Abstract
Objectives
To evaluate the CT scores and fibrotic pattern changes in interstitial lung disease (ILD) patients, with and without previous COVID-19 pneumonia.
Methods
Patients with ILD (idiopathic pulmonary fibrosis (IPF) and connective tissue disease-associated ILD (CTD-ILD)) were retrospectively enrolled in the study which consisted of patients who had COVID-19 pneumonia while the control group had not. All patients had two CT scans, initial and follow-up, which were evaluated semi-quantitatively for severity, extent, and total CT scores, fibrosis patterns, and traction bronchiectasis.
Results
A total of 102 patients (pneumonia group n = 48; control group n = 54) were enrolled in the study. For both groups, baseline characteristics were similar and CT scores were increased. While there was a 4.5 ± 4.6 point change in the total CT score of the COVID-19 group, there was a 1.2 ± 2.7 point change in the control group (p < 0.001). In the IPF subgroup, the change in total CT score was 7.0 points (95% CI: 4.1 to 9.9) in the COVID-19 group and 2.1 points (95% CI: 0.8 to 3.4) in the control group. Seven patients (14.6%) in the COVID-19 group progressed to a higher fibrosis pattern, but none in the control group.
Conclusions
Semi-quantitative chest CT scores in ILD patients demonstrated a significant increase after having COVID-19 pneumonia compared to ILD patients who had not had COVID-19 pneumonia. The increase in CT scores was more prominent in the IPF subgroup. There was also a worsening in the fibrosis pattern in the COVID-19 group.
Key Points
• The impact of COVID-19 pneumonia on existing interstitial lung diseases and fibrosis is unclear.
• COVID-19 pneumonia may worsen existing interstitial lung involvement with direct lung damage and indirect inflammatory effect.
• COVID-19 pneumonia may affect existing lung fibrosis by triggering inflammatory pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease 2019 (COVID-19) has become a global pandemic, and the World Health Organisation (WHO) declared it a public health emergency of international concern in January 2020 [1]. Computed tomography (CT) most commonly reveals ground-glass opacities (GGOs) or consolidations in a peripheral and subpleural distribution in the acute phase of the disease [2, 3]. The extent of lung involvement is dependent on the degree of underlying inflammation, with greater inflammation associated with a worse outcome [4,5,6].
The recovery process is variable after COVID-19 pneumonia and the radiologic changes have been described in both early and late courses although the prevalence and abnormalities vary depending on the interval after infection, the severity of initial illness, comorbidities, and the study population [3, 7, 8]. The most common residual abnormalities at follow-up CT were interlobular septal thickening, focal/multifocal GGOs, subpleural parenchymal bands, and fibrotic-like changes including traction bronchiectasis, and parenchymal distortion and/or honeycombing [7, 9].
Interstitial lung disease (ILD) is a large group of diffuse lung diseases characterized by similar clinical, pathological, and radiological features. CT is a high-sensitivity imaging tool for the assessment of the severity and extent of lung changes and fibrosis. Semi-quantitative CT scoring analysis in ILD has the potential to standardize the role of imaging in ILD and correlates well with the mortality rate [10,11,12].
Superimposing COVID-19 pneumonia on pre-existing ILD may complicate the radiological picture. There is limited information concerning whether COVID-19 pneumonia changes the expected radiological findings during the follow-up of ILD. This is a critical but difficult issue due to the potential long-term sequelae of COVID-19 pneumonia to simulate fibrosis, secondary to ILD.
The purpose of this study was to investigate if subsequent COVID-19 pneumonia causes a change in the radiological appearance of the pre-existing ILD findings and leads to a progression in severity and extent of the ILD disease beyond the expected state.
Materials and methods
Study design
This was a cross-sectional study of retrospectively collected single-center data. All patients with previous ILD and diagnosed with definite COVID-19 pneumonia at the inpatient and outpatient clinics of a tertiary care facility between March 2020 and January 2022 were enrolled in the study. Ethical committee approval was obtained before the study started.
Study protocol
ILD patients were diagnosed according to the latest international consensus guideline and were separated into those with idiopathic pulmonary fibrosis (IPF) and connective tissue disease-associated ILD (CTD-ILD) [13, 14]. Medical records of these patients including demographic characteristics (age, gender), comorbidities, hospitalization status, need for oxygen therapy, and need for intensive care unit were evaluated.
The study cohort consists of two groups. The COVID-19 group consisted of pre-existing ILD patients with subsequent COVID-19 pneumonia, designated the study group, and the control group included ILD patients without COVID-19 pneumonia. The cases with positive real-time fluorescence polymerase chain reaction (PCR) test results according to the WHO COVID-19 case definition sheet were defined as confirmed COVID-19 cases [15]. PCR test data were identified using the local hospital and national electronic health recording system. The COVID-19 group included adult patients who had confirmed diagnoses of COVID-19 and had two serial CT scans. All patients underwent the initial CT examination within 18 months before COVID-19 pneumonia diagnosis. The duration from the onset of COVID-19 pneumonia to follow-up CT was between four months to one year. The control group included adult patients with two serial CT scans, the time between the initial and follow-up CT scans was at most three years. Patients were selected from the period before the COVID-19 pandemic. Those patients were randomly chosen from the local hospital picture archiving and communications system between 2017 and 2019 before the pandemic period.
Patients were excluded if they did not have at least two serial CT scans or if the CT scans were insufficient for interpretation.
CT technique and interpretation
Patients underwent chest CT with a 64-detector scanner (Aquillion 64; Canon Medical Systems—previously Toshiba Medical Systems) in a supine position with full inspiration. Scanning parameters were as follows: 120 kV, variable mAs according to the patient size using an automatic exposure control system, slice thickness of 1 mm, and an average CTDIvol 21 mGy. The images were reconstructed in a 512 × 512 matrix with 1 mm non-overlapping slices, applying a standard high-resolution CT reconstruction algorithm. Prone scans were obtained when there were gravitational opacities in limited patients. All images were viewed with a window level of − 600 Hounsfield units (HU) and a width of 1.600 HU. Images were reconstructed with a lung parenchyma FC52 kernel and a mediastinal FC13 kernel. No iterative reconstruction techniques were applied. The CT images were interpreted independently by two thoracic radiologists, one with 15 and the other with 4 years of experience in thoracic radiology.
Both the initial and follow-up CT images in COVID-19 and control subgroups were assessed with a semi-quantitative method proposed by Warrick et al [16]. The severity score was assessed by recording five elementary lesions. These were GGOs, irregularities in the pleural margins, septal lines, honeycombing, and subpleural cysts. The basic CT terminology for elementary lesions described by the Fleischner Society glossary was used [17].
Each elementary lesion was rated from 1 to 5 and a total severity score was generated by summing the values for each lesion observed on CT images. The severity score had a possible range of 0 (no elementary lesions) to 15 (all elementary lesions present). The extent score was assessed according to the number of segments involved for each elementary lesion. Each lesion type was assigned a score from 1 to 3 where 1 indicated that the lesion was present in one to three segments, 2 indicated that the lesion was present in four to nine segments, and 3 indicated that the lesion was present in more than nine segments. The extent score had a possible range of 0–15 according to the quantification. A total Warrick score was then calculated by adding up the severity and extent scores. The total Warrick score has a range between 0 and 30. The criteria used for calculating the Warrick score are shown in Table 1.
In addition to the Warrick scoring system, we also evaluated traction bronchiectasis and fibrosis patterns. We defined the fibrosis pattern by using standard terminology to categorise the usual interstitial pneumonia (UIP) pattern in lung tissue: definite UIP, probable UIP, indeterminate for UIP, and features most likely consistent with an alternative diagnosis [18, 19].
Outcome measures
The primary outcome measure in this study was the change in the difference between the initial and follow-up total CT scores in both groups. Secondary outcome measures were the changes in individual severity and extent scores in COVID-19 and control groups and the effect of having COVID-19 in patients with IPF or without a diagnosis of IPF.
Statistical analysis
Data analysis of the current study was performed using SPSS for Windows, version 15.0. (SPSS Inc.). The Kolmogorov–Smirnov test was used to test the normal distribution of continuous variables. Continuous variables with normal distribution were tested using the Independent samples t-test for independent variables and paired samples t-test for dependent variables. Data is presented as mean and standard deviation (SD). Non-parametric calculations for continuous data were performed using the Mann–Whitney U test and data were expressed as medians and interquartile range (IQR). Categorical variables were tested using the chi-square test. Mean differences were expressed with 95% confidence intervals (CIs). All the statistical analyses were two-sided. An alpha value of 0.05 was considered to be the nominal level of significance.
Results
Baseline characteristics
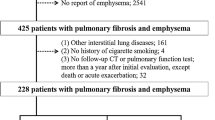
A total of 160 patients were evaluated for enrolment in the study. After the exclusion criteria were implemented, 102 patients were included, with 48 (47.1%) in the COVID-19 group and 54 (52.9%) in the control group. The COVID-19 group flowchart is shown in Fig. 1. In the COVID-19 group, 15 (31.3%) patients were diagnosed with IPF, and 33 patients (68.7%) were diagnosed with CTD-ILD. In the control group, 27 patients (50%) were diagnosed with IPF, and 27 patients (50%) were diagnosed with CTD-ILD. CTD-ILD patients included rheumatoid arthritis, scleroderma, systemic lupus erythematosus, Sjögren’s syndrome, and mixed connective tissue disease. The median interstitial disease duration from diagnosis was 60 months (IQR: 39–60) for the COVID-19 group and 48 months (IQR: 36–48) for the control group.
Table 2 depicts the characteristics of the study population in terms of age, gender, diagnosis of the patients, and their distribution according to COVID-19 status. The COVID-19 and the control groups were well balanced in terms of gender. The mean age of the control group was 68 ± 9 years, whereas the COVID-19 group was younger with a mean age of 63 ± 12 years (p = 0.022). GGOs, subpleural reticulations, and traction bronchiectasis were more frequently seen in patients with COVID-19 (Table 2).
A summary of comorbidities is depicted in Table 3. About 52% of all patients had comorbidities. The most prevalent comorbidity was hypertension (50%), followed by diabetes mellitus (21.5%). Analysis by stratifying patients according to their comorbidities did not reveal significant differences in any category between COVID-19 and control groups. No co-infection was detected in any of the patients in both groups.
Of the COVID-19 group, 81.2% had a history of hospitalization and 50% had oxygen therapy during hospitalization. Only two patients required non-invasive mechanical ventilation and were therefore admitted to the intensive care unit.
Comparison of CT findings and scores
The median (IQR) time interval between initial and follow-up CT scans was 17 (13–24) months for the COVID-19 group and 13 (9–22) months for the control group (p = 0.011). In the COVID-19 group, the patients underwent follow-up CT scans 4–9 months after positive PCR testing, with 85.4% of the CT examinations being obtained after 6 months.
The severity, extent, and total CT scores in the follow-up scans were significantly higher in both groups compared with the baseline CT scores (Table 4). There was a 4.5 ± 4.6 point change in the total CT score of the COVID-19 group and there was a 1.2 ± 2.7 point change in the control group. The difference in the increases was significant (p < 0.001) (Fig. 2). There was also a difference in the scores for both severity and extent between the two groups. Patients with IPF constituted 15 (31.25%) of the COVID-19 group and 27 (50%) of the control group. The change in total CT score in IPF patients in the COVID-19 group was 7.0 points (95% CI: 4.1 to 9.9), while the change in total CT score in the control group was 2.1 points (95% CI: 0.8 to 3.4). Table 5 illustrates changes in individual scores in IPF and CTD-ILD patients. Changes in the initial and follow-up total scores using COVID-19 status are shown in Fig. 3a and b.
The pattern of fibrosis changed in seven patients (14.6%) in the COVID-19 group. The indeterminate UIP pattern changed to probable UIP in 4/7 (57.1%) patients, while in the remaining three patients probable UIP pattern changed to a definite UIP pattern in 1, and normal scans changed to probable (n = 1) and indeterminate (n = 1) UIP patterns. The CT pattern did not change in any of the patients in the control group (Fig. 4).
A 64-year-old man with idiopathic pulmonary fibrosis (IPF) presenting with progressive dyspnea after COVID-19 pneumonia. a Baseline CT images obtained 2 months before COVID-19 pneumonia show peripheral reticulations and minimal traction bronchiectasis (arrow) in the lower lobes consistent with a probable UIP pattern. The Warrick CT scores were; severity score:6, extent score:9, total score: 15. b Follow-up CT images obtained 8 months after COVID-19 pneumonia show more prominent traction bronchiectasis and new subpleural cyst (arrow). The Warrick CT scores were progressed in the follow-up images; severity score:11, extent score:12, total score: 23
Discussion
Serial CT scanning plays a key role in ILD patients for monitoring progression, during follow-up, and assessing prognosis. Semi-quantitative CT scoring analysis has been reported to be more reliable than visual qualitative assessment in this setting [11, 20]. In our study, all semi-quantitative CT scores were increased between the initial and follow-up CTs in both the COVID-19 and the control groups. However, the increase in the COVID-19 group was found to be significantly greater than in the control group. The increase in CT scores was most prominent in the group of IPF patients with a history of COVID-19 pneumonia.
The natural course of progression in ILD patients is variable [21]. It is known that viral agents can play a triggering role in autoimmune diseases and change the course [22]. Many different viral agents may play a role in the etiology and progression of pulmonary fibrosis and IPF acute exacerbation [23,24,25,26,27]. The interaction of COVID-19, caused by the SARS-CoV-2 virus, with pre-existing ILD, has been investigated since the beginning of the pandemic, and the virus is thought to destruct the lung tissue by creating an inflammatory response in the form of immune-mediated injury as well as by direct damage [7, 9]. It appears that ILD patients are more susceptible to contracting COVID-19, and more likely to have severe disease with fatal outcomes [28,29,30,31]. Lee et al [29] reported in their 8070 patients cohort that patients with pre-existing ILD are more prone to develop symptomatic COVID-19 disease and more likely to have worse outcomes (49.3% versus 13.1%, adjusted OR 2.32, 95% CI 1.24–4.01). Similar data were noted by Drake et al [30] that patients with pre-existing ILD but particularly those with a diagnosis of IPF rather than CTD-ILD were at very high risk of death when hospitalized with COVID-19. Although the mechanisms leading to a worse prognosis are not clearly known, reduced lung reserve, impaired gas exchange, and poor pulmonary functions in ILD patients may be the most predictable causes. The increase in SARS-CoV-2 entry genes such as angiotensin-converting enzyme-2, interleukin-6, and type 1 interferon response genes, as well as the increase of avb6 integrin levels in the alveolar epithelium, which is associated with a poor prognosis, especially in IPF patients, can be other factors of the damage in the lung parenchyma in COVID-19 patients [31]. Damaged lung parenchyma may be associated with persistent symptoms and functional impairment in patients after infection.
Long-term sequelae of COVID-19 pneumonia can be classified as reversible and ‘fibrotic like’ irreversible changes. GGOs and subpleural reticulations constitute most of the reversible changes and the incidence has been reported at different rates ranging from 7 to 92%. It may take up to one year for GGOs to disappear completely [32,33,34]. In our study, GGOs and subpleural reticulations were found to be increased, being more prominent in the COVID-19 group. Progression in GGOs was observed in 52% of patients in the COVID-19 group and 20% in the control group. Newly developed GGOs were not in the form of separate areas of involvement in most patients in both groups but were largely superposed to reticulations and fibrosis. It is radiologically impossible to say whether this increase was due to COVID-19 pneumonia or to the progression of interstitial disease. To understand the cause of the increased GGOs, whether by viral pneumonia or interstitial disease, it would be helpful to compare the opacities that develop acutely during pneumonia and in the control CTs. This was not possible in our study, as CT imaging was not performed in all patients during episodes of acute pneumonia.
Irreversible sequelae changes developing after COVID-19 pneumonia are generally termed ‘fibrotic-like’. The presence of parenchymal bands, reticulations, traction bronchiectasis, and/or honeycombing have been described as defining features [8, 10, 28].
In our study, we examined fibrosis pattern changes, in addition to CT scoring, to identify fibrotic changes. When fibrosis patterns were evaluated, none of the patients in the control group showed any change, whereas seven patients in the COVID-19 group showed pattern change, which may indicate a worsening of fibrosis.
‘Fibrotic-like’ changes are considered somewhat confusing lesions as there are not enough histopathological data yet for robust classification and the long-term outcome of the lesions is not yet known. The rates given for ‘fibrotic-like’ changes vary between 13 and 27% [8, 10, 35, 36]. These rates vary according to the comorbidities of the patients, the severity of COVID-19 pneumonia, and the duration of control imaging [35]. In the literature, these ‘fibrotic-like’ changes have been described mostly in moderate-severe COVID-19 patients who usually required intensive care unit admission [8, 10, 28]. In contrast, our COVID-19 patient group consisted of mild-to-moderate pneumonia clinically, and only two had severe COVID-19 pneumonia and needed intensive care unit admission. Thus, it seems reasonable to assign the increased fibrotic changes in our patient group to the coexistence of COVID-19 and ILD.
Traction bronchiectasis was evaluated separately because it was not considered an elementary lesion in the Warrick scoring system and is an important CT feature in the radiological definition of fibrosis. A significant progression was detected in the COVID-19 group in traction bronchiectasis in follow-up CTs.
Drake et al [30] discussed treatment differences in hospitalized COVID-19 patients with ILD. Their study showed that more patients with ILD received oral corticosteroids in the hospital than patients without ILD and there was no significant difference in outcome between those receiving corticosteroids or anti-fibrotic therapy and those who did not. We did not evaluate and compare patients’ medications in terms of corticosteroids or anti-fibrotic drugs. In the future, it may be valuable to study the long-term effects of corticosteroids or anti-fibrotic therapy on fibrotic progression in ILD patients.
Our study had the following limitations. Firstly, a prospective design for the study would be more appropriate to set for more accurate time intervals in CT scanning. The control CT imaging was performed within 6 months after pneumonia in 10.4% of the COVID-19 patients. Although the number of these patients undergoing control CT within 6 months was very low, this may still be a cause of false-positive evaluation of GGO and reticulation increase. This can lead to misjudgment in pattern change. The mean time difference between initial and follow-up CTs between the two groups was 4 months. However, considering that ILD patients are often followed up with annual CTs, this time difference may not be considered long enough to explain the observed CT score increase. Secondly, the study was conducted in a single center with a limited sample size, which may limit its generalizability. Lastly, it may also be useful to support the radiological findings observed in ILD patients with detailed clinical, histopathological, and immunological data. Since we do not have complete clinical and radiological data for the period when patients had COVID-19, we could not include information on the severity of pneumonia at that time. However, considering that some patients did not have a CT scan when they had COVID-19, it can be speculated that they may have had a relatively mild disease.
In conclusion, a significant increase in CT scores was detected in a semi-quantitative analysis of follow-up CTs of ILD patients who had COVID-19 pneumonia compared to the group without pneumonia. Worsening of CT scores and fibrosis patterns was more prominent in IPF patients. Most of these patients had a mild to moderate clinical course of acute COVID-19 pneumonia. COVID-19 pneumonia may accelerate the progression of pre-existing interstitial involvement in ILD patients.
Abbreviations
- COVID-19:
-
Coronavirus Disease 2019
- CTD-ILD:
-
Connective tissue disease-associated ILD
- GGOs:
-
Ground-glass opacities
- HU:
-
Hounsfield units
- ILD:
-
Interstitial lung disease
- IPF:
-
Idiopathic pulmonary fibrosis
- IQR:
-
Interquartile range
- PCR:
-
Real-time fluorescence polymerase chain reaction
- SD:
-
Standard deviation
- UIP:
-
Usual interstitial pneumonia
- WHO:
-
World Health Organisation
References
Web site: https://covid19.who.int/ (Accessed on: July 10, 2022)
Wilson N, Kvalsvig A, Barnard LT, Baker MG (2020) Case-fatality risk estimates for COVID-19 calculated by using a lag time for fatality. Emerg Infect Dis 26:1339–1441. https://doi.org/10.3201/eid2606.200320
Hu Q, Guan H, Sun Z et al (2020) Early CT features and temporal lung changes in COVID-19 pneumonia in Wuhan. China. Eur J Radiol 128:109017. https://doi.org/10.1016/j.ejrad.2020.109017
Henkel M, Weikert T, Marston K et al (2020) Lethal COVID-19: radiological-pathological correlation of the lungs. Radiol Cardiothorac Imaging 2:200406. https://doi.org/10.1148/ryct.2020200406
Solomon JJ, Heyman B, Ko JP, Condos R, Lynch DA (2021) CT of post-acute lung complications of COVID-19. Radiology 301:383–395. https://doi.org/10.1148/radiol.2021211396
Do TD, Skornitske S, Merle U (2022) Covid-19 pneumonia: prediction of patient outcome by CT-based quantitative lung parencyhma analysis combined with laboratory parameters. PLoS One 17:e0271787. https://doi.org/10.1371/journal.pone.0271787
Han X, Fan Y, Alwalid O et al (2021) Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology 299:177–186. https://doi.org/10.1148/radiol.2021203153
Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B (2022) Global prevalence of post-acute sequelae of COVID-19 (PASC) or long COVID: a meta-analysis and systematic review. J Infect Dis 226:1593–1607. https://doi.org/10.1093/infdis/jiac136
Caruso D, Guido G, Zerunian M et al (2021) Post-acute sequelae of COVID-19 pneumonia: 6-month chest CT follow-up. Radiology 301:396–405. https://doi.org/10.1148/radiol.2021210834
Park YS, Seo JB, Kim N et al (2008) Texture-based quantification of pulmonary emphysema on high-resolution computed tomography: comparison with density-based quantification and correlation with pulmonary function test. Invest Radiol 43:395–402. https://doi.org/10.1097/RLI.0b013e31816901c7
Weatherley ND, Eaden JA, Stewart NJ et al (2019) Experimental and quantitative imaging techniques in interstitial lung disease. Thorax 74:611–619. https://doi.org/10.1136/thoraxjnl-2018-211779
Yilmazer B, Gumustas S, Cosan F et al (2016) High-resolution computed tomography and rheumatoid arthritis: semi-quantitative evaluation of lung damage and its correlation with clinical and functional abnormalities. Radiol Med 121:181–189. https://doi.org/10.1007/s11547-015-0590-5
Travis WD, Costabel U, Hansell DM et al (2013) An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188:733–748. https://doi.org/10.1164/rccm.201308-1483ST
Kondoh Y, Makino S, Ogura T et al (2021) 2020 guide for the diagnosis and treatment of interstitial lung disease associated with connective tissue disease. Respir Investig 59:709–740. https://doi.org/10.1016/j.resinv.2021.04.011
WHO /2019-nCoV/Surveillance Case_Definition/2020.2 (Accessed on: 27.12.2020)
Warrick JH, Bhalla M, Schabel SI, Silver RM (1991) High-resolution computed tomography in early scleroderma lung disease. J Rheumatol Oct 18:1520–1528 PMID: 1765976
Hansell DM, Bankier AA, MacMahon H, McLoud T, Müller NL, Remy J (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246:697–722. https://doi.org/10.1148/radiol.2462070712
Lynch DA, Sverzellati N, Travis WD et al (2018) Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med 6:138–153. https://doi.org/10.1016/S2213-2600(17)30433-2
Raghu G, Collard HR, Egan JJ et al (2011) An Official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183:788–824. https://doi.org/10.1164/rccm.2009-040GL
Walsh SLF, Devaraj A, Enghelmayer JI et al (2018) Role of imaging in progressive-fibrosing interstitial lung diseases. Eur Respir Rev 27:180073. https://doi.org/10.1183/16000617.0073-2018
Martinez FJ, Safrin S, Weycker D et al (2005) The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med 142:963–967. https://doi.org/10.7326/0003-4819-142-12_part_1-200506210-00005
Hussein HM, Rahal EA (2019) The role of viral infections in the development of autoimmune diseases. Crit Rev Microbiol 45:394–412. https://doi.org/10.1080/1040841X.2019.1614904
Moore BB, Moore TA (2015) Viruses in idiopathic pulmonary fibrosis. Etiology and Exacerbation. Ann Am Thorac Soc 12:186–192. https://doi.org/10.1513/AnnalsATS.201502-088AW
Ueda T, Ohta K, Suzuki N et al (1992) Idiopathic pulmonary fibrosis and high prevalence of serum antibodies to hepatitis C virus. Am Rev Respir Dis 146:266–268. https://doi.org/10.1164/ajrccm/146.1.266
Arase Y, Suzuki F, Suzuki Y et al (2008) Hepatitis C virus enhances incidence of idiopathic pulmonary fibrosis. World J Gastroenterol 14:5880–5886. https://doi.org/10.3748/wjg.14.5880
Hayashi S, Hogg JC (2007) Adenovirus infections and lung disease. Curr Opin Pharmacol 7:237–243. https://doi.org/10.1016/j.coph.2006.11.014
Wells AU, Devaraj A, Desai SR (2021) Interstitial lung disease after COVID-19 infection: a catalog of uncertainties. Radiology 299:216–218. https://doi.org/10.1148/radiol.2021204482
Esposito AJ, Menon AA, Ghosh AJ et al (2020) Increased odds of death for patients with interstitial lung disease and COVID-19: a case–control study. Am J Respir Crit Care Med 202:1710–1713. https://doi.org/10.1164/rccm.202006-2441LE
Lee H, Choi H, Yang B et al (2021) Interstitial lung disease increases susceptibility to and severity of COVID-19. Eur Respir J 58:2004125. https://doi.org/10.1183/13993003.04125-2020
Drake TM, Docherty AB, Harrison EM et al (2020) Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. An International Multicenter Study. Am J Respir Crit Care Med 202:1656–1665. https://doi.org/10.1164/rccm.202007-2794OC
Kondoh Y, Kataoka K, Ando M et al (2021) COVID-19 and acute exacerbation of interstitial lung disease. Respir Investig 59:675–678. https://doi.org/10.1016/j.resinv.2021.06.007
Wu X, Liu X, Zhou Y et al (2021) 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med 9:747–754. https://doi.org/10.1016/S2213-2600(21)00174-0
Huang C, Huang L, Wang Y et al (2021) 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397:220–232. https://doi.org/10.1016/S0140-6736(20)32656-8
Frija-Masson J, Debray MP, Boussouar S et al (2021) Residual ground glass opacities three months after Covid-19 pneumonia correlate to alteration of respiratory function: the post Covid M3 study. Respir Med 184:106435. https://doi.org/10.1016/j.rmed.2021.106435
Montani D, Savale L, Noel N et al (2022) Post-acute COVID-19 syndrome. Eur Respir Rev 31:210185. https://doi.org/10.1183/16000617.0185-2021
Zhao YM, Shang YM, Song WB et al (2020) Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. Clin Med 25:100463. https://doi.org/10.1016/j.eclinm.2020.100463
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is the Ethical Review Committee of Kocaeli University, Faculty of Medicine, Kocaeli, Turkey.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
-
retrospective
-
cross-sectional study
-
performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Doğan, S., Güldiken, G.S., Alpaslan, B. et al. Impact of COVID-19 pneumonia on interstitial lung disease: semi-quantitative evaluation with computed tomography. Eur Radiol 33, 4758–4766 (2023). https://doi.org/10.1007/s00330-023-09441-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09441-2