Abstract
Objective
To introduce novel parameters in determining directions of os odontoideum (OO) with atlantoaxial displacement (AAD) and compensations of cervical sagittal alignment after displacement.
Methods
Analysis was performed on 96 cases receiving surgeries for upper cervical myelopathy caused by OO with AAD from 2011 to 2021. Twenty-four patients were included in the OO group and divided into the OO-anterior displacement (AD) group and the OO-posterior displacement (PD) group by displacement. Seventy-two patients were included as the control (Ctrl) group and divided into Ctrl-positive (Ctrl-P) group and Ctrl-negative (Ctrl-N) group by axial superior facet slope (ASFS) in a neutral position. ASFS, the sum of C2 slope (C2S) and axial superior facet endplate angle (ASFEA), was measured and calculated by combining cervical supine CT with standing X-ray. Cervical sagittal parameters were measured to analyse the atlantoaxial facet and compensations after AAD.
Results
Atlas inferior facet angle (AIFA), ASFS, and ASFEA in Ctrl-P significantly differed from OO-AD.C0-C1, C1-C2, C0-C2, C2-C7, C2-C7 SVA, and C2S in Ctrl-P significant differed from the OO-AD group. C2-C7 SVA and C2S in Ctrl-N significantly were smaller than the OO-PD group. C1-C2 correlated with C0-C1 and C2-C7 negatively in the OO group. Slight kyphosis of C1-C2 in OO-AD was compared with lordosis of C1-C2 in Ctrl-P, inducing increased extension of C0-C1 and C2-C7. Mildly increased lordosis of C1-C2 in OO-PD was compared with C1-C2 in Ctrl-N, triggering augmented flexion of C0-C1 and C2-C7.
Conclusion
ASFS was vital in determining directions of OO with AAD and explaining compensations. ASFS and ASFEA could provide pre- and intraoperative guidelines.
Key Points
• ASFS may determine the directions and compensatory mechanisms of AAD secondary to OO.
• ASFS could be achieved by the sum of ASFEA and C2S.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aetiologies of atlantoaxial displacement (AAD, including instability, subluxation and dislocation) include trauma, inflammation, and genetics, among which os odontoideum (OO) is an important factor [1]. Although OO triggers AAD, which is characterised by higher morbidities of anterior displacement (AD) than posterior displacement (PD), by regulating lateral atlantoaxial joints (LAJs) [2,3,4], the elements determining displacement directions are still unclear.
The atlantooccipital complex can serve as the moving object during AAD based on the recognition of the axial superior facet (ASF) as the sliding surface. While few reports have focused on ASF, it is vital to explore ASF characteristics based on significance by comparing AD with PD.
Nontraumatic L5 spondylolisthesis features forwards translation and rotational displacement of L5 vertebrae on the S1 upper endplate, while posterior sliding of L5 is seldom mentioned (except for the loss of L5/S1 lordosis and retrolisthesis in slump-sitting posture [5]). The reason for this is the orientation of the upper sacral endplate inclined from anteroinferior to posterosuperior in the upright position [6]. This made us consider whether the displacement is related to the anatomy and position of the ASF in the upright position. Therefore, the axial superior facet endplate angle (ASFEA), an anatomical parameter derived from supine cervical CT, and the axial superior facet slope (ASFS) were proposed to explore the relationships of AAD directions to recognize the ASF in standing spinal X-ray examinations and limit the use of standing cervical CT examinations.
Hitherto, their little attention has been given to the regulation of cervical-global sagittal balance [7], especially compensations of the atlantooccipital joint and subaxial cervical spine after AAD. However, whether AAD can trigger compensations as does spondylolisthesis remains unknown. Herein, we found that the ASFS might determine the displacement orientation of AAD secondary to OO and trigger compensations between the atlantooccipital joint and subaxial cervical spine, thereby providing guidelines for treating AAD.
Methods
Data collection
Research was performed on patients admitted from 2011 to 2021 undergoing surgery for upper cervical myelopathy caused by OO with AAD. Patients who underwent surgery for spinal compression caused by OO with AAD and who had clear radiographical data were included. Patients with AAD caused by trauma, rheumatoid arthritis (RA), inflammation, infection, tumour, history of surgery on the cervical spine and OO combined with atlas occipitalisation were excluded. Twenty-four patients diagnosed with OO with AAD were included in the OO group. Seventy-two patients with cervical degeneration without an occipitocervical disorder were included in the control group (Ctrl) at a 1:3 ratio with matching by age and sex to the OO group. Patients in the Ctrl group had cervical disc degenerative diseases with supine cervical CT data, standing cervical lateral radiographs and no occipitocervical abnormalities.
Measurements of cervical sagittal parameters
Cervical sagittal parameters are shown in Table 1 and Fig. 1. C7S can be used to replace T1S to avoid an unclear upper T1 endplate [8, 9].
ASFEA measurements are shown in Fig. 2, and the specific steps are as follows: (1) Mid-sagittal CT reconstructions of the axis and ASF were captured. (2) Two images were aligned, overlapped and cropped according to positioning lines such that the final image clearly demonstrated the axial inferior endplate and ASF simultaneously. (3) ASFEA was measured as the angle between the line connecting the anterior and posterior points of the ASF and the extension line of the axial lower endplate. In addition, C2S was measured, and ASFS was obtained as the sum of ASFEA and C2S (Fig. 3).
Method for ASFEA measurement. A Mid-sagittal CT reconstruction of the axis (red frame) and ASF (orange frame) (B) were obtained. C Two images were aligned, overlapped and cropped to demonstrate the axial inferior endplate and ASF simultaneously. The ASFEA was the angle between the line connecting the anterior and posterior points of the ASF and the extension line of the axial lower endplate
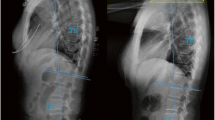
Measurement of ASFEA in AAD patients. Herein, C2S + ASFEA = ASFS. A Cervical X-ray of an AD patient in a neutral position and measurement of C2S. B Measurement of left ASFEA in an AD patient. C Measurement of right ASFEA in an AD patient; (left ASFEA (28°) + right ASFEA (31°))/2 + C2S (− 15°)) = ASFS (14.5°). D of Cervical X-ray of a PD patient in a neutral position and measurement of C2S. E Measurement of left ASFEA in a PD patient. F Measurement of right ASFEA in a PD patient; (left ASFEA (− 15°) + right ASFEA (− 14°))/2 + C2S (12°)) = ASFS (− 2.5°)
When the angle vertex lay in front of or behind the cervical spine, the values were positive or negative, respectively. Positive values of C0-C1, C1-C2, C0-C2, C2-C7, C0-C7, and ASFEA indicated kyphosis, and negative values indicated lordosis. Positive values of C2S, C7S, and ASFS implied that the lower endplate of C2, C7, and ASF were inclined from anteroinferior to posterosuperior compared with the horizontal plane (AIPS), while negative values represented inclination from anterosuperior to posteroinferior (ASPI).
Consistency analysis
Two surgeons measured and calculated ASFS independently every 2 weeks. Mean measurements were used for further accuracy. Intraobserver and interobserver reliability were analysed.
Statistical analysis
Data were analysed using SPSS 28.0 (SPSS, Inc.). Parameters in the two groups were compared by t tests. Reliability was calculated by the intraclass correlation coefficient (ICC) [10]. Correlation analysis was performed using the Pearson method. A p value < 0.05 was considered to indicate statistical significance.
Results
Demographic information
The OO group included 14 men and 10 women, aged 51.13 ± 13.50 years, as well as 20 OO-AD cases (83.3%) and 4 OO-PD cases (16.7%). The Ctrl group included 43 men and 29 women, aged 45.79 ± 20.29 years; there were four cervical sagittal curve types in the Ctrl group, including 43 lordosis cases (59.7%), 16 neutral cases (22.2%), 8 kyphosis cases (11.1%), and 5 S-shaped cases (7.0%) according to a previous report [11].
Reliability analysis of ASFS
Table 2 shows that the intraobserver and interobserver reliability were both excellent for ASFS.
ASFEA, sagittal inferior C1 facet, and ASFS
ASFEA, ASFS and AIFA are shown in Table 3.
Positive ASFS values indicated AIPS of the ASF, and the atlantooccipital complex tended to slide forwards; conversely, it tended to slide backwards. OO-AD was compared with Ctrl-P (positive, ASFS > 0), and OO-PD was compared with Ctrl-N (negative, ASFS < 0) to analyse the inferior C1 facet and ASF morphology.
The OO group included 19 positive ASFEA cases (79.2%) and 5 negative cases (20.8%). All cases in the Ctrl group had positive ASFEA values (100%); the OO group included 20 positive ASFS cases (83.3%) and 4 negative ASFS cases (16.7%). In the neutral position, the Ctrl group had 57 positive ASFS cases (79.2%) and 15 negative ASFS cases (20.8%). In the overflexion position, the Ctrl group had 68 positive ASFS cases (94.4%) and 4 negative ASFS cases (5.6%).
Compensations of the atlantooccipital joint and lower cervical spine for OO-AD and OO-PD
Comparisons of cervical sagittal parameters are shown in Table 4, and the correlation analysis results are shown in Table 5.
Comparison between the OO-AD and Ctrl-P groups demonstrated that the former had significantly larger C1-C2 and C0-C2 and smaller C0-C1, C2-C7, C2-C7 SVA and C2S values, with no significant differences in C7S or C0-C7 values. Comparison between the OO-PD and Ctrl-N groups demonstrated that the former had significantly larger C2S and C2-C7 SVA values, with no significance in C0-C1, C1-C2, C0-C2, C2-C7, C0-C7, and C7S values.
Correlation analysis showed that C1-C2 correlated positively with C0-C2; C0-C1, C2-C7, C2-C7 SVA, C2S, and C7S values correlated positively with each other except for a weak negative correlation between C2-C7 and C7S; and C1-C2 and C0-C2 correlated negatively with C0-C1, C2-C7, C2-C7 SVA, C2S, and C7S values. The weakest correlations were in the OO-PD group, possibly due to sample size limitations, nonobvious compensations and slight PD. Strong correlations were observed in the OO-AD group, possibly due to sufficient sample size, thus inducing apparent compensations. The strongest correlations were observed in the OO group.
Discussion
AAD has been described in diseases such as traumatic odontoid fracture, OO, and RA [12], featuring higher morbidity in AD than in PD. Morphologic remodelling of axial LAJs during AAD has attracted much attention. LAJs with non-union odontoid fractures gradually remodel into a fish-lip or dome-shape during AAD, resulting in irreducibility [13, 14]. Salunke found that the AIFA was linked with disease progression and irreducibility [15]. Ma also classified the morphology of lateral C1-C2 joint facets in congenital AAD patients based on the AIFA [16]. These results reflect the atlantoaxial facet morphology but fail to consider OO patients and positional parameters of atlantoaxial facets.
In AAD, the ASF is the sliding surface, while the atlantooccipital complex is the sliding object. Hence, the ASF better reflects the mechanisms of AAD. A previous study assessed the atlantoaxial superior facet morphology in AAD and showed the significance of sagittal joint inclination, similar to ASFEA, in determining AAD severity [17]. However, it was more anatomical than positional. Yuan proposed a novel cervical parameter, sagittal atlantoaxial joint inclination (SAAJI), illustrating the atlantoaxial articular surface on the parasagittal view for irreducible AAD, which resembled ASFS [18]. However, horizontal lines were difficult to determine on supine CT, which restricted its clinical application. Additionally, the sagittal slippage angle of the atlantooccipital inferior joint facet uses the eye-ear plane as the horizontal plane, which fails to reflect real conditions while standing [19]. In addition, the specificity of patients and the ASF were not mentioned.
These studies focused on morphologic changes in LAJs after long-term remodelling in AAD while neglecting initial factors propelling AD rather than PD. A previous study revealed that PD was usually found with odontoid destruction in RA [20]. Mechanisms of irreducible PD with OO showed that once the C1 facet posteriorly crosses the medial hump of the C2 facet, it tends to slide further and locks in this position, making the PD irreducible. Theoretically, the odds of AD should equal those of PD, which is in contrast to the clinical observation that AD cases outnumber PD cases.
The displacement in patients with OO-related fracture or RA is accidental to some extent based on the trauma to or pathological destruction of the atlantoaxial joints. Thus, only in cases of OO and type II transverse odontoid fracture without obvious displacement would the atlantoaxial vertebrae experience a long process ranging from instability and subluxation to irreducibility, during which the displacement of the atlas compared with the axis and the remodelling of LAJs advance together and have mutual effects. When traced back to the initial process of displacement, however, the facet surface of LAJs showed no significant changes, but AD was obviously more prevalent than PD, prompting us to consider the relationship between ASFS and the direction of AAD.
To determine the cause-effect relationship between ASFS and OO diagnosed with AAD, it was advisable to conduct a cohort study for the measurement of ASFS before displacement and analyse their correlation. However, these patients are normally diagnosed with neurologic symptoms after AAD, or even if they are occasionally diagnosed in the early stage, surgery is immediately recommended to avoid neurologic sequelae [1], making it difficult to collect cases from prospective cohort observations. The alternative was to study the ASFS in people without AAD and compare it with the ASFS in patients with OO with AAD to indirectly understand the relationship between the ASFS and the direction of AAD in OO. Patients with cervical disc degenerative disease were included in the Ctrl group, which could better reflect the true conditions under physiological circumstances. Therefore, we studied patients with and without AAD to indirectly demonstrate the crosstalk between ASFS and displacement directions.
Herein, ASFEA and ASFS were consequences of AAD after remodelling, which cannot represent the initial conditions of the atlantoaxial facet surfaces. The displacement of the atlantooccipital complex on the ASF and the remodelling of LAJs have mutual influences, which cannot be interpreted as only causes or results [3, 21,22,23]. Moreover, their relationship should be recognised as an interaction, as one condition could significantly affect the other while also accelerating the displacement process. Since it was impossible to track ASFEA and ASFS before AAD, atlantoaxial parameters from non-AAD patients were used as a substitute approach to reflect them before AAD. Notably, the skeletal system remains steady in adults, leading to presumptions that ASFEA and ASFS stay constant in adults, which corresponded to pelvic inclination (PI) and neutral sacral slope (SS) in a previous report [24]. OO patients were an average of 51.13 ± 13.50 years old (we were not a specialised children’s hospital), suggesting that AAD might start following adulthood. Therefore, it was better to measure ASFS and ASFEA in the non-AAD group to simulate conditions before AAD. In this study, the displacement in OO patients (AD (20 cases, 83.3%), PD (4 cases, 16.7%)) was consistent with that in previous reports [1, 4]. In the control group (neutral), a positive ASFS was found in 57 (79.2%) patients, and a negative ASFS was found in 15 (20.8%), whereas a positive ASFS was found in 68 (94.4%) patients on hyperflexion radiography. These findings indicate that the distribution of displacement in OO was approximate to that of the ASFS in the Ctrl group, underlying the potential clinical significance of ASFS for determining displacement direction in OO patients.
Specifically, when the ASFS was 0° in OO patients before AAD, the ASF remained relatively horizontal, maintaining the stability of the LAJs and surrounding structures. Less mechanical loading would be applied to LAJs in the absence of remodelling. However, a positive ASFS would enable the tendency to slide forwards given that the facet surfaces were AIPS based on gravity. In turn, LAJs with AD would result in further remodelling of facet surfaces to increase the ASFS and contracture of periarticular stabilisers to induce abnormalities of LAJs, leading to anterior instability, subluxation, and luxation of the LAJs [19]. Conversely, a negative ASFS tended to slide backwards under gravity for ASPI.
Most AAD patients were AD with a positive ASFS and PD with a negative ASFS, leading to a higher prevalence of AD than PD, which was aggravated by head-lowering movements during daily work [25]. Kauppi reported that during neck hyperextension, the contact between the posterior arch of the atlas and the spinous process restrained PD, while such restriction did not completely work during flexion, partially explaining the lower incidence of PD [26]. We further illustrated that AAD initiated even in a neutral position, possibly due to ASFS and ASFEA imbalances.
Furthermore, sacral osteotomy has been reported in the correction of pelvic parameters (PI) for maintaining spinal-pelvic sagittal balance [27], hinting at possibilities of adjusting the ASFS via ASF osteotomy or C2-C3 fusion, thereby preventing AAD and preserving atlantoaxial functions without spinal syndromes. Osteotomy on the inferior C1 facet and ASF could convert AAD irreducibility and thereby independently realise an intraoperative reduction of LAJs by a posterior approach [15]. This further shows the significance of the ASFEA and ASFS intraoperatively; otherwise, the opposite osteotomy may be performed, which would aggravate the irreducibility of AAD or deteriorate the deformity of LAJs. Generally, the ASFS and ASFEA could help surgeons adjust treatments appropriately in early stages to delay or reverse AAD progression, especially to facilitate the osteotomy direction based on the ASFEA [18, 28]. More importantly, the ASFS and ASFEA predicted the direction of AAD, which was useful for instructing patients to adjust their neck posture and effectively suspend displacement.
Lordosis of the ASFEA became kyphosis with an average of 25°, and the AIFA decreased by approximately 5° due to remodelling after OO-AD. The ASFS in OO-AD increased, which further explained the crosstalk between the ASF and displacement direction. Atlantoaxial parameters in the comparison between the Ctrl-P and OO-PD groups were not significantly different, possibly due to the sample size and blockage of the odontoid process.
Notably, sagittal imbalance of the cervical spine has been shown to induce compensations in adjacent segments. Cervical parameters have been used to analyse sagittal compensations of the cervical spine, such as in C0-C2, C1-C2, C2S, and T1S [29, 30], and crosstalk between C2S and cranial slope (CS) has been reported in the superior cervical spine [7]. Plain standing spinal films enabled the measurement of C2S, propelling us to further determine ASFS and ASFEA. We showed that ASFS = C2S + ASFEA, where ASFEA was an anatomical parameter that remained stable regardless of position, indicating a positive correlation between ASFS and the functional parameter C2S. The results showed that both ASFS and C2S correlated with C1-C2, C0-C2, and C2-C7, indicating that AIPS facets of a positive ASFS induced larger C1-C2 and smaller C0-C1 and C2-C7 values in AD patients, while ASPI facets of a negative ASFS induced smaller C1-C2 and larger C0-C1 and C2-C7 values in PD patients, thereby maintaining balance during standing. A larger ASFS in AD patients led to lordosis of C0-C2 and C2-C7, showing that craniocervical compensations were involved in maintaining a horizontal view. However, patients with an excessively large ASFS developed severe cervical lordosis in the late stages of AD, which triggered unsustainable compensations, leading to failure to maintain a horizontal gaze while standing.
To maintain an upright standing posture and horizontal gaze, the spine as a whole system is balanced by adjusting the spinal segments. Consequently, the musculoskeletal system coordinates spinal alignment to adjust physiologic curves and maintain a horizontal view [31]. Compensations in adjacent vertebrae usually arise when spinal misalignment appears in localised segments [32]. Herein, sagittal deformity of C1-C2 led to compensations of C0-C1, C2-C7, C2-C7 SVA, C2S, and C7S, indicating that sagittal parameters exerted apparent effects on adjacent segments but minor impacts on distal segments. A negative C1-C2 in Ctrl-P represented lordosis, while a positive C1-C2 in OO-AD indicated kyphosis, with an approximate difference of 33°. Changes in the C0-C1 and C2-C7 curves indicated compensations of approximately 10° and 30°, respectively, driven by C1-C2 kyphosis, which further resulted in C2-C7 SVA and C2S decreases. No significance was found in C7S, showing that the thoracolumbar spine was not involved in compensations for C1-C2 deformity. Significance was found in C2-C7 SVA and C2S when OO-PD was compared to Ctrl-N, indicating that OO-PD patients developed slight morphologic alterations of atlantoaxial joints, inducing compensations in atlantoaxial adjacencies. However, this finding is carries less meaning considering the sample size of OO-PD patients.
Understanding C1-C2 deformity and compensations in AAD can help surgeons adjust fusion angles intraoperatively. Although the reported fusion angle was 20–22° for AAD with OO, another study argued that it was unnecessary to recover the C0-C2 fusion angle to the normal range intraoperatively [33, 34]. Herein, a horizontal gaze was maintained by C2-C7 adjustments due to the absence of C0-C1 compensations in C0-C2 fusion. The fixation angle of C0-C2 should be emphasised to avoid lower cervical spine degeneration. In contrast, the C1-C2 fixation angle allowed a larger range to maintain a horizontal view after C1-C2 fusion by adjusting C0-C1 and C2-C7 to compensate for C1-C2 deformity. Limitations of this study include the sample size and inherent limitations of cohort studies. Therefore, relationships between the ASFS and the direction of AAD in OO patients remain speculative.
Conclusions
The ASFS and ASFEA were introduced for determining the direction of AAD. A positive ASFS indicated AIPS, suggesting the tendency of AD due to forwards sliding, while a negative ASFS indicated ASPI in PD. Compensations after displacement included OO-AD inducing increased flexion of C0-C1 and C2-C7 and decreased flexion of C1-C2; OO-PD triggered opposite compensations. Therefore, recognition of the ASFS and ASFEA provided referential guidelines pre- and intraoperatively.
Abbreviations
- AAD:
-
Atlantoaxial displacement
- AD:
-
Anterior displacement
- AIFA:
-
Atlas inferior facet angle
- AIPS:
-
Incline from anteroinferior to posterosuperior compared with the horizontal plane
- ASF:
-
Axial superior facet
- ASFEA:
-
Axial superior face endplate angle
- ASFS:
-
Axial superior facet slope
- ASPI:
-
Incline from anterosuperior to posteroinferior compared with the horizontal plane
- C2S:
-
C2 slope
- C7S:
-
C7 slope
- LAJ:
-
Lsateral atlantoaxial joint
- OO:
-
Os odontoideum
- PD:
-
Posterior displacement
References
Klimo P Jr, Kan P, Rao G, Apfelbaum R, Brockmeyer D (2008) Os odontoideum: presentation, diagnosis, and treatment in a series of 78 patients. J Neurosurg Spine 9(4):332–342
Klimo P Jr, Coon V, Brockmeyer D (2011) Incidental os odontoideum: current management strategies. Neurosurg Focus 31(6):E10
Shirasaki N, Okada K, Oka S, Hosono N, Yonenobu K, Ono K (1991) Os odontoideum with posterior atlantoaxial instability. Spine (Phila Pa 1976), 16(7):706–15
Fielding JW, Hensinger RN, Hawkins RJ (1980) Os Odontoideum. J Bone Joint Surg Am 62(3):376–383
Hey HWD, Lim JCL, Law GW, Liu GK, Wong HK (2021) Understanding the pathophysiology of L5-S1 loss of lordosis and retrolisthesis: an EOS study of lumbopelvic movement between standing and slump sitting postures. World Neurosurg
McCarty ME, Mehlman CT, Tamai J, Do TT, Crawford AH, Klein G (2009) Spondylolisthesis: intraobserver and interobserver reliability with regard to the measurement of slip percentage. J Pediatr Orthop 29(7):755–759
Gerilmez A, Naderi S (2021) A novel perspective for analyzing craniocervical sagittal balance and horizontal gaze. World Neurosurg 149:e924–e930
Ye IB, Tang R, Cheung ZB, White SJW, Cho SK (2018) Can C7 slope substitute the T1 slope?: an analysis using cervical radiographs and kinematic MRIs. Spine (Phila Pa 1976), 43(7):520–525
Inoue T, Ando K, Kobayashi K et al (2021) Age-related changes in T1 and C7 slope and the correlation between them in more than 300 asymptomatic subjects. Spine (Phila Pa 1976), 46(8):E474-E481
Lachin JM (2004) The role of measurement reliability in clinical trials. Clin Trials 1(6):553–566
Teo AQA, Thomas AC, Hey HWD (2020) Sagittal alignment of the cervical spine: do we know enough for successful surgery? J Spine Surg 6(1):124–135
Mingsheng T, Long G, Ping Y T et al (2020) New classification and its value evaluation for atlantoaxial dislocation. Orthop Surg 12(4):1199–1204
Wang S, Wang C, Yan M, Zhou H, Dang G (2013) Novel surgical classification and treatment strategy for atlantoaxial dislocations. Spine (Phila Pa 1976), 38(21): E1348–56
Ma F, Liao Y, Tang Q et al (2021) Morphometric analysis of the lateral atlantoaxial joints in patients with an old type II odontoid fracture and atlantoaxial dislocation: a study based on CT analysis. Spine (Phila Pa 1976), 46(11): 726–733
Salunke P, Sharma M, Sodhi HB, Mukherjee KK, Khandelwal NK (2011) Congenital atlantoaxial dislocation: a dynamic process and role of facets in irreducibility. J Neurosurg Spine 15(6):678–685
Ma F, He H, Liao Y et al (2020) Classification of the facets of lateral atlantoaxial joints in patients with congenital atlantoaxial dislocation. Eur Spine J 29(11):2769–2777
Chandra PS, Goyal N, Chauhan A, Ansari A, Sharma BS, Garg A (2014) The severity of basilar invagination and atlantoaxial dislocation correlates with sagittal joint inclination, coronal joint inclination, and craniocervical tilt: a description of new indexes for the craniovertebral junction. Neurosurgery 10 Suppl 4: 621–9; discussion 629–30
Yuan SL, Xu HM, Fu LC, Cao J, Yang JK, Xi YM (2018) Sagittal atlantoaxial joint inclination and reduction index values for diagnosis and treatment of irreducible atlantoaxial dislocation. Indian J Orthop 52(2):190–195
Yin YH, Yu XG, Zhou DB et al (2012) Three-dimensional configuration and morphometric analysis of the lateral atlantoaxial articulation in congenital anomaly with occipitalization of the atlas. Spine (Phila Pa 1976), 37(3): E170–3
Ferrante A, Ciccia F, Giammalva GR et al (2019) The craniovertebral junction in rheumatoid arthritis: state of the art. Acta Neurochir Suppl 125:79–86
Behari S, Jaiswal A, Srivastava A, Rajput D, Jain VK (2010) Os odontoideum with “free-floating” atlantal arch causing C1–2 anterolisthesis and retrolisthesis with cervicomedullary compression. Indian J Orthop 44(4):417–423
Goel A, Jain S, Shah A et al (2018) Atlantoaxial fixation for odontoid fracture: analysis of 124 surgically treated cases. World Neurosurg 110:558–567
Meng H, Gao Y, Li M, Luo Z, Du J (2014) Posterior atlantoaxial dislocation complicating odontoid fracture without neurologic deficit: a case report and review of the literature. Skeletal Radiol 43(7):1001–1006
Mac-Thiong JM, Berthonnaud E, Dimar JR II, Betz RR, Labelle H (2004) Sagittal alignment of the spine and pelvis during growth. Spine (Phila Pa 1976), 29(15): 1642–7
Morningstar M (2002) Cervical curve restoration and forward head posture reduction for the treatment of mechanical thoracic pain using the pettibon corrective and rehabilitative procedures. J Chiropr Med 1(3):113–115
Kauppi M (1994) A method for classification of the posterior atlanto-axial subluxation. Clin Rheumatol 13(3):492–495
Khalife M, Khalife P, Pascal-Moussellard H (2020) Sacrum osteotomy to change pelvic parameters: surgical technique and 3D modeling. Orthop Traumatol Surg Res 106(6):1227–1232
Salunke P, Sahoo SK (2019) Comprehensive drilling of C1–2 facets and multiplanar realignment for atlanto-axial dislocation and basilar invagination: 2-dimensional operative video. Oper Neurosurg (Hagerstown) 16(2):55–57
Le Huec JC, Demezon H, Aunoble S (2015) Sagittal parameters of global cervical balance using EOS imaging: normative values from a prospective cohort of asymptomatic volunteers. Eur Spine J 24(1):63–71
Divi SN, Bronson WH, Canseco JA et al (2021) How do C2 tilt and C2 slope correlate with patient reported outcomes in patients after anterior cervical discectomy and fusion? Spine J 21(4):578–585
Barrey C, Roussouly P, Perrin G, Le Huec JC (2011) Sagittal balance disorders in severe degenerative spine. Can we identify the compensatory mechanisms? Eur Spine J, 20 Suppl 5(Suppl 5): 626–33
Lamartina C, Berjano P (2014) Classification of sagittal imbalance based on spinal alignment and compensatory mechanisms. Eur Spine J 23(6):1177–1189
Choi BW, Park JB, Kang JW, Kim DG, Chang H (2019) Influence of atlantoaxial fusion on sagittal alignment of the occipitocervical and subaxial spines in Os odontoideum with atlantoaxial instability. Asian Spine J 13(4):556–562
Tang C, Li GZ, Kang M, Liao YH, Tang Q, Zhong J (2020) Is it suitable to fix the occipito-C2 angle and the posterior occipitocervical angle in a normal range during occipitocervical fusion? Clin Spine Surg 33(7):E342-e351
Acknowledgements
We are grateful to Haijun Tian and Xiao Yang for their reading and advice on the manuscript.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Changqing Zhao.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because the biological information and data of the imaging data used in the study have been de-labelled, which meant they were not individually identifiable and traceable. Exemption from obtaining the informed consent of the subject would not have a negative impact on the rights and interests of the subject.
Ethical approval
This study was approved by the ethics review committee of our hospital (SH9H-2021-T459-2). The biological information and imaging data used in the study have been anonymised, which means they are not individually identifiable or traceable. Exemption from obtaining informed consent from the subjects will not have a negative impact on the rights or interests of the subjects.
Methodology
• Retrospective
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Du, H., Cheng, X. et al. Axial superior facet slope may determine anterior or posterior atlantoaxial displacement secondary to os odontoideum and compensatory mechanisms of the atlantooccipital joint and subaxial cervical spine. Eur Radiol 33, 5606–5614 (2023). https://doi.org/10.1007/s00330-023-09544-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09544-w