Abstract
Objectives
Amyloid deposition is considered the initial pathology in Alzheimer’s disease (AD). Personalized management requires investigation of amyloid pathology and the risk factors for both amyloid pathology and cognitive decline in the Chinese population. We aimed to investigate amyloid positivity and deposition in AD patients, as well as factors related to amyloid pathology in Chinese cities.
Methods
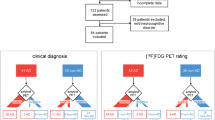
This cross-sectional multicenter study was conducted in Shanghai and Zhengzhou, China. All participants were recruited from urban communities and memory clinics. Amyloid positivity and deposition were analyzed based on amyloid positron emission tomography (PET). We used partial least squares (PLS) models to investigate how related factors contributed to amyloid deposition and cognitive decline.
Results
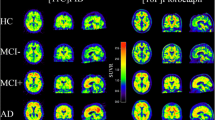
In total, 1026 participants were included: 768 participants from the community-based cohort (COMC) and 258 participants from the clinic-based cohort (CLIC). The overall amyloid-positive rates in individuals with clinically diagnosed AD, mild cognitive impairment (MCI), and normal cognition (NC) were 85.8%, 44.5%, and 26.9%, respectively. The global amyloid deposition standardized uptake value ratios (SUVr) (reference: cerebellar crus) were 1.44 ± 0.24, 1.30 ± 0.22, and 1.24 ± 0.14, respectively. CLIC status, apolipoprotein E (ApoE) ε4, and older age were strongly associated with amyloid pathology by PLS modeling.
Conclusion
The overall amyloid-positive rates accompanying AD, MCI, and NC in the Chinese population were similar to those in published cohorts of other populations. ApoE ε4 and CLIC status were risk factors for amyloid pathology across the AD continuum. Education was a risk factor for amyloid pathology in MCI. Female sex and age were risk factors for amyloid pathology in NC.
Clinical relevance statement
This study provides new details about amyloid pathology in the Chinese population. Factors related to amyloid deposition and cognitive decline can help to assess patients’ AD risk.
Key Points
• We studied amyloid pathology and related risk factors in the Chinese population.
•·The overall amyloid-positive rates in individuals with clinically diagnosed AD, MCI, and NC were 85.8%, 44.5%, and 26.9%, respectively.
• These overall amyloid-positive rates were in close agreement with the corresponding prevalence for other populations.




Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- AFT:
-
Animal fluency test
- ANCOVA:
-
Analysis of covariance
- ApoE :
-
Apolipoprotein E
- AVLT-LDR:
-
Long-delayed recall of the Auditory Verbal Learning Test
- Aβ:
-
β-Amyloid
- BH:
-
Benjamini and Hochberg
- BNT:
-
Boston Naming Test
- CLIC:
-
Clinic-based cohort
- COMC:
-
Community-based cohort
- CT:
-
Computed tomography
- FBP:
-
Filtered back-projection
- FDR:
-
False discovery rate
- FWHM:
-
Full width at half maximum
- MCI:
-
Mild cognitive impairment
- MMSE:
-
Mini-Mental State Examination
- MNI:
-
Montreal Neurological Institute
- MoCA-B:
-
Montreal Cognitive Assessment-Basic
- MP-RAGE:
-
Magnetization-prepared rapid gradient echo
- MRI:
-
Magnetic resonance imaging
- NC:
-
Normal cognition
- NIA-AA:
-
National Institute on Aging and Alzheimer’s Association
- PET:
-
Positron emission tomography
- PLS:
-
Partial least squares
- PVC:
-
Partial volume error correction
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- STT:
-
Shape Trail Test
- SUVr:
-
Standardized uptake value ratio
References
Jia L, Du Y, Chu L et al (2020) Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health 5:e661–e671
Kern S, Zetterberg H, Kern J et al (2018) Prevalence of preclinical Alzheimer disease: comparison of current classification systems. Neurology 90:e1682–e1691
Ding D, Zhao Q, Guo Q et al (2015) Prevalence of mild cognitive impairment in an urban community in China: a cross-sectional analysis of the Shanghai Aging Study. Alzheimers Dement 11:300-309.e302
Jia J, Zhou A, Wei C et al (2014) The prevalence of mild cognitive impairment and its etiological subtypes in elderly Chinese. Alzheimers Dement 10:439–447
Silva MVF, Loures CMG, Alves LCV, de Souza LC, Borges KBG, Carvalho MDG (2019) Alzheimer’s disease: risk factors and potentially protective measures. J Biomed Sci 26:33
Day GS, Cruchaga C, Wingo T, Schindler SE, Coble D, Morris JC (2019) Association of acquired and heritable factors with intergenerational differences in age at symptomatic onset of Alzheimer disease between offspring and parents with dementia. JAMA Netw Open 2:e1913491
Jack CR Jr, Holtzman DM (2013) Biomarker modeling of Alzheimer’s disease. Neuron 80:1347–1358
Bateman RJ, Xiong C, Benzinger TLS et al (2012) Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367:795–804
Shi Z, Fu LP, Zhang N et al (2020) Amyloid PET in dementia syndromes: a Chinese multicenter study. J Nucl Med 61:1814–1819
Ossenkoppele R, Jansen WJ, Rabinovici GD et al (2015) Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA 313:1939–1949
Jansen WJ, Ossenkoppele R, Knol DL et al (2015) Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313:1924–1938
Braak H, Braak E (1997) Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 18:351–357
Iadanza MG, Jackson MP, Hewitt EW, Ranson NA, Radford SE (2018) A new era for understanding amyloid structures and disease. Nat Rev Mol Cell Biol 19:755–773
Yasuno F, Kazui H, Morita N et al (2015) Low amyloid-beta deposition correlates with high education in cognitively normal older adults: a pilot study. Int J Geriatr Psychiatry 30:919–926
Rentz DM, Locascio JJ, Becker JA et al (2010) Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol 67:353–364
Wada M, Noda Y, Shinagawa S et al (2018) Effect of education on Alzheimer’s disease-related neuroimaging biomarkers in healthy controls, and participants with mild cognitive impairment and Alzheimer’s disease: a cross-sectional study. J Alzheimers Dis 63:861–869
Rawlings AM, Sharrett AR, Mosley TH, Wong DF, Knopman DS, Gottesman RF (2019) Cognitive reserve in midlife is not associated with amyloid-beta deposition in late-life. J Alzheimers Dis 68:517–521
Lim YY, Kalinowski P, Pietrzak RH et al (2018) Association of beta-amyloid and apolipoprotein E epsilon4 with memory decline in preclinical Alzheimer disease. JAMA Neurol 75:488–494
Mattsson N, Zetterberg H, Hansson O et al (2009) CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302:385–393
Serrano-Pozo A, Qian J, Monsell SE, Betensky RA, Hyman BT (2015) APOEepsilon2 is associated with milder clinical and pathological Alzheimer disease. Ann Neurol 77:917–929
Cui L, Huang L, Pan FF et al (2023) Chinese preclinical Alzheimer’s disease study (C-PAS): design and challenge from PET acceptance. J Prev Alzheimers Dis 10:571–580
Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW (2015) Subtle cognitive decline and biomarker staging in preclinical Alzheimer’s disease. J Alzheimers Dis 47:231–242
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269
Jak AJ, Bondi MW, Delano-Wood L et al (2009) Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 17:368–375
Pan FF, Huang L, Chen KL, Zhao QH, Guo QH (2020) A comparative study on the validations of three cognitive screening tests in identifying subtle cognitive decline. BMC Neurol 20:78
Huang Y, Pan FF, Huang L, Guo Q (2023) The value of clock drawing process assessment in screening for mild cognitive impairment and Alzheimer’s dementia. Assessment 30:364–374
Huang Y, Li Y, Xie F, Guo Q (2022) Associations of plasma phosphorylated tau181 and neurofilament light chain with brain amyloid burden and cognition in objectively defined subtle cognitive decline patients. CNS Neurosci Ther 28:2195–2205
Ren S, Li J, Huang L et al (2023) Brain functional alterations and association with cognition in people with preclinical subjective cognitive decline and objective subtle cognitive difficulties. Neuroscience 513:137–144
Ding D, Zhao Q, Guo Q et al (2016) Progression and predictors of mild cognitive impairment in Chinese elderly: a prospective follow-up in the Shanghai Aging Study. Alzheimers Dement 4:28–36
Guo Q, Zhao Q, Chen M, Ding D, Hong Z (2009) A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alzheimer Dis Assoc Disord 23:253–259
Zhao Q, Guo Q, Hong Z (2013) Clustering and switching during a semantic verbal fluency test contribute to differential diagnosis of cognitive impairment. Neurosci Bull 29:75–82
Zhao Q, Guo Q, Liang X et al (2015) Auditory verbal learning test is superior to rey-osterrieth complex figure memory for predicting mild cognitive impairment to Alzheimer’s disease. Curr Alzheimer Res 12:520–526
Ren S, Pan Y, Li J et al (2023) The necessary of ternary amyloid classification for clinical practice: an alternative to the binary amyloid definition. View. https://doi.org/10.1002/viw.20220080
Su J, Huang Q, Ren S et al (2019) Altered brain glucose metabolism assessed by (18)F-FDG PET imaging is associated with the cognitive impairment of CADASIL. Neuroscience 417:35–44
Huang Q, Ren S, Jiang D et al (2019) Changes in brain glucose metabolism and connectivity in somatoform disorders: an 18F-FDG PET study. Eur Arch Psychiatry Clin Neurosci 270:881–891
Razifar P, Sandstrom M, Schnieder H et al (2005) Noise correlation in PET, CT, SPECT and PET/CT data evaluated using autocorrelation function: a phantom study on data, reconstructed using FBP and OSEM. BMC Med Imaging 5:5
Lilly E Full prescribing information. Available via https://pi.lilly.com/us/amyvid-uspi.pdf. Accessed 05/2023
Jack CR Jr, Bennett DA, Blennow K et al (2018) NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14:535–562
Wang J, Wang M, Ren S et al (2023) The effect of gender and APOE ɛ4 status on brain amyloid-β deposition in different age groups of mild cognitively impaired individuals: a PET-CT study. J Alzheimers Dis 94:763–775
Jansen WJ, Janssen O, Tijms BM et al (2022) Prevalence estimates of amyloid abnormality across the Alzheimer disease clinical spectrum. JAMA Neurol 79:228–243
Crary JF, Trojanowski JQ, Schneider JA et al (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766
Serrano-Pozo A, Qian J, Monsell SE et al (2014) Mild to moderate Alzheimer dementia with insufficient neuropathological changes. Ann Neurol 75:597–601
Barkhof F, Polvikoski TM, van Straaten EC et al (2007) The significance of medial temporal lobe atrophy: a postmortem MRI study in the very old. Neurology 69:1521–1527
Beekly DL, Ramos EM, Lee WW et al (2007) The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord 21:249–258
Winblad B, Palmer K, Kivipelto M et al (2004) Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256:240–246
Wong CG, Thomas KR, Edmonds EC et al (2018) Neuropsychological criteria for mild cognitive impairment in the framingham heart study’s old-old. Dement Geriatr Cogn Disord 46:253–265
Bondi MW, Edmonds EC, Jak AJ et al (2014) Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis 42:275–289
Buckley RF, Mormino EC, Rabin JS et al (2019) Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol 76:542–551
Ferretti MT, Iulita MF, Cavedo E et al (2018) Sex differences in Alzheimer disease - the gateway to precision medicine. Nat Rev Neurol 14:457–469
Russell JK, Jones CK, Newhouse PA (2019) The role of estrogen in brain and cognitive aging. Neurotherapeutics 16:649–665
Schupf N, Lee JH, Pang D et al (2018) Epidemiology of estrogen and dementia in women with Down syndrome. Free Radic Biol Med 114:62–68
Xiong J, Kang SS, Wang Z et al (2022) FSH blockade improves cognition in mice with Alzheimer’s disease. Nature 603:470–476
Zhu W, Li X, Li X et al (2021) The protective impact of education on brain structure and function in Alzheimer’s disease. BMC Neurol 21:423
Stern Y (2012) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11:1006–1012
Stern Y, Arenaza-Urquijo EM, Bartres-Faz D et al (2020) Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement 16:1305–1311
Serrano-Pozo A, Das S, Hyman BT (2021) APOE and Alzheimer’s disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol 20:68–80
Deane R, Sagare A, Hamm K et al (2008) apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 118:4002–4013
Castellano JM, Kim J, Stewart FR et al (2011) Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med 3:89ra57
Bertram L, Tanzi RE (2008) Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci 9:768–778
Cho H, Seo SW, Kim JH et al (2013) Amyloid deposition in early onset versus late onset Alzheimer’s disease. J Alzheimers Dis 35:813–821
Perrotin A, La Joie R, de La Sayette V et al (2017) Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: differential affective and imaging correlates. Alzheimers Dement 13:550–560
Hu C, Yu D, Sun X, Zhang M, Wang L, Qin H (2017) The prevalence and progression of mild cognitive impairment among clinic and community populations: a systematic review and meta-analysis. Int Psychogeriatr 29:1595–1608
Collij LE, Salvadó G, Wottschel V et al (2022) Data‐driven evidence for three distinct patterns of amyloid‐β accumulation. Alzheimers Dement 17(S4):e055417
Collij LE, Salvadó G, Wottschel V et al (2022) Spatial-temporal patterns of β-amyloid accumulation: a subtype and stage inference model analysis. Neurology 98:e1692–e1703
Acknowledgements
The authors thank Jianfei Xiao, Xiangqing Xie, Yue Qian, and Zhiwei Pan for their generous assistance with this study.
Funding
This study has received funding by the National Key R&D Program of China (2016YFC1306305, 2018YFE0203600); STI2030-Major Projects (2022ZD0213800); the National Science Foundation of China (81801752, 81571345); the Shanghai Sailing Program (18YF1403200, 19YF1405300); the startup fund of Huashan Hospital, Fudan University (2017QD081); Shanghai Municipal Key Clinical Specialty (shslczdzk03402); Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01); and Shanghai Rising-Star Program (21QA1405800) and ZJLab.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Fang Xie.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained. This study has been approved by the Institutional Review Board of Huashan Hospital, Fudan University.
Study subjects or cohorts overlap
Some study subjects or cohorts have not been previously reported.
Methodology
• retrospective
• cross sectional study
• multicenter study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, K., Li, B., Huang, L. et al. Positive rate and quantification of amyloid pathology with [18F]florbetapir in the urban Chinese population. Eur Radiol (2023). https://doi.org/10.1007/s00330-023-10366-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-023-10366-z