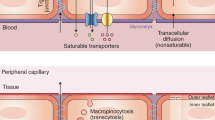
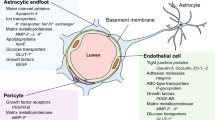
Abstract
Life expectancy has increased in most developed countries, which has led to an increase in the proportion of elderly people in the world’s population. However, this increase in life expectancy is not accompanied by a lengthening of the health span since aging is characterized with progressive deterioration in cellular and organ functions. The brain is particularly vulnerable to disease, and this is reflected in the onset of age-related neurodegenerative diseases such as Alzheimer’s disease. Research shows that dysfunction of two barriers in the central nervous system (CNS), the blood–brain barrier (BBB) and the blood–cerebrospinal fluid (CSF) barrier (BCSFB), plays an important role in the progression of these neurodegenerative diseases. The BBB is formed by the endothelial cells of the blood capillaries, whereas the BCSFB is formed by the epithelial cells of the choroid plexus (CP), both of which are affected during aging. Here, we give an overview of how these barriers undergo changes during aging and in Alzheimer’s disease, thereby disturbing brain homeostasis. Studying these changes is needed in order to gain a better understanding of the mechanisms of aging at the brain barriers, which might lead to the development of new therapies to lengthen the health span (including mental health) and reduce the chances of developing Alzheimer’s disease.

Similar content being viewed by others
References
Abbott NJ (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45:545–552
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood–brain barrier. Neurobiol Dis 37:13–25
Balusu S, Brkic M, Libert C, Vandenbroucke RE (2016) The choroid plexus-cerebrospinal fluid interface in Alzheimer’s disease: more than just a barrier. Neural Regen Res 11(4):534–537
Baruch K, Ron-Harel N, Gal H, Deczkowska A, Shifrut E, Ndifon W, Mirlas-Neisberg N, Cardon M, Vaknin I, Cahalon L, Berkutzki T, Mattson MP, Gomez-Pinilla F, Friedman N, Schwartz M (2013) CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci 110:2264–2269
Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, Amit I, Schwartz M (2014) Aging. aging-induced type i interferon response at the choroid plexus negatively affects brain function. Science 346:89–93
Baruch K, Kertser A, Porat Z, Schwartz M (2015) Cerebral nitric oxide represses choroid plexus NFkappaB-dependent gateway activity for leukocyte trafficking. EMBO J 34:1816–1828
Begley DJ (2004) ABC transporters and the blood–brain barrier. Curr Pharm Des 10:1295–1312
Begley DJ, Brightman MW (2003) Structural and functional aspects of the blood–brain barrier. Prog Drug Res 61:39–78
Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68:409–427
Bellingham SA, Guo BB, Coleman BM, Hill AF (2012) Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol 3:124
Blennow K, Fredman P, Wallin A, Gottfries CG, Karlsson I, Langstrom G, Skoog I, Svennerholm L, Wikkelso C (1993) Protein analysis in cerebrospinal fluid. II. Reference values derived from healthy individuals 18–88 years of age. Eur Neurol 33:129–133
Brinker T, Stopa E, Morrison J, Klinge P (2014) A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11:10
Brkic M, Balusu S, Van Wonterghem E, Gorle N, Benilova I, Kremer A, Van Hove I, Moons L, De Strooper B, Kanazir S, Libert C, Vandenbroucke RE (2015) Amyloid beta oligomers disrupt blood–CSF barrier integrity by activating matrix metalloproteinases. J Neurosci 35:12766–12778
Brown WR, Thore CR (2011) Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol 37:56–74
Buchweitz-Milton E, Weiss HR (1987) Perfused capillary morphometry in the senescent brain. Neurobiol Aging 8:271–276
Chen RL, Kassem NA, Redzic ZB, Chen CP, Segal MB, Preston JE (2009) Age-related changes in choroid plexus and blood-cerebrospinal fluid barrier function in the sheep. Exp Gerontol 44:289–296
Chiaretti A, Antonelli A, Genovese O, Pezzotti P, Rocco CD, Viola L, Riccardi R (2008) Nerve growth factor and doublecortin expression correlates with improved outcome in children with severe traumatic brain injury. J Trauma 65:80–85
Cottrell DA, Blakely EL, Johnson MA, Ince PG, Borthwick GM, Turnbull DM (2001) Cytochrome c oxidase deficient cells accumulate in the hippocampus and choroid plexus with age. Neurobiol Aging 22:265–272
Crone C, Christensen O (1981) Electrical resistance of a capillary endothelium. J Gen Physiol 77:349–371
Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C, Paquet N, Begdouri H, Bentourkia M, Turcotte E, Allard M, Barberger-Gateau P, Fulop T, Rapoport SI (2011) Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition 27:3–20
De Bock M, Vandenbroucke RE, Decrock E, Culot M, Cecchelli R, Leybaert L (2014) A new angle on blood-CNS interfaces: a role for connexins? FEBS Lett 588:1259–1270
De Bock M, Van Haver V, Vandenbroucke RE, Decrock E, Wang N, Leybaert L (2016) Into rather unexplored terrain-transcellular transport across the blood-brain barrier. Glia
Deczkowska A, Baruch K, Schwartz M (2016) Type I/II Interferon Balance in the Regulation of Brain Physiology and Pathology. Trends Immunol 37:181–192
Demeestere D, Libert C, Vandenbroucke RE (2015) Clinical implications of leukocyte infiltration at the choroid plexus in (neuro)inflammatory disorders. Drug Discov Today 20:928–941
Dickson PW, Aldred AR, Marley PD, Bannister D, Schreiber G (1986) Rat choroid plexus specializes in the synthesis and the secretion of transthyretin (prealbumin). Regulation of transthyretin synthesis in choroid plexus is independent from that in liver. J Biol Chem 261:3475–3478
Dietrich MO, Spuch C, Antequera D, Rodal I, de Yebenes JG, Molina JA, Bermejo F, Carro E (2008) Megalin mediates the transport of leptin across the blood-CSF barrier. Neurobiol Aging 29:902–912
Dziegielewska KM, Ek J, Habgood MD, Saunders NR (2001) Development of the choroid plexus. Microsc Res Tech 52:5–20
El Andaloussi S, Mager I, Breakefield XO, Wood MJA (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12:347–357
Elahy M, Jackaman C, Mamo JC, Lam V, Dhaliwal SS, Giles C, Nelson D, Takechi R (2015) Blood–brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing 12:2
Emerich DF, Skinner SJ, Borlongan CV, Vasconcellos AV, Thanos CG (2005) The choroid plexus in the rise, fall and repair of the brain. BioEssays 27:262–274
Endo H, Sasaki K, Tonosaki A, Kayama T (1998) Three-dimensional and ultrastructural ICAM-1 distribution in the choroid plexus, arachnoid membrane and dural sinus of inflammatory rats induced by LPS injection in the lateral ventricles. Brain Res 793:297–301
Engelhardt B, Coisne C (2011) Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS 8:10
Engelhardt B, Ransohoff RM (2005) The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol 26:485–495
Eriksson L, Westermark P (1986) Intracellular neurofibrillary tangle-like aggregations. A constantly present amyloid alteration in the aging choroid plexus. Am J Pathol 125:124–129
Eriksson L, Westermark P (1990) Characterization of intracellular amyloid fibrils in the human choroid plexus epithelial cells. Acta Neuropathol 80:597–603
Falcao AM, Marques F, Novais A, Sousa N, Palha JA, Sousa JC (2012) The path from the choroid plexus to the subventricular zone: go with the flow! Front Cell Neurosci 6:34
Farkas E, Luiten PG (2001) Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 64:575–611
Farrall AJ, Wardlaw JM (2009) Blood–brain barrier: ageing and microvascular disease—systematic review and meta-analysis. Neurobiol Aging 30:337–352
Ferrante F, Amenta F (1987) Enzyme histochemistry of the choroid plexus in old rats. Mech Ageing Dev 41:65–72
Franceschi C, Campisi J (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69(Suppl 1):S4–S9. doi:10.1093/gerona/glu057
Fuchs HE, Bullard DE (1988) Immunology of transplantation in the central nervous system. Appl Neurophysiol 51:278–296
Galea I, Bechmann I, Perry VH (2007) What is immune privilege (not)? Trends Immunol 28:12–18
Garton MJ, Keir G, Lakshmi MV, Thompson EJ (1991) Age-related changes in cerebrospinal fluid protein concentrations. J Neurol Sci 104:74–80
Gemechu JM, Bentivoglio M (2012) T cell recruitment in the brain during normal aging. Front Cell Neurosci 6:38
Goldman H, Berman RF, Gershon S, Murphy S, Morehead M, Altman HJ (1992) Cerebrovascular permeability and cognition in the aging rat. Neurobiol Aging 13:57–62
Gonzalez-Marrero I, Gimenez-Llort L, Johanson CE, Carmona-Calero EM, Castaneyra-Ruiz L, Brito-Armas JM, Castaneyra-Perdomo A, Castro-Fuentes R (2015) Choroid plexus dysfunction impairs beta-amyloid clearance in a triple transgenic mouse model of Alzheimer’s disease. Front Cell Neurosci 9:17
Grammas P, Ovase R (2001) Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol Aging 22:837–842
Grammas P, Samany PG, Thirumangalakudi L (2006) Thrombin and inflammatory proteins are elevated in Alzheimer’s disease microvessels: implications for disease pathogenesis. J Alzheimers Dis 9:51–58
Grapp M, Wrede A, Schweizer M, Huwel S, Galla HJ, Snaidero N, Simons M, Buckers J, Low PS, Urlaub H, Gartner J, Steinfeld R (2013) Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun 4:2123
Greenwood J, Heasman SJ, Alvarez JI (2011) Review: leucocyte–endothelial cell crosstalk at the blood–brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathology and Applied Neurobiology
Haqqani AS, Delaney CE, Tremblay TL, Sodja C, Sandhu JK, Stanimirovic DB (2013) Method for isolation and molecular characterization of extracellular microvesicles released from brain endothelial cells. Fluids Barriers CNS 10:4
Hawkins BT, Davis TP (2005) The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57:173–185
Hoyer S (1990) Brain glucose and energy metabolism during normal aging. Aging (Milano) 2:245–258
Hunt A, Schonknecht P, Henze M, Seidl U, Haberkorn U, Schroder J (2007) Reduced cerebral glucose metabolism in patients at risk for Alzheimer’s disease. Psychiatry Res 155:147–154
Iadecola C (2004) Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5:347–360
Johanson CE, Duncan JA 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD (2008) Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res 5:10
Kalani A, Tyagi A, Tyagi N (2014) Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol Neurobiol 49:590–600
Keep RF, Jones HC (1990) A morphometric study on the development of the lateral ventricle choroid plexus, choroid plexus capillaries and ventricular ependyma in the rat. Brain Res Dev Brain Res 56:47–53
Kim SU, de Vellis J (2005) Microglia in health and disease. J Neurosci Res 81:302–313
Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M (2004) T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci 101:8180–8185
Kleine TO, Hackler R, Lutcke A, Dauch W, Zofel P (1993) Transport and production of cerebrospinal fluid (CSF) change in aging humans under normal and diseased conditions. Z Gerontol 26:251–255
Kokubo H, Saido TC, Iwata N, Helms JB, Shinohara R, Yamaguchi H (2005) Part of membrane-bound Abeta exists in rafts within senile plaques in Tg2576 mouse brain. Neurobiol Aging 26:409–418
Kumagai N, Chiba Y, Hosono M, Fujii M, Kawamura N, Keino H, Yoshikawa K, Ishii S, Saitoh Y, Satoh M, Shimada A, Hosokawa M (2007) Involvement of pro-inflammatory cytokines and microglia in an age-associated neurodegeneration model, the SAMP10 mouse. Brain Res 1185:75–85
Kunis G, Baruch K, Rosenzweig N, Kertser A, Miller O, Berkutzki T, Schwartz M (2013) IFN-γ-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain 136:3427–3440
Li M, Shang DS, Zhao WD, Tian L, Li B, Fang WG, Zhu L, Man SM, Chen YH (2009) Amyloid beta interaction with receptor for advanced glycation end products up-regulates brain endothelial CCR5 expression and promotes T cells crossing the blood–brain barrier. J Immunol 182:5778–5788
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217
Marchesi VT (2011) Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J 25:5–13
Marques F, Sousa JC, Correia-Neves M, Oliveira P, Sousa N, Palha JA (2007) The choroid plexus response to peripheral inflammatory stimulus. Neuroscience 144:424–430
Marques F, Sousa JC, Coppola G, Geschwind DH, Sousa N, Palha JA, Correia-Neves M (2009) The choroid plexus response to a repeated peripheral inflammatory stimulus. BMC Neurosci 10:135
Marques F, Sousa JC, Sousa N, Palha JA (2013) Blood-brain-barriers in aging and in Alzheimer’s disease. Mol Neurodegener 8:38
Masseguin C, LePanse S, Corman B, Verbavatz JM, Gabrion J (2005) Aging affects choroidal proteins involved in CSF production in Sprague-Dawley rats. Neurobiol Aging 26:917–927
McLachlan MS (1978) The ageing kidney. Lancet 2:143–145
Mesquita SD, Ferreira AC, Gao F, Coppola G, Geschwind DH, Sousa JC, Correia-Neves M, Sousa N, Palha JA, Marques F (2015) The choroid plexus transcriptome reveals changes in type I and II interferon responses in a mouse model of Alzheimer’s disease. Brain Behav Immun 49:280–292
Miguel-Hidalgo JJ, Nithuairisg S, Stockmeier C, Rajkowska G (2007) Distribution of ICAM-1 immunoreactivity during aging in the human orbitofrontal cortex. Brain Behav Immun 21:100–111
Miklossy J, Kraftsik R, Pillevuit O, Lepori D, Genton C, Bosman FT (1998) Curly fiber and tangle-like inclusions in the ependyma and choroid plexus—a pathogenetic relationship with the cortical Alzheimer-type changes? J Neuropathol Exp Neurol 57:1202–1212
Modic MT, Weinstein MA, Rothner AD, Erenberg G, Duchesneau PM, Kaufman B (1980) Calcification of the choroid plexus visualized by computed tomography. Radiology 135:369–372
Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV (2015) blood–brain barrier breakdown in the aging human hippocampus. Neuron 85:296–302
Mooradian AD (1994) Potential mechanisms of the age-related changes in the blood–brain barrier. Neurobiol Aging 15:751–755 (discussion 761–762, 767)
Mooradian AD, Chung HC, Shah GN (1997) GLUT-1 expression in the cerebra of patients with Alzheimer’s disease. Neurobiol Aging 18:469–474
Nazari Z, Nabiuni M, Safaei Nejad Z, Delfan B, Irian S (2015) Expression of aquaporins in the rat choroid plexus. Arch Neurosci 2:e17312
Nilsson C, Hultberg BM, Gammeltoft S (1996) Autocrine role of insulin-like growth factor II secretion by the rat choroid plexus. Eur J Neurosci 8:629–635
Ohara Y, McCarron RM, Hoffman TT, Sugano H, Bembry J, Lenz FA, Spatz M (2000) Adrenergic mediation of TNF alpha-stimulated ICAM-1 expression on human brain microvascular endothelial cells. Acta Neurochir Suppl 76:117–120
Pascale CL, Miller MC, Chiu C, Boylan M, Caralopoulos IN, Gonzalez L, Johanson CE, Silverberg GD (2011) Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent. Fluids Barriers CNS 8:21
Pelegri C, Canudas AM, del Valle J, Casadesus G, Smith MA, Camins A, Pallas M, Vilaplana J (2007) Increased permeability of blood–brain barrier on the hippocampus of a murine model of senescence. Mech Ageing Dev 128:522–528
Perry VH (1998) A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol 90:113–121
Petzold A, Psotta L, Brigadski T, Endres T, Lessmann V (2015) Chronic BDNF deficiency leads to an age-dependent impairment in spatial learning. Neurobiol Learn Mem 120:52–60
Preston JE (2001) Ageing choroid plexus-cerebrospinal fluid system. Microsc Res Tech 52:31–37
Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K (2006) Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci 103:11172–11177
Ransohoff RM, Engelhardt B (2012) The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 12:623–635
Ransohoff RM, Kivisakk P, Kidd G (2003) Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 3:569–581
Rapoport SI, Ohno K, Pettigrew KD (1979) Blood–brain barrier permeability in senescent rats. J Gerontol 34:162–169
Redzic Z (2011) Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS 8:3
Redzic ZB, Segal MB (2004) The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv Drug Deliv Rev 56:1695–1716
Redzic ZB, Preston JE, Duncan JA, Chodobski A, Szmydynger-Chodobska J (2005) The choroid plexus-cerebrospinal fluid system: from development to aging. Curr Top Dev Biol 71:1–52
Redzic ZB, Malatiali SA, Craik JD, Rakic ML, Isakovic AJ (2009) Blood–brain barrier efflux transport of pyrimidine nucleosides and nucleobases in the rat. Neurochem Res 34:566–573
Roberts LM, Woodford K, Zhou M, Black DS, Haggerty JE, Tate EH, Grindstaff KK, Mengesha W, Raman C, Zerangue N (2008) Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood–brain barrier. Endocrinology 149:6251–6261
Rubenstein E (1998) Relationship of senescence of cerebrospinal fluid circulatory system to dementias of the aged. Lancet 351:283–285
Rudick RA, Buell SJ (1983) Integrity of blood–brain barrier to peroxidase in senescent mice. Neurobiol Aging 4:283–287
Saito Y, Wright EM (1984) Regulation of bicarbonate transport across the brush border membrane of the bull-frog choroid plexus. J Physiol 350:327–342
Sanchez-Covarrubias L, Slosky LM, Thompson BJ, Davis TP, Ronaldson PT (2014) Transporters at CNS barrier sites: obstacles or opportunities for drug delivery? Curr Pharm Des 20:1422–1449
Saunders NR, Habgood MD, Dziegielewska KM (1999) Barrier mechanisms in the brain, II Immature brain. Clin Exp Pharmacol Physiol 26:85–91
Saunders NR, Ek CJ, Habgood MD, Dziegielewska KM (2008) Barriers in the brain: a renaissance? Trends Neurosci 31:279–286
Saunders NR, Daneman R, Dziegielewska KM, Liddelow SA (2013) Transporters of the blood-brain and blood-CSF interfaces in development and in the adult. Mol Aspects Med 34:742–752
Schindowski K, Eckert A, Peters J, Gorriz C, Schramm U, Weinandi T, Maurer K, Frolich L, Muller WE (2007) Increased T-cell reactivity and elevated levels of CD8+ memory T-cells in Alzheimer’s disease-patients and T-cell hyporeactivity in an Alzheimer’s disease-mouse model: implications for immunotherapy. Neuromolecular Med 9:340–354
Schinkel AH (1999) P-Glycoprotein, a gatekeeper in the blood–brain barrier. Adv Drug Deliv Rev 36:179–194
Schwarzman AL, Gregori L, Vitek MP, Lyubski S, Strittmatter WJ, Enghilde JJ, Bhasin R, Silverman J, Weisgraber KH, Coyle PK et al (1994) Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci 91:8368–8372
Sedlakova R, Shivers RR, Del Maestro RF (1999) Ultrastructure of the blood-brain barrier in the rabbit. J Submicrosc Cytol Pathol 31:149–161
Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV (2013) Deficiency in mural vascular cells coincides with blood–brain barrier disruption in Alzheimer’s disease. Brain Pathol 23:303–310
Serot JM, Christmann D, Dubost T, Couturier M (1997) Cerebrospinal fluid transthyretin: aging and late onset Alzheimer’s disease. J Neurol Neurosurg Psychiatry 63:506–508
Serot JM, Bene MC, Foliguet B, Faure GC (2000) Morphological alterations of the choroid plexus in late-onset Alzheimer’s disease. Acta Neuropathol 99:105–108
Serot JM, Bene MC, Faure GC (2003) Choroid plexus, aging of the brain, and Alzheimer’s disease. Front Biosci 8:s515–s521
Shearer GM (1997) Th1/Th2 changes in aging. Mech Ageing Dev 94:1–5
Shechter R, London A, Schwartz M (2013) Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol 13:206–218
Silverberg GD, Mayo M, Saul T, Rubenstein E, McGuire D (2003) Alzheimer’s disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol 2:506–511
Silverberg GD, Miller MC, Messier AA, Majmudar S, Machan JT, Donahue JE, Stopa EG, Johanson CE (2010) Amyloid deposition and influx transporter expression at the blood–brain barrier increase in normal aging. J Neuropathol Exp Neurol 69:98–108
Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27:1766–1791
Solito E, Sastre M (2012) Microglia function in Alzheimer’s disease. Front Pharmacol 3:14
Sousa JC, Marques F, Dias-Ferreira E, Cerqueira JJ, Sousa N, Palha JA (2007) Transthyretin influences spatial reference memory. Neurobiol Learn Mem 88:381–385
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV et al (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:280–292
Stichel CC, Luebbert H (2007) Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. Neurobiol Aging 28:1507–1521
Storck SE, Meister S, Nahrath J, Meißner JN, Schubert N, Di Spiezio A, Baches S, Vandenbroucke RE, Bouter Y, Prikulis I, Korth C, Weggen S, Heimann A, Schwaninger M, Bayer TA, Pietrzik CU (2016) Endothelial LRP1 transports amyloid-β1-42 across the blood–brain barrier. J Clin Invest 126:123–136
Strazielle N, Ghersi-Egea JF (2000) Choroid plexus in the central nervous system: biology and physiopathology. J Neuropathol Exp Neurol 59:561–574
Thouvenot E, Lafon-Cazal M, Demettre E, Jouin P, Bockaert J, Marin P (2006) The proteomic analysis of mouse choroid plexus secretome reveals a high protein secretion capacity of choroidal epithelial cells. Proteomics 6:5941–5952
Tietje A, Maron KN, Wei Y, Feliciano DM (2014) Cerebrospinal fluid extracellular vesicles undergo age dependent declines and contain known and novel non-coding RNAs. PLoS One 9:e113116
Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K (2002) Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol 124:83–92
Tripathy D, Sanchez A, Yin X, Luo J, Martinez J, Grammas P (2013) Thrombin, a mediator of cerebrovascular inflammation in AD and hypoxia. Front Aging Neurosci 5:19
Tsilioni I, Panagiotidou S, Theoharides TC (2014) Exosomes in neurologic and psychiatric disorders. Clin Ther 36:882–888
Tuszynski MH, Blesch A (2004) Nerve growth factor: from animal models of cholinergic neuronal degeneration to gene therapy in Alzheimer’s disease. Prog Brain Res 146:441–449
Ujiie M, Dickstein DL, Carlow DA, Jefferies WA (2003) Blood–brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation 10:463–470
Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR et al (2011) The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477:90–94
Wadhwani KC, Koistinaho J, Balbo A, Rapoport SI (1991) Blood-nerve and blood–brain barrier permeabilities and nerve vascular space in Fischer-344 rats of different ages. Mech Ageing Dev 58:177–190
Walter HJ, Berry M, Hill DJ, Cwyfan-Hughes S, Holly JM, Logan A (1999) Distinct sites of insulin-like growth factor (IGF)-II expression and localization in lesioned rat brain: possible roles of IGF binding proteins (IGFBPs) in the mediation of IGF-II activity. Endocrinology 140:520–532
Wang Y, Liu J, Zhang Z, Wang X, Zhang C (2011) Structure and permeability changes of the blood–brain barrier in APP/PS1 mice: an Alzheimer’s disease animal model. Neurochem J 5:220–222
Wen GY, Wisniewski HM, Kascsak RJ (1999) Biondi ring tangles in the choroid plexus of Alzheimer’s disease and normal aging brains: a quantitative study. Brain Res 832:40–46
Weng NP (2006) Aging of the immune system: how much can the adaptive immune system adapt? Immunity 24:495–499
Wijnholds J, deLange EC, Scheffer GL, van den Berg DJ, Mol CA, van der Valk M, Schinkel AH, Scheper RJ, Breimer DD, Borst P (2000) Multidrug resistance protein 1 protects the choroid plexus epithelium and contributes to the blood-cerebrospinal fluid barrier. J Clin Invest 105:279–285
Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, Sengillo JD, Hillman S, Kong P, Nelson AR, Sullivan JS, Zhao Z, Meiselman HJ, Wenby RB, Soto J, Abel ED, Makshanoff J, Zuniga E, De Vivo DC, Zlokovic BV (2015) GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci 18:521–530
Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G (2009) CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol 182:3979–3984
Woods SC, Seeley RJ, Baskin DG, Schwartz MW (2003) Insulin and the blood–brain barrier. Curr Pharm Des 9:795–800
Yang YM, Shang DS, Zhao WD, Fang WG, Chen YH (2013) Microglial TNF-alpha-dependent elevation of MHC class I expression on brain endothelium induced by amyloid-beta promotes T cell transendothelial migration. Neurochem Res 38:2295–2304
Yang Y, Keene CD, Peskind ER, Galasko DR, Hu SC, Cudaback E, Wilson AM, Li G, Yu CE, Montine KS, Zhang J, Baird GS, Hyman BT, Montine TJ (2015) Cerebrospinal fluid particles in Alzheimer disease and Parkinson disease. J Neuropathol Exp Neurol 74:672–687
Young VG, Halliday GM, Kril JJ (2008) Neuropathologic correlates of white matter hyperintensities. Neurology 71:804–811
Yuyama K, Sun H, Mitsutake S, Igarashi Y (2012) Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J Biol Chem 287:10977–10989
Zhang Y, Pardridge WM (2001) Rapid transferrin efflux from brain to blood across the blood–brain barrier. J Neurochem 76:1597–1600
Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M (2006) Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 9:268–275
Zlokovic BV (2008) The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12:723–738
Author information
Authors and Affiliations
Corresponding author
Glossary
- Alzheimer’s disease
-
Most common form of dementia, characterized by the aggregation of Aβ in extracellular senile plaques and the formation of intraneuronal neurofibrillary tangles composed of hyperphosphorylated tau protein
- Biondi rings
-
Biondi rings are intracellular neurofibrillar inclusions found in the choroid plexus during aging
- Blood–brain barrier (BBB)
-
One of the brain barriers, formed by tightly connected tight junctions between the endothelial cells in the brain. This barrier is supported by associated pericytes and astrocyte end-feet
- Blood–cerebrospinal fluid barrier (BCSFB)
-
One of the brain barriers, formed by the choroid plexus epithelium. Tight junctions prevent contact between blood and cerebrospinal fluid (CSF)
- Dementia
-
Progressive loss of cognitive functions, caused by severe neuronal death
- Inflammaging
-
An important process during aging. It is a progressive low inflammatory state causing age-related diseases and death
- Lipofuscin
-
Lipofuscins are granules filled with pigment composed of lipid-containing residues of lysosomal degradation. They can be found during aging in the liver, kidneys, neurons, and choroid plexus
- Pericyte
-
Cells associated with the endothelial cells of capillaries and venules. They regulate blood flow and are important for the integrity of the blood–brain barrier
- Secretome
-
Refers to all molecules secreted by cells, tissues, and organs
Rights and permissions
About this article
Cite this article
Gorlé, N., Van Cauwenberghe, C., Libert, C. et al. The effect of aging on brain barriers and the consequences for Alzheimer’s disease development. Mamm Genome 27, 407–420 (2016). https://doi.org/10.1007/s00335-016-9637-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-016-9637-8