf V, Mumby PJ, Hardtke LA, Rodolfo-Metalpa R, Smith DJ, Suggett DJ (2018b) The future of coral reefs subject to rapid climate change: lessons from natural extreme environments. Front Mar Sci 5:4
Cant J, Salguero-Gómez R, Kim SW, Sims CA, Sommer B, Brooks M, Malcolm HA, Pandolfi JM, Beger M (2021) The projected degradation of subtropical coral assemblages by recurrent thermal stress. J Anim Ecol 90:233–247
Carreón-Palau L, Özdemir NŞ, Parrish CC, Parzanini C (2020) Sterol composition of sponges, cnidarians, arthropods, mollusks, and echinoderms from the deep northwest Atlantic: a comparison with shallow coastal Gulf of Mexico. Mar Drugs 18:598
Cheung WWL, Meeuwig JJ, Feng M, Harvey E, Lam VWY, Langlois T, Slawinski D, Sun C, Pauly D (2012) Climate-change induced tropicalisation of marine communities in Western Australia. Mar Freshw Res 63:415–427
Crandall JB, Teece MA, Estes BA, Manfrino C, Ciesla JH (2016) Nutrient acquisition strategies in mesophotic hard corals using compound specific stable isotope analysis of sterols. J Expl Mar Bio Ecol 474:133–141
Davy SK, Withers KJT, Hinde R (2006) Effects of host nutritional status and seasonality on the nitrogen status of zooxanthellae in the temperate coral Plesiastrea versipora (Lamarck). J Expl Mar Bio Ecol 335:256–265
Einat DL, Nanette EC (2006) Long-term effects of competition on coral growth and sweeper tentacle development. Mar Ecol Prog Ser 313:115–123
Figueira WF, Booth DJ (2010) Increasing ocean temperatures allow tropical fishes to survive overwinter in temperate waters. Glob Chang Bio 16:506–516
Figueiredo J, Baird AH, Connolly SR (2013) Synthesizing larval competence dynamics and reef-scale retention reveals a high potential for self-recruitment in corals. Ecology 94:650–659
Goyen S, Camp EF, Fujise L, Lloyd A, Nitschke MR, LaJeunensse T, Kahlke T, Ralph PJ, Suggett D (2019) Mass coral bleaching of P. versipora in Sydney Harbour driven by the 2015–2016 heatwave. Coral Reefs 38:815–830
Grottoli AG, Toonen RJ, van Woesik R, Vega Thurber R, Warner ME, McLachlan RH, Price JT, Bahr KD, Baums IB, Castillo K, Coffroth MA, Cunning R, Dobson K, Donahue M, Hench JL, Iglesias-Prieto R, Kemp DW, Kenkel CD, Kline DI, Kuffner IB, Matthews JL, Mayfield A, Padilla-Gamino J, Palumbi S, Voolstra CR, Weis VM, Wu HC (2021) Increasing comparability among coral bleaching experiments. Ecol Appl 31(4):e2262
Grover R, Maguer J-F, Allemand D, Ferrier-Pagès C (2006) Urea uptake by the scleractinian coral Stylophora pistillata. J Expl Mar Bio Ecol 332:216–225
Harriott VJ (1999) Coral growth in subtropical eastern Australia. Coral Reefs 18:281–291
Hillyer KE, Dias DA, Lutz A, Wilkinson SP, Roessner U, Davy SK (2017) Metabolite profiling of symbiont and host during thermal stress and bleaching in the coral Acropora aspera. Coral Reefs 36:105–118
Hillyer KE, Dias D, Lutz A, Roessner U, Davy SK (2018) 13C metabolomics reveals widespread change in carbon fate during coral bleaching. Metabolomics 14:12
Howe SA, Marshall AT (2001) Thermal compensation of metabolism in the temperate coral, Plesiastrea versipora (Lamarck, 1816). J Expl Mar Bio Ecol 259:231–248
Howe SA, Marshall AT (2002) Temperature effects on calcification rate and skeletal deposition in the temperate coral, Plesiastrea versipora (Lamarck). J Expl Mar Bio Ecol 275:63–81
Hume BCC, Smith EG, Ziegler M, Warrington HJM, Burt JA, LaJeunesse TC, Wiedenmann J, Voolstra CR (2019) SymPortal: A novel analytical framework and platform for coral algal symbiont next-generation sequencing ITS2 profiling. Mol Ecol Res 19:1063–1080
Johnston NK, Campbell JE, Paul VJ, Hay ME (2020) Effects of future climate on coral-coral competition. PLoS ONE 15(8):e0235465
Kavousi J, Keppel G (2017) Clarifying the concept of climate change refugia for coral reefs. ICES J Mar Sci 75:43–49
Kim SW, Sampayo EM, Sommer B, Sims CA, Gómez-Cabrera MdC, Dalton SJ, Beger M, Malcolm HA, Ferrari R, Fraser N, Figueira WF, Smith SDA, Heron SF, Baird AH, Byrne M, Eakin CM, Edgar R, Hughes TP, Kyriacou N, Liu G, Matis PA, Skirving WJ, Pandolfi JM (2019) Refugia under threat: mass bleaching of coral assemblages in high-latitude eastern Australia. Glob Chang Biol 25:3918–3931
Lachs L, Sommer B, Cant J, Hodge JM, Malcolm HA, Pandolfi JM, Beger M (2021) Linking population size structure, heat stress and bleaching responses in a subtropical endemic coral. Coral Reefs 40:777–790
Lõhelaid H, Teder T, Samel N (2015) Lipoxygenase-allene oxide synthase pathway in octocoral thermal stress response. Coral Reefs 34:143–154
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Let 4:122–131
Lybolt M, Neil D, Zhao J, Feng Y, Yu K-F, Pandolfi J (2011) Instability in a marginal coral reef: the shift from natural variability to a human-dominated seascape. Front Ecol Environ 9:154–160
Madin JS, Kuo C-Y, Martinelli JC, Mizerek T, Baird AH (2015) Very high coral cover at 36°S on the east coast of Australia. Coral Reefs 34:327–327
Madsen A, Madin JS, Tan CH, Baird AH (2014) The reproductive biology of the scleractinian coral Plesiastrea versipora in Sydney harbour, Australia. SEDAO 1:25–33
Malcolm HA, Davies PL, Jordan A, Smith SD (2011) Variation in sea temperature and the east Australian current in the solitary Islands region between 2001–2008. Deep Sea Res Part II 58(5):616–627
Matthews JL, Crowder CM, Oakley CA, Lutz A, Roessner U, Meyer E, Grossman AR, Weis VM, Davy SK (2017) Optimal nutrient exchange and immune responses operate in partner specificity in the cnidarian-dinoflagellate symbiosis. PNAS 114:13194–13199
Matthews JL, Oakley CA, Lutz A, Hillyer KE, Roessner U, Grossman AR, Weis VM, Davy SK (2018) Partner switching and metabolic flux in a model cnidarian-dinoflagellate symbiosis. Proc Royal Soc B 285:20182336
Matthews JL, Cunning R, Ritson-Williams R, Oakley CA, Lutz A, Roessner U, Grossman AR, Weis VM, Gates RD, Davy SK (2020) Metabolite pools of the reef building coral Montipora capitata are unaffected by Symbiodiniaceae community composition. Coral Reefs 39:1727–1737
Mayer-Pinto M, Johnston EL, Hutchings PA, Marzinelli EM, Ahyong ST, Birch G, Booth DJ, Creese RG, Doblin MA, Figueira W, Gribben PE, Pritchard T, Roughan M, Steinberg PD, Hedge LH (2015) Sydney Harbour: a review of anthropogenic impacts on the biodiversity and ecosystem function of one of the world’s largest natural harbours. Mar Freshw Res 66:1088–1105
Mizerek TL, Madin JS, Benzoni F, Huang D, Luiz OJ, Mera H, Schmidt-Roach S, Smith SDA, Sommer B, Baird AH (2021) No evidence for tropicalization of coral assemblages in a subtropical climate change hot spot. Coral Reef 40(5):1451–1461. https://doi.org/10.1007/s00338-021-02167-x
Papina M, Meziane T, van Woesik R (2007) Acclimation effect on fatty acids of the coral Montipora digitata and its symbiotic algae. Comp Biochem Physiol B Biochem Mol Bio 147:583–589
Parkinson JE, Baums IB (2014) The extended phenotypes of marine symbioses: ecological and evolutionary consequences of intraspecific genetic diversity in coral–algal associations. Front Microbiol. https://doi.org/10.3389/fmicb.2014.00445
Pinzón JH, Dornberger L, Beach-Letendre J, Weil E, Mydlarz LD (2014) The link between immunity and life history traits in scleractinian corals. PeerJ 2:e628
Poloczanska ES, Brown CJ, Sydeman WJ, Kiessling W, Schoeman DS, Moore PJ, Brander K, Bruno JF, Buckley LB, Burrows MT, Duarte CM, Halpern BS, Holding J, Kappel CV, O’Connor MI, Pandolfi JM, Parmesan C, Schwing F, Thompson SA, Richardson AJ (2013) Global imprint of climate change on marine life. Nat Clim Chang 3:919–925
Poloczanska ES, Burrows MT, Brown CJ, García Molinos J, Halpern BS, Hoegh-Guldberg O, Kappel CV, Moore PJ, Richardson AJ, Schoeman DS, Sydeman WJ (2016) Responses of marine organisms to climate change across oceans. Front Mar Sci. https://doi.org/10.3389/fmars.2016.00062
Pratchett MS, Anderson KD, Hoogenboom MO, Widman E, Baird AH, Pandolfi JM, Edmunds PJ, Lough JM (2015) Spatial, temporal and taxonomic variation in coral growth—implications for the structure and function of coral reef ecosystems. In: Hughes R, Hughes D, Smith I, Dale A (eds) Oceanography and Marine Biology: An Annual Review. CRC Press, Boca Raton, pp 215–295
Precoda K, Baird AH, Madsen A, Mizerek T, Sommer B, Su SN, Madin JS (2018) How does a widespread reef coral maintain a population in an isolated environment? Mar Ecol Prog Ser 594:85–94
Preston KL, Rotenberry JT, Redak RA, Allen MF (2008) Habitat shifts of endangered species under altered climate conditions: importance of biotic interactions. Glob Chang Biol 14:2501–2515
Rädecker N, Pogoreutz C, Gegner HM, Cárdenas A, Roth F, Bougoure J, Guagliardo P, Wild C, Pernice M, Raina J-B, Meibom A, Voolstra CR (2021) Heat stress destabilizes symbiotic nutrient cycling in corals. PNAS 118:e2022653118
Roach TNF, Dilworth J, H CM, Jones AD, Quinn RA, Drury C, (2021) Metabolomic signatures of coral bleaching history. Nat Ecol Evol 5:495–503
Rodriguez-Lanetty M, Loh W, Carter D, Hoegh-Guldberg O (2001) Latitudinal variability in symbiont specificity within the widespread scleractinian coral Plesiastrea versipora. Mar Biol 138:1175–1181
Ros M, Suggett DJ, Edmondson J, Haydon T, Hughes DJ, Kim M, Guagliardo P, Bougoure J, Pernice M, Raina J-B, Camp EF (2021) Symbiont shuffling across environmental gradients aligns with changes in carbon uptake and translocation in the reef-building coral Pocillopora acuta. Coral Reefs. https://doi.org/10.1007/s00338-021-02066-1
Rosset SL, Oakley CA, Ferrier-Pagès C, Suggett DJ, Weis VM, Davy SK (2021) The molecular language of the cnidarian–dinoflagellate symbiosis. Trends Microbiol 29:320–333
Schmidt-Roach S, Miller KJ, Andreakis N (2013) Pocillopora aliciae: a new species of scleractinian coral (Scleractinia, Pocilloporidae) from subtropical Eastern Australia. Zootaxa 3626:576–582
Smart KF, Aggio RBM, Van Houtte JR, Villas-Bôas SG (2010) Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography–mass spectrometry. Nat Protoc 5:1709–1729
Sommer B, Harrison PL, Beger M, Pandolfi JM (2014) Trait-mediated environmental filtering drives assembly at biogeographic transition zones. Ecology 95:1000–1009
Sommer B, Sampayo EM, Beger M, Harrison PL, Babcock RC, Pandolfi JM (2017) Local and regional controls of phylogenetic structure at the high-latitude range limits of corals. Proc Royal Soc B 284(1861):20170915
Sommer B, Beger M, Harrison PL, Babcock RC, Pandolfi JM (2018) Differential response to abiotic stress controls species distributions at biogeographic transition zones. Ecography 41:478–490
Suggett DJ, Smith DJ (2020) Coral bleaching patterns are the outcome of complex biological and environmental networking. Glob Chang Biol 26:68–79
Suggett DJ, Warner ME, Leggat W (2017) Symbiotic dinoflagellate functional diversity mediates coral survival under ecological crisis. Trends Ecol Evol 32:735–745
Tchernov D, Gorbunov MY, de Vargas C, Narayan Yadav S, Milligan AJ, Häggblom M, Falkowski PG (2004) Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. PNAS 101:13531–13535
Treignier C, Tolosa I, Grover R, Reynaud S, Ferrier-Pagés C (2009) Carbon isotope composition of fatty acids and sterols in the scleractinian coral Turbinaria reniformis: Effect of light and feeding. Limnol Oceanogr 54:1933–1940
Tusher VG, Tibshirani R, Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. PNAS 98:5116–5121
Varkey D, Mazard S, Jeffries TC, Hughes DJ, Seymour J, Paulsen IT, Ostrowski M (2018) Stormwater influences phytoplankton assemblages within the diverse, but impacted Sydney Harbour estuary. PLoS ONE 13:e0209857
Vergés A, Steinberg PD, Hay ME, Poore AGB, Campbell AH, Ballesteros E, Heck KL, Booth DJ, Coleman MA, Feary DA, Figueira W, Langlois T, Marzinelli EM, Mizerek T, Mumby PJ, Nakamura Y, Roughan M, Ev S, Gupta AS, Smale DA, Tomas F, Wernberg T, Wilson SK (2014) The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc R Soc B 281:20140846
Wang J, Douglas A (1998) Nitrogen recycling or nitrogen conservation in an alga-invertebrate symbiosis? J Exp Biol 201:2445–2453
Xia J, Wishart DS (2011) Metabolomic data processing, analysis, and interpretation using MetaboAnalyst. Curr Protoc Bioinformatics. https://doi.org/10.1002/0471250953.bi1410s34
Yamano H, Sugihara K, Nomura K (2011) Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures. Geophys Res Lett 38:4
Yang K, Li J, Gao H (2006) The impact of sample imbalance on identifying differentially expressed genes. BMC Bioinformatics 7:S8
Acknowledgements
This project was funded by a 2019 Ethel Mary Read Research Grant from the Royal Zoological Society of New South Wales awarded to JM and a PADI Foundation grant awarded to JM, BS, DB and WF. JM was support by a Human Frontiers Science Programme Long-term Postdoctoral fellowship (LT000625/2018-L). RAGP was supported by the Early Career Training Program of the Coral Bleaching Research Coordination Network and by a Career Development Scholarship Extension of The University of Queensland. BS was supported by a Chancellor’s Postdoctoral Research Fellowship from the University of Technology Sydney and a Research Fellowship from The University of Sydney.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Mark Vermeij
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file3 (MOV 307513 kb)
Supplementary file4 (MOV 453635 kb)
Supplementary file5 (MOV 257920 kb)
Supplementary file6 (MOV 257324 kb)
Supplementary file7 (MOV 258940 kb)
Supplementary file8 (MOV 307304 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
González-Pech, R.A., Hughes, D.J., Strudwick, P. et al. Physiological factors facilitating the persistence of Pocillopora aliciae and Plesiastrea versipora in temperate reefs of south-eastern Australia under ocean warming. Coral Reefs 41, 1239–1253 (2022). https://doi.org/10.1007/s00338-022-02277-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02277-0