Abstract
Objectives
Non-contrast computed tomography (NCCT) markers are robust predictors of parenchymal hematoma expansion in intracerebral hemorrhage (ICH). We investigated whether NCCT features can also identify ICH patients at risk of intraventricular hemorrhage (IVH) growth.
Methods
Patients with acute spontaneous ICH admitted at four tertiary centers in Germany and Italy were retrospectively included from January 2017 to June 2020. NCCT markers were rated by two investigators for heterogeneous density, hypodensity, black hole sign, swirl sign, blend sign, fluid level, island sign, satellite sign, and irregular shape. ICH and IVH volumes were semi-manually segmented. IVH growth was defined as IVH expansion > 1 mL (eIVH) or any delayed IVH (dIVH) on follow-up imaging. Predictors of eIVH and dIVH were explored with multivariable logistic regression. Hypothesized moderators and mediators were independently assessed in PROCESS macro models.
Results
A total of 731 patients were included, of whom 185 (25.31%) suffered from IVH growth, 130 (17.78%) had eIVH, and 55 (7.52%) had dIVH. Irregular shape was significantly associated with IVH growth (OR 1.68; 95%CI [1.16–2.44]; p = 0.006). In the subgroup analysis stratified by the IVH growth type, hypodensities were significantly associated with eIVH (OR 2.06; 95%CI [1.48–2.64]; p = 0.015), whereas irregular shape (OR 2.72; 95%CI [1.91–3.53]; p = 0.016) in dIVH. The association between NCCT markers and IVH growth was not mediated by parenchymal hematoma expansion.
Conclusions
NCCT features identified ICH patients at a high risk of IVH growth. Our findings suggest the possibility to stratify the risk of IVH growth with baseline NCCT and might inform ongoing and future studies.
Clinical relevance statement
Non-contrast CT features identified ICH patients at a high risk of intraventricular hemorrhage growth with subtype-specific differences. Our findings may assist in the risk stratification of intraventricular hemorrhage growth with baseline CT and might inform ongoing and future clinical studies.
Key Points
• NCCT features identified ICH patients at a high risk of IVH growth with subtype-specific differences.
• The effect of NCCT features was not moderated by time and location or indirectly mediated by hematoma expansion.
• Our findings may assist in the risk stratification of IVH growth with baseline NCCT and might inform ongoing and future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 5.5 million patients suffer from intracerebral hemorrhage (ICH) annually [1] and ICH represents the most severe form of stroke, with mortality and severe disability rates approaching 50% at 1 month and exceeding 75% at 1 year, respectively [2, 3]. Therapeutic strategies have failed to show overall beneficial treatment effects in previous ICH trials over the past years. Therefore, new candidates for potential treatment strategies are holding vast research attention. Intraventricular extension of intracerebral hemorrhage (IVH) occurs in 40% of ICH patients and is a well-established predictor of poor functional outcome [4]. IVH is a dynamic and potentially modifiable process (IVH growth) [5, 6] which can present either as interval increases in IVH volume (expansion IVH, eIVH) or as a delayed development of IVH (dIVH) on subsequent neuroimaging [7, 8]. Furthermore, both have shown a strong relationship with a poor functional outcome [5, 6] and hold potential to improve outcome prediction models for clinical ICH management [9]. Recent evidence suggested that non-contrast computed tomography (NCCT) features might help identify patients at risk of IVH growth; however, the exact relationship with eIVH and dIVH remains unclear [10]. Therefore, we hypothesized that both subgroups are independently predicted by distinct NCCT features. Recognizing that eIVH and dIVH vary in terms of chronological order and ICH location on the one hand, and on the other hand are common phenomena in patients with acute HE, we additionally hypothesized a potential link with the effects exerted by the NCCT features [8]. To test and evaluate our hypotheses, we performed a three-fold approach: Firstly, we tested and validated the association of NCCT features and IVH growth in an independent multicenter cohort. Secondly, we conducted two subgroup analyses to identify differences in NCCT markers independently associated with eIVH and dIVH. Thirdly, we evaluated if the effects were mediated by acute HE and moderated by ICH location and time.
Material and methods
This multicenter retrospective study was approved by the ethics committee (Charité Berlin, Germany [protocol number EA1/035/20], University Medical-Center Hamburg, Germany [protocol number WF-054/19], University Hospital Muenster, Germany [protocol number 2017–233-f-S], and IRCCS Mondino Foundation, Pavia, Italy [protocol number 20190099462]), and written informed consent was waived by the institutional review boards. All study protocols and procedures were conducted in accordance with the Declaration of Helsinki. Patient consent was not needed due to the retrospective nature of the study.
Study population
We retrospectively selected ICH patients admitted at four tertiary stroke centers in Germany and Italy (Charité University Hospital, Berlin, Germany (2015–2019); University Medical Center Hamburg-Eppendorf (2015–2019), Germany; University Hospital Muenster (2011–2015), Germany; and IRCCS Mondino Foundation, Pavia, Italy (2017–2019)). Patients were selected according to the following inclusion criteria: (1) primary, spontaneous ICH, (2) age > 18 years, (3) baseline NCCT images acquired within 24 h from onset/last seen well (LSW) with or without CT angiography (CTA); patients with (1) secondary ICH, (2) missing follow-up imaging or (3) follow-up NCCT performed after 72 h, and (4) surgical procedures including craniotomy and placement of external ventricular drain (EVD). A patients’ selection flowchart is provided in Fig. 1.
Clinical variables
Clinical data obtained from medical records included age, sex, history of hypertension and diabetes mellitus, systolic blood pressure, anticoagulation and antiplatelet treatment, Glasgow Coma Scale (GCS) at admission, time from symptom onset/LSW to imaging, and modified Rankin Scale (mRS) at 90 days.
Image analysis
NCCT images were acquired based on local CT protocols at each participating site. Imaging data were retrieved in Digital Imaging and Communications in Medicine (DICOM) format from the local picture archiving and communication system (PACS) servers and anonymized in compliance with the local guidelines. DICOM data were transformed into Neuroimaging Informatics Technology Initiative (NifTI) format and were independently reviewed by two stroke imaging experienced readers (Berlin: J.N. and L.V.; Hamburg: J.N. and S.E.; Muenster: J.N. and S.E.; Pavia: J.N. and F.M.). All readers independently reviewed images in a random order, were blinded to all demographic and outcome data, and were not directly involved in the clinical care of the enrolled patients. Images were analyzed for the presence of IVH and ICH location. Supratentorial bleedings in cortical and subcortical locations were classified as lobar whether hemorrhages involving the thalamus, basal ganglia, internal capsule, and deep periventricular white matter were classified as deep [11]. Brainstem and cerebellar bleedings were classified as infratentorial [12].Volume quantifications of ICH and IVH were performed on NCCT images with semimanual planimetric measurements. The region of interest (ROI) histogram for ICH and IVH segmentation was sampled between 20 and 80 Hounsfield units (HU) to exclude voxels that likely belonged to the cerebrospinal fluid or calcification. ROIs were delineated using the Analyze 11.0 Software and ITK-SNAP 3.8.0 Software (Penn Image Computing and Science Laboratory, 2019) [13, 14]. All NCCT markers were rated on axial NCCT images by two raters (J.N. with 6 years of experience in ICH imaging research and L.V. with 3 years of experience in ICH research) to determine the following nine markers as previously reported [15]: (1) irregular shape, (2) satellite sign, (3) island sign which were characterized as markers of shape [16]. Markers of density consisted of (1) heterogeneous density, (2) swirl sign, (3) black hole sign, (4) blend sign, (5) fluid sign, and (6) hypodensities [16]. All markers were rated inconsideration of the consensus definitions proposed by the International NCCT ICH Study Group [16]: In brief, irregular shape was defined as foci of hematoma margin irregularities in the largest hematoma region according to the Barras shape scales I–V [16, 17]. The presence of irregular shape was defined after dichotomization with a Barras scale of ≥ III [16]. Satellite sign was defined as a hematoma separate from the main hematoma (1–20 mm distance) with a maximum diameter of 10 mm [16, 18]. The island sign consisted of at least three scattered small hematomas all separate from the main hematoma or at least four small hematomas, some or all of which may connect with the main hematoma [16, 19]. Heterogeneous density was evaluated as foci of hypoattenuation in within the largest hematoma compared to the brain parenchyma according to the Barras density scales I–V [16, 17]. The presence of heterogeneous density was defined after dichotomization with a Barras scale of ≥ III [16]. The swirl sign was defined as a region of hypo- or isoattenuation compared with the brain parenchyma. The region may be rounded, streak-like, or irregular and does not require a strict encapsulation within the hematoma [20, 21]. The black hole sign consisted of a relatively hypodense area which is encapsulated within a hyperdense area and which is not connected with the adjacent brain tissue [21, 22]. The relatively hypodense area has an identifiable border and a difference of at least 28 HU between the two density regions [22, 23]. Blend sign was defined as a hypoattenuating area adjacent to a hyperattenuating area of the hematoma, with a clear separation between them at a density difference of at least 18 Hounsfield Units (HU) [23, 24]. Fluid sign referred similarly to the presence of one distinct hypoattenuating area above and one hyperattenuating area below a discrete straight line of separation, yet irrespective of its density measurements [16, 25]. The imaging sign hypodensities were defined as any hypodense region strictly encapsulated within the hemorrhage with any shape, size, and density which does not require a density measurement [16, 26]. Illustrative examples on NCCT marker ratings are provided in Supplementary Figure 1. A subset of patients (n = 100) was randomly selected and presented again for a second reading to one rater (J.N. with 6 years of experience in ICH imaging research). Images for the second reading were presented in a random order 3 months later to minimize the recall of the images.
Outcomes
The main outcome of the analysis was the occurrence of any IVH growth. In secondary analysis, the same regression models were repeatedly stratified by the IVH growth type, distinguishing eIVH from dIVH.
Statistical analysis
Data were tested for normality and homogeneity of variance using histogram plots and the Shapiro–Wilk test. Descriptive statistics are presented as counts (percentages [%]) for categorical variables and compared with the χ2 test, mean (standard deviation [SD]) for continuous normally distributed variables, and medians (interquartile range [IQR]) for non-normal continuous variables and compared with the Mann–Whitney test, respectively. Interrater agreement for ICH and IVH volume quantifications was calculated and expressed as intraclass correlation coefficient (ICC) with 95% upper and lower confidence intervals (CI) from pairs of two readers (E.V. and L.V.) [27, 28]. Interrater and intrarater agreements for the readings of NCCT markers were calculated and expressed as Cohen’s κ statistic with 95% upper and lower CI [29].
Logistic regression analysis
Multivariate regression analysis was performed to identify independent variables associated with eIVH and dIVH in patients with IVH growth. Candidate variables were selected based on prior knowledge of clinical significance and sample size for the following: age, sex, hypertension, admission GCS, use of oral anticoagulation, and supratentorial ICH location [30]. Collinear covariates, as expressed by a variance inflation factor (VIF) of greater than 3, were identified and removed from the model if necessary [31]. Variables were fitted together using backward elimination with a p value criterion of less than 0.05 [32]. Given for selected variables are odds ratios (OR) with 95%CI and corresponding beta coefficients. For visual display, adjusted beta coefficients of significant independent variables were plotted. A statistically significant difference was set at a p value of less than 0.05.
Moderation and mediation analysis
Time [8, 33] and location [34] were hypothesized to moderate the effect of the NCCT markers on IVH growth and subgroups (eIVH and dIVH). The PROCESS SPSS macro version 2.13 model 2 for moderation analysis was used to calculate the regression coefficients for the respective NCCT marker in each group (IVH growth, eIVH, dIVH) independently (Supplementary Material) [35]. Hematoma expansion (defined as continuous growth > 6 mL or relative growth > 33%) hypothesized to mediate the effect of NCCT markers on IVH growth and subgroups was assessed in a mediation model (Supplementary Material). The PROCESS SPSS macro version 2.13 model 4 for mediation analysis was used to calculate three pathways (Supplementary Material) [36]. Pathway a determined the regression coefficients for the effect of the NCCT marker on the mediator, pathway b examined the association between the mediator and IVH growth or subgroups, and pathway c estimated the total and direct effect of the NCCT marker on IVH growth and subgroups, respectively. Pathway ab calculated the indirect intervention effects. To test the significance of the indirect effect, the macro generated bias-corrected bootstrapped 95%CI [36]. Significant mediation was determined if the CI around the indirect effect did not include zero [36]. Analyses were performed using the statistical software package SPSS version 25® (IBM Corporation, 2019) and R Statistics® Version 3.5.1 (R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2018).
Supplementary analysis
A second multivariate regression analysis was performed to identify the effect of eIVH and dIVH on the functional outcome (Supplementary Table 4). A receiver operating curve (ROC) analysis was performed to evaluate and compare the discriminatory accuracies of IVH growth, HE, and revised HE in the prediction of functional outcome and mortality (Supplementary Material Table 5).
Data availability statement
The datasets that support the findings of our study are available upon reasonable request from the corresponding author; however, prior approval of proposals may apply by our institution’s data security management and a signed data sharing agreement will then be approved.
Results
A total of 731 out of 1399 patients met the inclusion criteria, as shown in Fig. 1, and 185 (25.31%) patients suffered from IVH growth, of whom 130 (17.78%) had an eIVH and 55 (7.52%) a dIVH. Patients with IVH growth had a lower admission GCS, shorter time from symptom onset/LSW to imaging, smaller hematoma volumes on both admission and follow-up imaging, and a higher frequency of hematomas in the basal ganglia. Within the IVH growth subgroups, patients with eIVH also had more deep bleedings, in contrast to dIVH that showed with higher frequencies of lobar hematomas. IVH volumes were higher in patients with eIVH compared to those in patients with dIVH on follow-up imaging. A detailed summary of the study population’s characteristic is provided in Table 1. Interobserver agreement for both ICH and IVH segmentations was excellent, as presented in the supplementary material. Interrater agreements for the NCCT features were substantial to excellent (n = 731; Cohen’s κ from 0.74 to 0.95) with good to excellent intrarater agreements (n = 100; Cohen’s κ from 0.81 to 0.98), as presented in the supplementary material. The presence of irregular shape was associated with 1.68 higher odds of IVH growth after adjustment for potential confounders in multivariable logistic regression (Table 2). In the subgroup analysis, irregular shape remained independently associated with an almost three times higher risk for dIVH (OR 2.72; 95%CI 1.91–3.33; p value 0.016), as shown in Table 3, whereas hypodensities were associated with increased odds of eIVH (OR 2.06; 95%CI 1.48–2.64; p value 0.0015). Finally, the moderator analysis demonstrated that the two interaction terms (Int. 1: NCCT marker × time; Int. 2: NCCT marker × location) had no significant effect on the direct association between the respective NCCT marker and IVH growth. The effect of the respective NCCT marker on IVH growth and its subtypes was not indirectly mediated by HE. Detailed results are presented in the supplementary material.
Discussion
In this study, we aimed to validate findings on the association of NCCT features with IVH growth and to determine subgroup-specific association with eIVH and dIVH in patients with acute spontaneous ICH. Our results confirmed that NCCT features were strong predictors of IVH growth [10]. In our subgroup analysis, eIVH was significantly associated with hypodensities whereas dIVH was significantly associated with irregular shape. Illustrative examples are given in Figures 2 and 3. Further, two important previous findings on the relationship of IVH growth and functional outcome were confirmed: Both eIVH and dIVH had a negative effect on the functional outcome as seen in our multivariate regression analysis (Supplementary Material Table 4) [7, 8]. Secondly, the revised HE definition had a significantly higher diagnostic accuracy in the prediction of poor functional outcome compared to the conventional definition of HE (Supplementary Table 5) [9, 10]. The pathophysiological mechanisms underlying these associations still remain unclear. Therefore, we hypothesized a mediation effect of parenchymal hematoma expansion, but our findings did not confirm this hypothesis, suggesting that the link between NCCT features and IVH growth is not a simple epiphenomenon of parenchymal bleeding [37]. Consistent with this finding, we also noted that most patients experiencing IVH growth did not have parenchymal hematoma expansion. We also explored a potential interaction with ICH location and time from onset to initial imaging but found no significant effect of these potential confounders on the association between NCCT features and increased risk of IVH growth [8, 33, 34]. From a clinical perspective, NCCT features may help clinicians in the stratification and early identification of patients at a high risk of neurological deterioration because of IVH growth. Recent results from the STOP-AUST trial indicate that the severity of IVH growth may be attenuated by tranexamic acid treatment following ICH [5]. Therefore, NCCT features may improve patients’ selection in future clinical trials, identifying subjects at a high risk of IVH growth and therefore more likely to benefit from hemostatic treatment. Some limitations of our analysis should be considered. First, our findings were derived from a retrospective analysis and require prospective validation. Second, the imaging protocol was not standardized across participating sites, although there is no evidence that the NCCT acquisition technique influences NCCT markers’ detection [16]. Third, imaging data were collected at two time points only and we are unable to assess potential dynamics of IVH volumes beyond this time frame. Fourth, NCCT markers were rated by well-experienced ICH researchers and may vary in raters with different levels of experience; however, results agreed well with our previous findings [15]. Finally, blood pressure control and coagulopathy reversal might have influenced the risk of IVH growth and were not accounted for. Finally, the described associations between NCCT markers and IVH growth may not necessarily imply causality, and further research is needed to characterize the underlying biological mechanisms.
An illustrative example of non-contrast computed tomography (NCCT) markers associated with intraventricular hemorrhage expansion in patients with acute intracerebral hemorrhage. An illustrative example of a patient with intraventricular hemorrhage expansion (eIVH) defined as absolute volume increase of intraventricular hemorrhage (IVH) between admission (left side) and follow-up NCCT imaging (right side); given with the presence of hypodensities and corresponding volumes for intracerebral hemorrhage (ICH) and IVH, admission Glasgow Coma Scale (GCS), and clinical outcome at 90 days with modified Rankin Scale. ICH, intracerebral hemorrhage; GCS, Glasgow Coma Scale; IVH, intraventricular hemorrhage; mL, milliliters; NCCT, non-contrast computed tomography
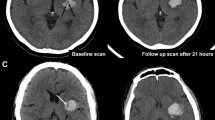
An illustrative example of non-contrast computed tomography (NCCT) markers associated with delayed intraventricular hemorrhage in patients with acute intracerebral hemorrhage. An illustrative example of a patient with delayed intraventricular hemorrhage (dIVH) defined as not present on admission imaging (left side) and any newly occurring IVH on follow-up NCCT imaging (right); given with the presence of irregular shape and corresponding volumes for intracerebral hemorrhage (ICH) and IVH, admission Glasgow Coma Scale (GCS), and clinical outcome at 90 days with modified Rankin Scale. ICH, intracerebral hemorrhage; GCS, Glasgow Coma Scale; IRR shape, irregular shape; IVH, intraventricular hemorrhage; mL, milliliters; NCCT, non-contrast computed tomography
Conclusions
NCCT features independently predict IVH growth, and this association was independent of imaging time and ICH location and not mediated by parenchymal hematoma growth HE. Our findings suggest the possibility to stratify the risk of IVH growth with baseline NCCT and might inform ongoing and future studies.
Change history
19 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00330-023-09896-3
Abbreviations
- %:
-
Percentage
- CI:
-
Confidence interval
- CTA:
-
Computed tomography angiography
- DICOM:
-
Digital Imaging and Communications in Medicine
- dIVH:
-
Delayed intraventricular hemorrhage
- eIVH:
-
Intraventricular hemorrhage expansion
- EVD:
-
External ventricular drain
- GCS:
-
Glasgow Coma Scale
- HE:
-
Hematoma expansion
- HET density:
-
Heterogeneous density
- HU:
-
Hounsfield units
- ICC:
-
Intraclass correlation coefficient
- ICH:
-
Intracerebral hemorrhage
- IQR:
-
Interquartile range
- IRR shape:
-
Irregular shape
- IVH:
-
Intraventricular hemorrhage
- LSW:
-
Last seen well
- mRS:
-
Modified Rankin Scale
- NCCT:
-
Non-contrast computed tomography
- NifTI:
-
Neuroimaging Informatics Technology Initiative
- OR:
-
Odds ratio
- PACS:
-
Picture archiving and communication system
- ROI:
-
Regions of interest
- SD:
-
Standard deviation
- VIF:
-
Variance inflation factor
References
Johnson CO, Nguyen M, Roth GA et al (2019) Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18:439–458
Drury I, Whisnant JP, Garraway WM (1984) Primary intracerebral hemorrhage: impact of CT on incidence. Neurology 34:653–657
Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G (1993) Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 24:987–993
Hanley DF, Fisher M, Lees K (2009) Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke 40:1533–1538
Yogendrakumar V, Wu TY, Churilov L et al (2022) Does tranexamic acid affect intraventricular hemorrhage growth in acute ICH? An analysis of the STOP-AUST trial. Eur Stroke J 7:15–19
Steiner T, Diringer MN, Schneider D et al (2006) Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery 59:767–773
Yogendrakumar V, Ramsay T, Fergusson D et al (2019) New and expanding ventricular hemorrhage predicts poor outcome in acute intracerebral hemorrhage. Neurology 93:e879
Maas MB, Nemeth AJ, Rosenberg NF, Kosteva AR, Prabhakaran S, Naidech AM (2013) Delayed intraventricular hemorrhage is common and worsens outcomes in intracerebral hemorrhage. Neurology 80:1295–1299
Yogendrakumar V, Ramsay T, Fergusson DA et al (2020) Redefining hematoma expansion with the inclusion of intraventricular hemorrhage growth. Stroke 51:1120–1127
Yang WS, Zhang SQ, Shen YQ (2021) Noncontrast computed tomography markers as predictors of revised hematoma expansion in acute intracerebral hemorrhage. J Am Heart Assoc 10:1–9
Falcone GJ, Biffi A, Brouwers HB et al (2013) Predictors of hematoma volume in deep and lobar supratentorial intracerebral hemorrhage. JAMA Neurol 70:988
Chen R, Wang X, Anderson CS et al (2019) Infratentorial intracerebral hemorrhage. Stroke 50:1257–1259
Yushkevich PA, Piven J, Hazlett HC et al (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31:1116–1128
Mishra M, Shukla P, Bodhit A et al (2014) Intracerebral hematoma volume measurement using ITK-SNAP software: initial experience (P7.145). Neurology 82
Nawabi J, Elsayed S, Kniep H et al (2020) Inter- and Intrarater agreement of spot sign and noncontrast CT markers for early intracerebral hemorrhage expansion. J Clin Med 9:1020
Morotti A, Boulouis G, Dowlatshahi D et al (2019) Standards for detecting, interpreting, and reporting noncontrast computed tomographic markers of intracerebral hemorrhage Expansion. Ann Neurol 86:480–492
Barras CD, Tress BM, Christensen S et al (2009) Density and shape as CT predictors of intracerebral hemorrhage growth. Stroke 40:1325–1331
Yu Z, Zheng J, Ali H et al (2017) Significance of satellite sign and spot sign in predicting hematoma expansion in spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg 162:67–71
Li Q, Liu QJ, Yang WS et al (2007) Island sign: an imaging predictor for early hematoma expansion and poor outcome in patients with intracerebral hemorrhage. Stroke 48:3019–3025
Selariu E, Zia E, Brizzi M, Abul-Kasim K (2012) Swirl sign in intracerebral haemorrhage: definition, prevalence, reliability and prognostic value. BMC Neurol 12:109
Xiong X, Li Q, Yang W-S et al (2018) Comparison of swirl sign and black hole sign in predicting early hematoma growth in patients with spontaneous intracerebral hemorrhage. Med Sci Monit 24:567–573
Li Q, Zhang G, Xiong X et al (2016) Black hole sign. Stroke 47:1777–1781
Sporns PB, Schwake M, Kemmling A et al (2017) Comparison of spot sign, blend sign and black hole sign for outcome prediction in patients with intracerebral hemorrhage. J Stroke 19:333–339
Li Q, Zhang G, Huang Y-J et al (2015) Blend sign on computed tomography. Stroke 46:2119–2123
Seo J-S, Nam T-K, Kwon J-T, Park Y-S (2014) Multiple spontaneous simultaneous intracerebral hemorrhages. J Cerebrovasc Endovasc Neurosurg 16:104
Morotti A, Dowlatshahi D, Boulouis G et al (2018) Predicting intracerebral hemorrhage expansion with noncontrast computed tomography. Stroke 49:1163–1169
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Schlunk F, Kuthe J, Harmel P et al (2022) Volumetric accuracy of different imaging modalities in acute intracerebral hemorrhage. BMC Med Imaging 22:9
Mchugh ML (2012) Interrater reliability: the kappa statistic. Biochem Medica 22:276–282
Heinze G, Dunkler D (2017) Five myths about variable selection. Transpl Int 30:6–10
Chen Q, Nian H, Zhu Y, Talbot HK, Griffin MR, Harrell FE (2016) Too many covariates and too few cases? – a comparative study. Stat Med 35:4546–4558
Multivariable model - building: a pragmatic approach to regression anaylsis based on fractional polynomials for modelling continuous variables | Wiley (2022) Available via https://www.wiley.com/en-sg/Multivariable+Model+Building:+A+Pragmatic+Approach+to+Regression+Anaylsis+based+on+Fractional+Polynomials+for+Modelling+Continuous+Variables-p-9780470028421. Accessed 22 Aug 2022
Maas MB (2015) Comment: intraventricular hemorrhage—it’s the blood that matters, not the timing. Neurology 84:993–993
Hallevi H, Albright KC, Aronowski J et al (2008) Intraventricular hemorrhage: anatomic relationships and clinical implications. Neurology 70:848–852
Hayes BJH, Andrew F (2013) Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York, NY: The Guilford Press. J Educ Meas 51:335–337
Preacher KJ, Hayes AF (2008) Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 40:879–891
Li Q, Li R, Zhao L-B et al (2020) Intraventricular hemorrhage growth: definition, prevalence and association with hematoma expansion and prognosis. Neurocrit Care 33:732–739
Acknowledgements
Jawed Nawabi is grateful for being part of the BIH Charité – Digital Clinician Scientist Program funded by Charité—Universitaetsmedizin Berlin, the Berlin Institute of Health and the German Research Foundation (DFG, Deutsche Forschungsgemeinschaft). Frieder Schlunk is grateful for being part of the BIH Charité – Clinician Scientist Program funded by Charité—Universitaetsmedizin Berlin, the Berlin Institute of Health. Tobias Penzkofer was supported by Berlin Institute of Health (Clinician Scientist Grant, Platform Grant), Ministry of Education and Research (BMBF, 01KX2021, 68GX21001A), German Research Foundation (DFG, SFB 1340/2), Horizon 2020 (952172).
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Andrea Morotti.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Tobias Penzkofer reports research agreements (no personal payments, outside of submitted work) with AGO, Aprea AB, ARCAGY-GINECO, Astellas Pharma Global Inc. (APGD), Astra Zeneca, Clovis Oncology, Inc., Dohme Corp, Holaira, Incyte Corporation, Karyopharm, Lion Biotechnologies, Inc., MedImmune, Merck Sharp, Millennium Pharmaceuticals, Inc., Morphotec Inc., NovoCure Ltd., PharmaMar S.A. and PharmaMar USA, Inc., Roche, Siemens Healthineers, and TESARO Inc., and fees for a book translation (Elsevier). All other authors have nothing to disclose.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Nawabi J, et al Imaging-based outcome prediction of acute intracerebral hemorrhage. Transl Stroke Res. 2021 Dec;12(6):958–967. https://doi.org/10.1007/s12975-021-00891-8. Epub 2021 Feb 6.
Methodology
• retrospective
• observational
• multicenter study
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article the affiliation details for Author Ulf Jensen-Kondering were incorrectly given as: Department of Radiology and Neuroradiology, University Hospital Schleswig-Holstein, Campus Kiel, Kiel, Germany
The correct affiliation should have been: Department of Neuroradiology, University Hospital Schleswig-Holstein, Campus Lübeck, Lübeck, Germany
Additionally, the author name Andrea Dell’Orco was given incorrectly as Andrea Dell Orco.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nawabi, J., Schlunk, F., Dell’Orco, A. et al. Non-contrast computed tomography features predict intraventricular hemorrhage growth. Eur Radiol 33, 7807–7817 (2023). https://doi.org/10.1007/s00330-023-09707-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09707-9