Abstract
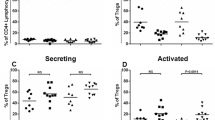
Neuroinflammation is observed in neurodegenerative diseases like Alzheimer’s disease (AD). However, a little is known about the mechanisms of neural-immune interactions. The involvement of peripheral T-cell function in AD is still far from clear, though it plays an important role in immunotherapy. The aim of this study was to determine peripheral T-cell reactivity in AD patients and in an AD mouse model. Mitogenic activation via ligation of the T-cell receptor (TCR) with PHA-L was measured in T lymphocytes from AD patients and Thy1(APP751SL) × HMG(PS1M146L)-transgenic mice (APP × PS1). In order to uncover failures in TCR signaling, the TCR was also bypassed by PMA and ionomycin treatment. All patients were sporadic late onset cases and the transgenic mice expressed no mutant APP in lymphocytes, so that direct interactions of mutant APP on T-cell function can be excluded. CD4+ and CD8+ T-cell showed increased reactivity (tyrosine phosphorylation, CD69 expression, and proliferation) in AD, while APP × PS1 transgenic mice displayed hyporeactive CD8+ T-cells after TCR ligation. Increased levels of CD8+ T memory cells and down regulation of CD8 receptor were found in AD and the animal model. Anergic TCR uncoupling was associated with loss of MAPK signaling (p38, ERK1 and ERK2) in APP × PS1. Our data implicate the generation of reactive memory T-cell in AD and of anergic memory T-cells in transgenic mice and should be taken into concern when designing immunotherapy.





Similar content being viewed by others
Abbreviations
- 7-AAD:
-
7-Amino actinomycin
- AD:
-
Alzheimer’s disease
- ApoE:
-
Apoliporotein E
- APP:
-
Amyloid precursor protein
- BrdU:
-
Brom-3′desoxy-uridine
- CD:
-
Cluster of differentiation
- CNS:
-
Central nervous system
- FITC:
-
Fluorescein isothiocyanate
- FSC:
-
Forward scatter
- I:
-
Ionomycin
- MMSE:
-
Mini mental state examination score
- MAPK:
-
Mitogen-activated protein kinase
- MS:
-
Multiple sclerosis
- n.s.:
-
Not statistically significant
- n.d.:
-
Not determined
- PBMC:
-
Peripheral blood mononuclear cells
- PE:
-
Phycoerythrin
- PerCp:
-
Peridin chlorophyll
- PHA-L:
-
Lectin from Phaseolus vulgaris Leucoagglutinin
- PMA:
-
Phorbol 12-myristate 13-acetate
- PS1:
-
Presenilin-1
- SSC:
-
Side scatter
- Th:
-
T helper
- vs.:
-
Versus
References
Adunsky, A., Baram, D., Hershkowitz, M., & Mekori, Y. A. (1991). Increased cytosolic free calcium in lymphocytes of Alzheimer patients. Journal of Neuroimmunology, 33, 167–172.
Adunsky, A., Diver-Haber, A., Becker, D., & Hershkowitz, M. (1995). Basal and activated intracellular calcium ion concentrations in mononuclear cells of Alzheimer’s disease and unipolar depression. The Journals of Gerontology. Series A Biological Sciences and Medical Sciences, 50, B201–B204.
Aloisi, F., Ria, F., Columba-Cabezas, S., Hess, H., Penna, G., & Adorini, L. (1999). Relative efficiency of microglia, astrocytes, dendritic cells and B cells in naive CD4+ T cell priming and Th1/Th2 cell restimulation. European Journal of Immunology, 29, 2705–2714.
Blanchard, V., Moussaoui, S., Czech, C., Touchet, N., Bonici, B., Planche, M., Canton, T., Jedidi, I., Gohin, M., Wirths, O., Bayer, T. A., Langui, D., Duyckaerts, C., Tremp, G., & Pradier, L. (2003). Time sequence of maturation of dystrophic neurites associated with Abeta deposits in APP/PS1 transgenic mice. Experimental Neurology, 184, 247–263.
Bongioanni, P., Boccardi, B., Borgna, M., Castagna, M., & Mondino, C. (1997a). T-cell interferon gamma binding in patients with dementia of the Alzheimer type. Archives of Neurology, 54, 457–462.
Bongioanni, P., Boccardi, B., Borgna, M., & Rossi, B. (1998). T-lymphocyte interleukin 6 receptor binding in patients with dementia of Alzheimer type. Archives of Neurology, 55, 1305–1308.
Bongioanni, P., Romano, M. R., Sposito, R., Castagna, M., Boccardi, B., & Borgna, M. (1997b). T-cell tumour necrosis factor-alpha receptor binding in demented patients. Journal of Neurology, 244, 418–425.
Carson, M. J., Reilly, C. R., Sutcliffe, J. G., & Lo, D. (1999). Disproportionate recruitment of CD8+ T cells into the central nervous system by professional antigen-presenting cells. American Journal of Pathology, 154, 481–494.
Carson, M. J., & Sutcliffe, J. G. (1999). Balancing function vs. self defense: The CNS as an active regulator of immune responses. Journal of Neuroscience Research, 55, 1–8.
Chandok, M. R., & Farber, D. L. (2004). Signaling control of memory T cell generation and function. Seminars in Immunology, 16, 285–293.
Chiodetti, L., Choi, S., Barber, D. L., & Schwartz, R. H. (2006). Adaptive tolerance and clonal anergy are distinct biochemical states. Journal of Immunology, 176, 2279–2291.
Cho, B. K., Wang, C., Sugawa, S., Eisen, H. N., & Chen, J. (1999). Functional differences between memory and naive CD8 T cells. Proceedings of the National Academy of Sciences of the United States of America, 96, 2976–2981.
Colton, C. A., Brown, C. M., & Vitek, M. P. (2005). Sex steroids, APOE genotype and the innate immune system. Neurobiology of Aging, 26, 363–372.
Cornet, A., Bettelli, E., Oukka, M., Cambouris, C., Avellana-Adalid, V., Kosmatopoulos, K., & Liblau, R. S. (2000). Role of astrocytes in antigen presentation and naive T-cell activation. Journal of Neuroimmunology, 106, 69–77.
Cribbs, D. H., Ghochikyan, A., Vasilevko, V., Tran, M., Petrushina, I., Sadzikava, N., Babikyan, D., Kesslak, P., Kieber-Emmons, T., Cotman, C. W., & Agadjanyan, M. G. (2003). Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. International Immunology, 15, 505–514.
Czech, C., Delaere, P., Macq, A. F., Reibaud, M., Dreisler, S., Touchet, N., Schombert, B., Mazadier, M., Mercken, L., Theisen, M., Pradier, L., Octave, J. N., Beyreuther, K., & Tremp, G. (1997). Proteolytical processing of mutated human amyloid precursor protein in transgenic mice. Brain Research. Molecular Brain Research, 47, 108–116.
Dai, Z., Nasr, I. W., Reel, M., Deng, S., Diggs, L., Larsen, C. P., Rothstein, D. M., & Lakkis, F. G. (2005). Impaired recall of CD8 memory T cells in immunologically privileged tissue. Journal of Immunology, 174, 1165–1170.
Das, P., Murphy, M. P., Younkin, L. H., Younkin, S. G., & Golde, T. E. (2001). Reduced effectiveness of Abeta1-42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiology of Aging, 22, 721–727.
DeSilva, D. R., Feeser, W. S., Tancula, E. J., & Scherle, P. A. (1996). Anergic T cells are defective in both jun NH2-terminal kinase and mitogen-activated protein kinase signaling pathways. The Journal of Experimental Medicine, 183, 2017–2023.
DeSilva, D. R., Jones, E. A., Feeser, W. S., Manos, E. J., & Scherle, P. A. (1997). The p38 mitogen-activated protein kinase pathway in activated and anergic Th1 cells. Cellular Immunology, 180, 116–123.
Dorszewska, J., Florczak, J., Rozycka, A., Jaroszewska-Kolecka, J., Trzeciak, W. H., & Kozubski, W. (2005). Polymorphisms of the CHRNA4 gene encoding the alpha4 subunit of nicotinic acetylcholine receptor as related to the oxidative DNA damage and the level of apoptotic proteins in lymphocytes of the patients with Alzheimer’s disease. DNA and Cell Biology, 24, 786–794.
Duff, K., Eckman, C., Zehr, C., Yu, X., Prada, C. M., Perez-tur, J., Hutton, M., Buee, L., Harigaya, Y., Yager, D., Morgan, D., Gordon, M. N., Holcomb, L., Refolo, L., Zenk, B., Hardy, J., & Younkin, S. (1996). Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature, 383, 710–713.
Ebstein, R. P., Nemanov, L., Lubarski, G., Dano, M., Trevis, T., & Korczyn, A. D. (1996). Changes in expression of lymphocyte amyloid precursor protein mRNA isoforms in normal aging and Alzheimer’s disease. Brain Research. Molecular Brain Research, 35, 260–268.
Eckert, A., Forstl, H., Hartmann, H., Czech, C., Monning, U., Beyreuther, K., & Muller, W. E. (1995). The amplifying effect of beta-amyloid on cellular calcium signalling is reduced in Alzheimer’s disease. Neuroreport, 6, 1199–1202.
Eckert, A., Oster, M., Zerfass, R., Hennerici, M., & Muller, W. E. (2001a). Elevated levels of fragmented DNA nucleosomes in native and activated lymphocytes indicate an enhanced sensitivity to apoptosis in sporadic Alzheimer’s disease. Specific differences to vascular dementia. Dementia and Geriatric Cognitive Disorders, 12, 98–105.
Eckert, A., Schindowski, K., Leutner, S., Luckhaus, C., Touchet, N., Czech, C., & Muller, W. E. (2001b). Alzheimer’s disease-like alterations in peripheral cells from presenilin-1 transgenic mice. Neurobiology of Disease, 8, 331–342.
Eisenbraun, M. D., Tamir, A., & Miller, R. A. (2000). Altered composition of the immunological synapse in an anergic, age-dependent memory T cell subset. Journal of Immunology, 164, 6105–6112.
Engelhardt, B., & Ransohoff, R. M. (2005). The ins and outs of T-lymphocyte trafficking to the CNS: Anatomical sites and molecular mechanisms. Trends in Immunology, 26, 485–495.
Esplugues, E., Sancho, D., Vega-Ramos, J., Martinez, C., Syrbe, U., Hamann, A., Engel, P., Sanchez-Madrid, F., & Lauzurica, P. (2003). Enhanced antitumor immunity in mice deficient in CD69. The Journal of Experimental Medicine, 197, 1093–1106.
Fiala, M., Liu, Q. N., Sayre, J., Pop, V., Brahmandam, V., Graves, M. C., & Vinters, H. V. (2002). Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood–brain barrier. European Journal of Clinical Investigation, 32, 360–371.
Fields, P. E., Gajewski, T. F., & Fitch, F. W. (1996). Blocked Ras activation in anergic CD4+ T cells. Science, 271, 1276–1278.
Ford, A. L., Foulcher, E., Lemckert, F. A., & Sedgwick, J. D. (1996). Microglia induce CD4 T lymphocyte final effector function and death. The Journal of Experimental Medicine, 184, 1737–1745.
Frey, C., Bonert, A., Kratzsch, T., Rexroth, G., Rosch, W., Muller-Spahn, F., Maurer, K., Muller, W. E., & Eckert, A. (2006). Apolipoprotein E epsilon 4 is associated with an increased vulnerability to cell death in Alzheimer’s disease. Journal of Neural Transmission.
Furlan, R., Brambilla, E., Sanvito, F., Roccatagliata, L., Olivieri, S., Bergami, A., Pluchino, S., Uccelli, A., Comi, G., & Martino, G. (2003). Vaccination with amyloid-beta peptide induces autoimmune encephalomyelitis in C57/BL6 mice. Brain, 126, 285–291.
Galimberti, D., Schoonenboom, N., Scheltens, P., Fenoglio, C., Venturelli, E., Pijnenburg, Y. A., Bresolin, N., & Scarpini, E. (2006). Intrathecal chemokine levels in Alzheimer disease and frontotemporal lobar degeneration. Neurology, 66, 146–147.
Gee, J. R., & Keller, J. N. (2005). Astrocytes: Regulation of brain homeostasis via apolipoprotein E. The International Journal of Biochemistry and Cell Biology, 37, 1145–1150.
Geginat, J., Lanzavecchia, A., & Sallusto, F. (2003). Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood, 101, 4260–4266.
Gilman, S., Koller, M., Black, R. S., Jenkins, L., Griffith, S. G., Fox, N. C., Eisner, L., Kirby, L., Rovira, M. B., Forette, F., & Orgogozo, J. M. (2005). Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology, 64, 1553–1562.
Giuliani, F., Goodyer, C. G., Antel, J. P., & Yong, V. W. (2003). Vulnerability of human neurons to T cell-mediated cytotoxicity. Journal of Immunology, 171, 368–379.
Grossmann, A., Kukull, W. A., Jinneman, J. C., Bird, T. D., Villacres, E. C., Larson, E. B., & Rabinovitch, P. S. (1993). Intracellular calcium response is reduced in CD4+ lymphocytes in Alzheimer’s disease and in older persons with Down’s syndrome. Neurobiology of Aging, 14, 177–185.
Guidi, L., Tricerri, A., Frasca, D., Vangeli, M., Errani, A. R., & Bartoloni, C. (1998). Psychoneuroimmunology and aging. Gerontology, 44, 247–261.
Hanisch, U. K., Neuhaus, J., Quirion, R., & Kettenmann, H. (1996). Neurotoxicity induced by interleukin-2: Involvement of infiltrating immune cells. Synapse, 24, 104–114.
Havenith, C. E., Askew, D., & Walker, W. S. (1998). Mouse resident microglia: Isolation and characterization of immunoregulatory properties with naive CD4+ and CD8+ T-cells. Glia, 22, 348–359.
Hubert, P., Grenot, P., Autran, B., & Debre, P. (1997). Analysis by flow cytometry of tyrosine-phosphorylated proteins in activated T-cell subsets on whole blood samples. Cytometry, 29, 83–91.
Iarlori, C., Gambi, D., Gambi, F., Lucci, I., Feliciani, C., Salvatore, M., & Reale, M. (2005). Expression and production of two selected beta-chemokines in peripheral blood mononuclear cells from patients with Alzheimer’s disease. Experimental Gerontology, 40, 605–611.
Ibarreta, D., Parrilla, R., & Ayuso, M. S. (1997). Altered Ca2+ homeostasis in lymphoblasts from patients with late-onset Alzheimer disease. Alzheimer Disease and Associated Disorders, 11, 220–227.
Ikeda, T., Yamamoto, K., Takahashi, K., Kaneyuki, H., & Yamada, M. (1991a). Interleukin-2 receptor in peripheral blood lymphocytes of Alzheimer’s disease patients. Acta Psychiatrica Scandinavica, 84, 262–265.
Ikeda, T., Yamamoto, K., Takahashi, K., & Yamada, M. (1991b). Immune system-associated antigens on the surface of peripheral blood lymphocytes in patients with Alzheimer’s disease. Acta Psychiatrica Scandinavica, 83, 444–448.
Itagaki, S., McGeer, P. L., & Akiyama, H. (1988). Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer’s disease brain tissue. Neuroscience Letters, 91, 259–264.
Jang, I. K., & Gu, H. (2003). Negative regulation of TCR signaling and T-cell activation by selective protein degradation. Current Opinion in Immunology, 15, 315–320.
Lassmann, H., & Ransohoff, R. M. (2004). The CD4-Th1 model for multiple sclerosis: A critical [correction of crucial] re-appraisal. Trends in Immunology, 25, 132–137.
Ledoux, S., Rebai, N., Dagenais, A., Shaw, I. T., Nalbantoglu, J., Sekaly, R. P., & Cashman, N. R. (1993). Amyloid precursor protein in peripheral mononuclear cells is up-regulated with cell activation. Journal of Immunology, 150, 5566–5575.
Lemere, C. A., Maron, R., Selkoe, D. J., & Weiner, H. L. (2001). Nasal vaccination with beta-amyloid peptide for the treatment of Alzheimer’s disease. DNA and Cell Biology, 20, 705–711.
Lemere, C. A., Maron, R., Spooner, E. T., Grenfell, T. J., Mori, C., Desai, R., Hancock, W. W., Weiner, H. L., & Selkoe, D. J. (2000). Nasal A beta treatment induces anti-A beta antibody production and decreases cerebral amyloid burden in PD-APP mice. Annals of the New York Academy of Sciences, 920, 328–331.
Leutner, S., Czech, C., Schindowski, K., Touchet, N., Eckert, A., & Muller, W. E. (2000). Reduced antioxidant enzyme activity in brains of mice transgenic for human presenilin-1 with single or multiple mutations. Neuroscience Letters, 292, 87–90.
Ligthart, G. J., Corberand, J. X., Fournier, C., Galanaud, P., Hijmans, W., Kennes, B., Muller-Hermelink, H. K., & Steinmann, G. G. (1984). Admission criteria for immunogerontological studies in man: The SENIEUR protocol. Mechanisms of Ageing and Development, 28, 47–55.
Lombardi, V. R., Garcia, M., Rey, L., & Cacabelos, R. (1999). Characterization of cytokine production, screening of lymphocyte subset patterns and in vitro apoptosis in healthy and Alzheimer’s Disease (AD) individuals. Journal of Neuroimmunology, 97, 163–171.
Maesaka, J. K., Palaia, T., Chowdhury, S. A., Shimamura, T., Fishbane, S., Reichman, W., Coyne, A., O’Rear, J. J., & El-Sabban, M. E. (1999). Partial characterization of apoptotic factor in Alzheimer plasma. The American Journal of Physiology, 276, F521–527.
Masliah, E., Hansen, L., Adame, A., Crews, L., Bard, F., Lee, C., Seubert, P., Games, D., Kirby, L., & Schenk, D. (2005). Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology, 64, 129–131.
Matyszak, M. K., Denis-Donini, S., Citterio, S., Longhi, R., Granucci, F., & Ricciardi-Castagnoli, P. (1999). Microglia induce myelin basic protein-specific T cell anergy or T cell activation, according to their state of activation. European Journal of Immunology, 29, 3063–3076.
McGeer, P. L., Akiyama, H., Itagaki, S., & McGeer, E. G. (1989). Immune system response in Alzheimer’s disease. The Canadian Journal of Neurological Sciences, 16, 516–527.
Mirshahidi, S., Ferris, L. C., & Sadegh-Nasseri, S. (2004). The magnitude of TCR engagement is a critical predictor of T cell anergy or activation. Journal of Immunology, 172, 5346–5355.
Mirshahidi, S., Huang, C. T., & Sadegh-Nasseri, S. (2001). Anergy in peripheral memory CD4(+) T cells induced by low avidity engagement of T cell receptor. The Journal of Experimental Medicine, 194, 719–731.
Monning, U., Konig, G., Banati, R. B., Mechler, H., Czech, C., Gehrmann, J., Schreiter-Gasser, U., Masters, C. L., & Beyreuther, K. (1992). Alzheimer beta A4-amyloid protein precursor in immunocompetent cells. The Journal of Biological Chemistry, 267, 23950–23956.
Monsonego, A., Maron, R., Zota, V., Selkoe, D. J., & Weiner, H. L. (2001). Immune hyporesponsiveness to amyloid beta-peptide in amyloid precursor protein transgenic mice: Implications for the pathogenesis and treatment of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America, 98, 10273–10278.
Monsonego, A., Zota, V., Karni, A., Krieger, J. I., Bar-Or, A., Bitan, G., Budson, A. E., Sperling, R., Selkoe, D. J., & Weiner, H. L. (2003). Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. The Journal of Clinical Investigation, 112, 415–422.
Orgogozo, J. M., Gilman, S., Dartigues, J. F., Laurent, B., Puel, M., Kirby, L. C., Jouanny, P., Dubois, B., Eisner, L., Flitman, S., Michel, B. F., Boada, M., Frank, A., & Hock, C. (2003). Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology, 61, 46–54.
Palotas, A., Kalman, J., Palotas, M., Juhasz, A., Janka, Z., & Penke, B. (2002). Beta-amyloid-induced increase in the resting intracellular calcium concentration gives support to tell Alzheimer lymphocytes from control ones. Brain Research Bulletin, 58, 203–205.
Panossian, L. A., Porter, V. R., Valenzuela, H. F., Zhu, X., Reback, E., Masterman, D., Cummings, J. L., & Effros, R. B. (2003). Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiology of Aging, 24, 77–84.
Park, E., Alberti, J., Mehta, P., Dalton, A., Sersen, E., & Schuller-Levis, G. (2000). Partial impairment of immune functions in peripheral blood leukocytes from aged men with Down’s syndrome. Clinical Immunology, 95, 62–69.
Pirttila, T., Mattinen, S., & Frey, H. (1992). The decrease of CD8-positive lymphocytes in Alzheimer’s disease. Journal of Neurological Sciences, 107, 160–165.
Ratts, R. B., Karandikar, N. J., Hussain, R. Z., Choy, J., Northrop, S. C., Lovett-Racke, A. E., & Racke, M. K. (2006). Phenotypic characterization of autoreactive T cells in multiple sclerosis. Journal of Neuroimmunology, 178, 100–110.
Robinson Agramonte, M., Dorta-Contreras, A. J., & Lorigados Pedre, L. (2001). Immune events in central nervous system of early and late onset Alzheimer’s disease patients. Revista de Neurologia, 32, 901–904.
Rogers, J., & Mufson, E. J. (1990). Demonstrating immune-related antigens in Alzheimer’s disease brain tissue. Neurobiology of Aging, 11, 477–479.
Rota, E., Bellone, G., Rocca, P., Bergamasco, B., Emanuelli, G., & Ferrero, P. (2006). Increased intrathecal TGF-beta1, but not IL-12, IFN-gamma and IL-10 levels in Alzheimer’s disease patients. Neurological Sciences, 27, 33–39.
Rubin, B., Llobera, R., Gouaillard, C., Alcover, A., & Arnaud, J. (2000). Dissection of the role of CD3gamma chains in profound but reversible T-cell receptor down-regulation. Scandinavian Journal of Immunology, 52, 173–183.
Sala, G., Galimberti, G., Canevari, C., Raggi, M. E., Isella, V., Facheris, M., Appollonio, I., & Ferrarese, C. (2003). Peripheral cytokine release in Alzheimer patients: Correlation with disease severity. Neurobiology of Aging, 24, 909–914.
Schenk, D., Barbour, R., Dunn, W., Gordon, G., Grajeda, H., Guido, T., Hu, K., Huang, J., Johnson-Wood, K., Khan, K., Kholodenko, D., Lee, M., Liao, Z., Lieberburg, I., Motter, R., Mutter, L., Soriano, F., Shopp, G., Vasquez, N., Vandevert, C., Walker, S., Wogulis, M., Yednock, T., Games, D., & Seubert, P. (1999). Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature, 400, 173–177.
Schindowski, K., Frohlich, L., Maurer, K., Muller, W. E., & Eckert, A. (2002). Age-related impairment of human T lymphocytes’ activation: Specific differences between CD4(+) and CD8(+) subsets. Mechanisms of Ageing and Development, 123, 375–390.
Schindowski, K., Kratzsch, T., Peters, J., Steiner, B., Leutner, S., Touchet, N., Maurer, K., Czech, C., Pradier, L., Frolich, L., Muller, W. E., & Eckert, A. (2003). Impact of aging: Sporadic, and genetic risk factors on vulnerability to apoptosis in Alzheimer’s disease. Neuromolecular Medicine, 4, 161–178.
Schindowski, K., Leutner, S., Muller, W. E., & Eckert, A. (2000). Age-related changes of apoptotic cell death in human lymphocytes. Neurobiology of Aging, 21, 661–670.
Schindowski, K., Peters, J., Gorriz, C., Schramm, U., Weinandi, T., Leutner, S., Maurer, K., Frölich, L., Muller, W. E., & Eckert, A. (2006). Apoptosis of CD4+ T and natural killer cells in Alzheimer’s disease. Pharmacopsychiatry, 39, 220–228.
Schlunck, T., Schraut, W., Riethmuller, G., & Ziegler-Heitbrock, H. W. (1990). Inverse relationship of CA2+ mobilization and cell proliferation in CD8+ memory and virgin T cells. European Journal of Immunology, 20, 1957–1963.
Schmid, I., Uittenbogaart, C. H., Keld, B., & Giorgi, J. V. (1994). A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. Journal of Immunological Methods, 170, 145–157.
Schuessel, K., Schafer, S., Bayer, T. A., Czech, C., Pradier, L., Muller-Spahn, F., Muller, W. E., & Eckert, A. (2005). Impaired Cu/Zn-SOD activity contributes to increased oxidative damage in APP transgenic mice. Neurobiology of Disease, 18, 89–99.
Seabrook, T. J., Thomas, K., Jiang, L., Bloom, J., Spooner, E., Maier, M., Bitan, G., & Lemere, C. A. (2007). Dendrimeric Abeta1-15 is an effective immunogen in wildtype and APP-tg mice. Neurobiology of Aging, 28, 813–823.
Sedgwick, J. D., Mossner, R., Schwender, S., & ter Meulen, V. (1991). Major histocompatibility complex-expressing nonhematopoietic astroglial cells prime only CD8+ T lymphocytes: Astroglial cells as perpetuators but not initiators of CD4+ T cell responses in the central nervous system. Journal of Experimental Medicine, 173, 1235–1246.
Shalit, F., Sredni, B., Brodie, C., Kott, E., & Huberman, M. (1995). T lymphocyte subpopulations and activation markers correlate with severity of Alzheimer’s disease. Clinical Immunology and Immunopathology, 75, 246–250.
Singh, V. K. (1990). Neuroimmune axis as a basis of therapy in Alzheimer’s disease. Progress in Drug Research, 34, 383–393.
Stieler, J. T., Lederer, C., Bruckner, M. K., Wolf, H., Holzer, M., Gertz, H. J., & Arendt, T. (2001). Impairment of mitogenic activation of peripheral blood lymphocytes in Alzheimer’s disease. Neuroreport, 12, 3969–3972.
Tan, J., Town, T., Abdullah, L., Wu, Y., Placzek, A., Small, B., Kroeger, J., Crawford, F., Richards, D., & Mullan, M. (2002). CD45 isoform alteration in CD4+ T cells as a potential diagnostic marker of Alzheimer’s disease. Journal of Neuroimmunology, 132, 164–172.
Tanchot, C., Rosado, M. M., Agenes, F., Freitas, A. A., & Rocha, B. (1997). Lymphocyte homeostasis. Seminars in Immunology, 9, 331–337.
Togo, T., Akiyama, H., Iseki, E., Kondo, H., Ikeda, K., Kato, M., Oda, T., Tsuchiya, K., & Kosaka, K. (2002). Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. Journal of Neuroimmunology, 124, 83–92.
Torres, P. S., Alcover, A., Zapata, D. A., Arnaud, J., Pacheco, A., Martin-Fernandez, J. M., Villasevil, E. M., Sanal, O., & Regueiro, J. R. (2003). TCR dynamics in human mature T lymphocytes lacking CD3 gamma. Journal of Immunology, 170, 5947–5955.
Town, T., Tan, J., Flavell, R. A., & Mullan, M. (2005). T-cells in Alzheimer’s disease. Neuromolecular Medicine, 7, 255–264.
Town, T., Tan, J., Sansone, N., Obregon, D., Klein, T., & Mullan, M. (2001). Characterization of murine immunoglobulin G antibodies against human amyloid-beta1-42. Neuroscience Letters, 307, 101–104.
Town, T., Vendrame, M., Patel, A., Poetter, D., DelleDonne, A., Mori, T., Smeed, R., Crawford, F., Klein, T., Tan, J., & Mullan, M. (2002). Reduced Th1 and enhanced Th2 immunity after immunization with Alzheimer’s beta-amyloid(1-42). Journal of Neuroimmunology, 132, 49–59.
Trieb, K., Ransmayr, G., Sgonc, R., Lassmann, H., & Grubeck-Loebenstein, B. (1996). APP peptides stimulate lymphocyte proliferation in normals, but not in patients with Alzheimer’s disease. Neurobiology of Aging, 17, 541–547.
Valitutti, S., Muller, S., Cella, M., Padovan, E., & Lanzavecchia, A. (1995). Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature, 375, 148–151.
Vingtdeux, V., Hamdane, M., Gompel, M., Begard, S., Drobecq, H., Ghestem, A., Grosjean, M. E., Kostanjevecki, V., Grognet, P., Vanmechelen, E., Buee, L., Delacourte, A., & Sergeant, N. (2005). Phosphorylation of amyloid precursor carboxy-terminal fragments enhances their processing by a gamma-secretase-dependent mechanism. Neurobiology of Disease, 20, 625–637.
Weishaupt, A., Jander, S., Bruck, W., Kuhlmann, T., Stienekemeier, M., Hartung, T., Toyka, K. V., Stoll, G., & Gold, R. (2000). Molecular mechanisms of high-dose antigen therapy in experimental autoimmune encephalomyelitis: Rapid induction of Th1-type cytokines and inducible nitric oxide synthase. Journal of Immunology, 165, 7157–7163.
Wenham, P. R., Price, W. H., & Blandell, G. (1991). Apolipoprotein E genotyping by one-stage PCR. Lancet, 337, 1158–1159.
Zhang, J., Kong, Q., Zhang, Z., Ge, P., Ba, D., & He, W. (2003). Telomere dysfunction of lymphocytes in patients with Alzheimer disease. Cognitive and Behavirol Neurology, 16, 170–176.
Acknowledgments
This work was supported by grants from Dr. Robert Pfleger Stiftung, Alzheimer Initiative e.V., Hirnliga e.V. and a Marie-Curie fellowship to KS. Disclosure statement: This study was approved by the responsible Ethical Committee and in accordance with standards of the guide for the care and use of laboratory animals and with respect to European Community rules.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schindowski, K., Eckert, A., Peters, J. et al. Increased T-cell Reactivity and Elevated Levels of CD8+ Memory T-cells in Alzheimer’s Disease-patients and T-cell Hyporeactivity in an Alzheimer’s Disease-mouse Model: Implications for Immunotherapy. Neuromol Med 9, 340–354 (2007). https://doi.org/10.1007/s12017-007-8015-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-007-8015-9