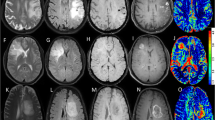
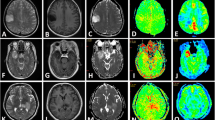
Abstract
Objectives
To comprehensively evaluate the glioma using quantitative susceptibility mapping (QSM).
Materials and methods
Forty-two patients (18 women; mean age, 45 years) with pathologically confirmed gliomas were retrospectively included. All the patients underwent conventional and advanced MRI examinations (QSM, DWI, MRS, etc.). Five patients underwent paired QSM (pre- and post-enhancement). Four Visually Accessible Rembrandt Image (VASARI) features and intratumoural susceptibility signal (ITSS) were observed. Three ROIs each were manually drawn separately in the tumour parenchyma with relatively high and low magnetic susceptibility. The association between the tumour’s magnetic susceptibility and other MRI parameters was also analysed.
Results
Morphologically, gliomas with heterogeneous ITSS were more similar to high-grade gliomas (p = 0.006, AUC: 0.72, sensitivity: 70%, and specificity: 73%). Heterogeneous ITSS was significantly associated with tumour haemorrhage, necrosis, diffusion restriction, and avid enhancement but did not change between pre- and post-enhanced QSM. Quantitatively, tumour parenchyma magnetic susceptibility had limited value in grading gliomas and identifying IDH mutation status, whereas the relatively low magnetic susceptibility of the tumour parenchyma helped identify oligodendrogliomas in IDH mutated gliomas (AUC = 0.78) with high specificity (100%). The relatively high tumour magnetic susceptibility significantly increased after enhancement (p = 0.039). Additionally, we found that the magnetic susceptibility of the tumour parenchyma was significantly correlated with ADC (r = 0.61) and Cho/NAA (r = 0.40).
Conclusions
QSM is a promising candidate for the comprehensive evaluation of gliomas, except for IDH mutation status. The magnetic susceptibility of tumour parenchyma may be affected by tumour cell proliferation.
Key Points
• Morphologically, gliomas with a heterogeneous intratumoural susceptibility signal (ITSS) are more similar to high-grade gliomas (p = 0.006; AUC, 0.72; sensitivity, 70%; and specificity, 73%). Heterogeneous ITSS was significantly associated with tumour haemorrhage, necrosis, diffusion restriction, and avid enhancement but did not change between pre- and post-enhanced QSM.
• Tumour parenchyma’s relatively low magnetic susceptibility helped identify oligodendroglioma with high specificity.
• Tumour parenchyma magnetic susceptibility was significantly correlated with ADC (r = 0.61) and Cho/NAA (r = 0.40).






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- ASL:
-
Arterial spin labelling
- Cho:
-
Choline
- DCE-MRI:
-
Dynamic contrast-enhanced MRI
- DWI:
-
Diffusion-weighted imaging
- HGGs:
-
High-grade gliomas
- ITSS:
-
Intratumoural susceptibility signal
- Kep:
-
Rate constant
- Ktrans :
-
Transfer constant
- LGGs:
-
Low-grade gliomas
- MRS:
-
Magnetic resonance spectrum
- NAA:
-
N-acetylaspartate
- QSM:
-
Quantitative susceptibility mapping
- relCBV:
-
Relative cerebral blood volume
- SWI:
-
Susceptibility-weighted imaging
- VASARI:
-
Visually Accessible Rembrandt Image
- Ve:
-
Extravascular-extracellular space volume fraction
References
Qazi MA, Vora P, Venugopal C et al (2017) Intratumoral heterogeneity: pathways to treatment resistance and relapse in human glioblastoma. Ann Oncol 28:1448–1456
DeCordova S, Shastri A, Tsolaki AG et al (2020) Molecular heterogeneity and immunosuppressive microenvironment in glioblastoma. Front Immunol 11:1402
Bulakbasi N, Paksoy Y (2019) Advanced imaging in adult diffusely infiltrating low-grade gliomas. Insights Imaging 10:122
Lieu AS, Hwang SL, Howng SL, Chai CY (1999) Brain tumors with hemorrhage. J Formos Med Assoc 98:365–367
Inano R, Oishi N, Kunieda T et al (2016) Visualization of heterogeneity and regional grading of gliomas by multiple features using magnetic resonance-based clustered images. Sci Rep 6:30344
Zhao J, Li J, Wang J et al (2018) Quantitative analysis of neurite orientation dispersion and density imaging in grading gliomas and detecting IDH-1 gene mutation status. Neuroimage Clin 19:174–181
Chu JP, Song YK, Tian YS et al (2021) Diffusion kurtosis imaging in evaluating gliomas: different region of interest selection methods on time efficiency, measurement repeatability, and diagnostic ability. Eur Radiol 31:729–739
Hu LS, Hawkins-Daarud A, Wang L, Li J, Swanson KR (2020) Imaging of intratumoral heterogeneity in high-grade glioma. Cancer Lett 477:97–106
Miller JJ, Gonzalez CL, McBrayer S et al (2023) Isocitrate dehydrogenase (IDH) mutant gliomas: a Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro Oncol 25:4–25
Leather T, Jenkinson MD, Das K, Poptani H (2017) Magnetic resonance spectroscopy for detection of 2-hydroxyglutarate as a biomarker for IDH mutation in gliomas. Metabolites 19:29
Park MJ, Kim HS, Jahng GH, Ryu CW, Park SM, Kim SY (2009) Semiquantitative assessment of intratumoral susceptibility signals using non-contrast-enhanced high-field high-resolution susceptibility-weighted imaging in patients with gliomas: comparison with MR perfusion imaging. AJNR Am J Neuroradiol 30:1402–1408
Deistung A, Schweser F, Wiestler B et al (2013) Quantitative susceptibility mapping differentiates between blood depositions and calcifications in patients with glioblastoma. PLoS One 8:e57924
Hsu CC, Watkins TW, Kwan GN, Haacke EM (2016) Susceptibility-weighted imaging of glioma: update on current imaging status and future directions. J Neuroimaging 26:383–390
Li X, Zhu Y, Kang H et al (2015) Glioma grading by microvascular permeability parameters derived from dynamic contrast-enhanced MRI and intratumoral susceptibility signal on susceptibility weighted imaging. Cancer Imaging 15:4
Legendre C, Garcion E (2015) Iron metabolism: a double-edged sword in the resistance of glioblastoma to therapies. Trends Endocrinol Metab 26:322–331
Huang R, Dong R, Wang N et al (2022) Adaptive changes allow targeting of ferroptosis for glioma treatment. Cell Mol Neurobiol 42:2055–2074
Liu C, Li W, Tong KA, Yeom KW, Kuzminski S (2015) Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging 42:23–41
Zhang S, Liu Z, Nguyen TD et al (2019) Clinical feasibility of brain quantitative susceptibility mapping. Magn Reson Imaging 60:44–51
Nikparast F, Ganji Z, Danesh DM, Faraji R, Zare H (2022) Brain pathological changes during neurodegenerative diseases and their identification methods: How does QSM perform in detecting this process? Insights Imaging 13:74
Thomas G, Leyland LA, Schrag AE, Lees AJ, Acosta-Cabronero J, Weil RS (2020) Brain iron deposition is linked with cognitive severity in Parkinson’s disease. J Neurol Neurosurg Psychiatry 91:418–425
Chang PD, Malone HR, Bowden SG et al (2017) A multiparametric model for mapping cellularity in glioblastoma using radiographically localized biopsies. AJNR Am J Neuroradiol 38:890–898
Anzalone N, Castellano A, Cadioli M et al (2018) Brain gliomas: multicenter standardized assessment of dynamic contrast-enhanced and dynamic susceptibility contrast MR images. Radiology 287:933–943
McKnight TR, Lamborn KR, Love TD et al (2007) Correlation of magnetic resonance spectroscopic and growth characteristics within grades II and III gliomas. J Neurosurg 106:660–666
Ravanfar P, Loi SM, Syeda WT et al (2021) Systematic review: Quantitative susceptibility mapping (QSM) of brain iron profile in neurodegenerative diseases. Front Neurosci 15:618435
Helmi A, Chan A, Towfighi S et al (2019) Incidence of dural venous sinus thrombosis in patients with glioblastoma and its implications. World Neurosurgery 125:e189–e197
Bale TA, Jordan JT, Rapalino O et al (2019) Financially effective test algorithm to identify an aggressive, EGFR-amplified variant of IDH-wildtype, lower-grade diffuse glioma. Neuro Oncol 21:596–605
Kong LW, Chen J, Zhao H et al (2019) Intratumoral susceptibility signals reflect biomarker status in gliomas. Sci Rep 9:17080
Wei H, Dibb R, Zhou Y et al (2015) Streaking artifact reduction for quantitative susceptibility mapping of sources with large dynamic range. NMR Biomed 28:1294–1303
Li W, Wang N, Yu F et al (2015) A method for estimating and removing streaking artifacts in quantitative susceptibility mapping. Neuroimage 108:111–122
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23:1231–1251
Sehgal V, Delproposto Z, Haacke EM et al (2005) Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging 22:439–450
Mohammed W, Xunning H, Haibin S, Jingzhi M (2013) Clinical applications of susceptibility-weighted imaging in detecting and grading intracranial gliomas: a review. Cancer Imaging 13:186–195
Wen Y, Spincemaille P, Nguyen T et al (2021) Multiecho complex total field inversion method (mcTFI) for improved signal modeling in quantitative susceptibility mapping. Magn Reson Med 86:2165–2178
Upadhyay N, Waldman AD (2011) Conventional MRI evaluation of gliomas. Br J Radiol 84 Spec No 2:S107-S111
Colgan TJ, Knobloch G, Reeder SB, Hernando D (2020) Sensitivity of quantitative relaxometry and susceptibility mapping to microscopic iron distribution. Magn Reson Med 83:673–680
Langkammer C, Schweser F, Krebs N et al (2012) Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage 62:1593–1599
Uchida Y, Kan H, Sakurai K et al (2020) Iron leakage owing to blood-brain barrier disruption in small vessel disease CADASIL. Neurology 95:e1188–e1198
Loudet C, Diller A, Grelard A, Oda R, Dufourc EJ (2010) Biphenyl phosphatidylcholine: a promoter of liposome deformation and bicelle collective orientation by magnetic fields. Prog Lipid Res 49:289–297
Hou W, Xue Y, Tang W et al (2019) Evaluation of tumor hypoxia in C6 glioma rat model with dynamic contrast-enhanced magnetic resonance imaging. Acad Radiol 26:e224–e232
Acknowledgements
This study was supported by the Guangdong Basic and Applied Basic Research Foundation, China, and the National Natural Science Foundation.
Funding
This study has received funding from the Guangdong Basic and Applied Basic Research Foundation, China (No.2020A1515011436, 2021A1515012279, 2022A1515011264), and the National Natural Science Foundation (NSFC 82172015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jing Zhao. Jianping Chu is a co-guarantor.
Conflict of interest
One of the authors (Mengzhu Wang) is an employee of Siemens Healthineers (Guangzhou, China). This author was not involved in data collection and/or management in any way that would influence the study. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because of the retrospective nature of this study.
Ethical approval
The Research Ethics Committee of the First Affiliated Hospital of Sun Yat-Sen University has approved this study (No. [2021]209).
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, S., Ma, H., Xie, D. et al. Quantitative susceptibility mapping evaluation of glioma. Eur Radiol 33, 6636–6647 (2023). https://doi.org/10.1007/s00330-023-09647-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09647-4