Abstract
Objective
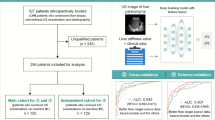
To develop and investigate a deep learning model with data integration of ultrasound contrast-enhanced micro-flow (CEMF) cines, B-mode images, and patients’ clinical parameters to improve the diagnosis of significant liver fibrosis (≥ F2) in patients with chronic hepatitis B (CHB).
Methods
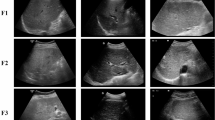
Of 682 CHB patients who underwent ultrasound and histopathological examinations between October 2016 and May 2020, 218 subjects were included in this retrospective study. We devised a data integration-based deep learning (DIDL) model for assessing ≥ F2 in CHB patients. The model contained three convolutional neural network branches to automatically extract features from ultrasound CEMF cines, B-mode images, and clinical data. The extracted features were fused at the backend of the model for decision-making. The diagnostic performance was evaluated across fivefold cross-validation and compared against the other methods in terms of the area under the receiver operating characteristic curve (AUC), with histopathological results as the reference standard.
Results
The mean AUC achieved by the DIDL model was 0.901 [95% CI, 0.857–0.939], which was significantly higher than those of the comparative methods, including the models trained by using only CEMF cines (0.850 [0.794–0.893]), B-mode images (0.813 [0.754–0.862]), or clinical data (0.757 [0.694–0.812]), as well as the conventional TIC method (0.752 [0.689–0.808]), APRI (0.792 [0.734–0.845]), FIB-4 (0.776 [0.714–0.829]), and visual assessments of two radiologists (0.812 [0.754–0.862], and 0.800 [0.739–0.849]), all ps < 0.01, DeLong test.
Conclusion
The DIDL model with data integration of ultrasound CEMF cines, B-mode images, and clinical parameters showed promising performance in diagnosing significant liver fibrosis for CHB patients.
Key Points
• The combined use of ultrasound contrast-enhanced micro-flow cines, B-mode images, and clinical data in a deep learning model has potential to improve the diagnosis of significant liver fibrosis.
• The deep learning model with the fusion of features extracted from multimodality data outperformed the conventional methods including mono-modality data-based models, the time-intensity curve-based recognizer, fibrosis biomarkers, and visual assessments by experienced radiologists.
• The interpretation of the feature attention maps in the deep learning model may help radiologists get better understanding of liver fibrosis-related features and hence potentially enhancing their diagnostic capacities.






Similar content being viewed by others
Data Availability
Data were available upon reasonable request to the corresponding authors.
Change history
24 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00330-023-09817-4
Abbreviations
- ≥ F2:
-
Significant liver fibrosis
- APRI:
-
Aspartate transaminase to platelet ratio index
- AUC :
-
Area under the receiver operating characteristic curve
- CA:
-
Contrast agent
- CEMF:
-
Contrast-enhanced micro-flow
- CHB:
-
Chronic hepatitis B
- CI :
-
Confidence interval
- CT:
-
Computed tomography
- DIDL:
-
Data integration-based deep learning
- FC:
-
Fully connected
- FIB-4:
-
Fibrosis index based on four factors
- MR:
-
Magnetic resonance
- ROC:
-
Receiver operating characteristic
- ROI :
-
Region of interest
- SD:
-
Standard deviation
- TIC:
-
Time-intensity curve
- US:
-
Ultrasound
References
Vittal A, Ghany MG (2019) WHO guidelines for prevention, care and treatment of individuals infected with HBV A US perspective. Clin Liver Dis 23(3):417–432
Lampertico P, Agarwal K, Berg T et al (2017) EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 67(2):370–398
Terrault NA, Lok ASF, McMahon BJ et al (2018) Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 67(4):1560–1599
Tan M, Bhadoria AS, Cui F et al (2021) Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: a systematic review and meta-analysis. Lancet Gastroenterol 6(2):106–119
Ferraioli G, Wong VW-S, Castera L et al (2018) Liver ultrasound elastography: an update to the World Federation for Ultrasound in Medicine and Biology guidelines and recommendations. Ultrasound Med Biol 44(12):2419–2440
Lee YA, Wallace MC, Friedman SL (2015) Pathobiology of liver fibrosis: a translational success story. Gut 64(5):830–841
European Association for the Study of the Liver (2017) EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 67(2):370–398
Wong SN, Lok AS (2006) Treatment of hepatitis B: who, when, and how? Arch Intern Med 166(1):9–12
Regev A, Berho M, Jeffers LJ et al (2002) Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 97(10):2614–2618
Bedossa P, Dargere D, Paradis V (2003) Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 38(6):1449–1457
Bravo AA, Sheth SG, Chopra S (2001) Current concepts: liver biopsy. N Engl J Med 344(7):495–500
Zheng R-Q, Wang Q-H, Lu M-D et al (2003) Liver fibrosis in chronic viral hepatitis: an ultrasonographic study. World J Gastroenterol 911:2484
Colli A, Fraquelli M, Andreoletti M et al (2003) Severe liver fibrosis or cirrhosis: accuracy of US for detection—analysis of 300 cases. Radiology 227(1):89–94
Salvatore V, Borghi A, Peri E et al (2012) Relationship between hepatic haemodynamics assessed by Doppler ultrasound and liver stiffness. Dig Liver Dis 44(2):154–159
Wai CT, Greenson JK, Fontana RJ et al (2003) A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38(2):518–526
Sterling RK, Lissen E, Clumeck N et al (2006) Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43(6):1317–1325
Wang K, Lu X, Zhou H et al (2019) Deep learning radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut 68(4):729–741
Lee JH, Joo I, Kang TW et al (2020) Deep learning with ultrasonography: automated classification of liver fibrosis using a deep convolutional neural network. Eur Radiol 30(2):1264–1273
Anteby R, Klang E, Horesh N, et al 2021 Deep learning for noninvasive liver fibrosis classification: a systematic review. Liver Int 41(10):2269–2278
Lu X, Zhou H, Wang K, et al 2021 Comparing radiomics models with different inputs for accurate diagnosis of significant fibrosis in chronic liver disease. Eur Radiol 31(11):8743–8754
Li W, Huang Y, Zhuang B-W et al (2019) Multiparametric ultrasomics of significant liver fibrosis: a machine learning-based analysis. Eur Radiol 29(3):1496–1506
Claudon M, Dietrich CF, Choi BI et al (2013) Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver—update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol 39(2):187–210
Li W, Wang W, Liu GJ et al (2015) Differentiation of atypical hepatocellular carcinoma from focal nodular hyperplasia: diagnostic performance of contrast-enhanced US and microflow imaging. Radiology 275(3):870–879
Hui AY, Chan HL, Wong VW et al (2005) Identification of chronic hepatitis B patients without significant liver fibrosis by a simple noninvasive predictive model. Am J Gastroenterol 100(3):616–623
Zeng MD, Lu LG, Mao YM et al (2005) Prediction of significant fibrosis in HBeAg-positive patients with chronic hepatitis B by a noninvasive model. Hepatology 42(6):1437–1445
Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24(2):289–293
Yushkevich PA, Piven J, Hazlett HC et al (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31(3):1116–1128
Tran D, Bourdev L, Fergus R, Torresani L, Paluri M. 2015 Learning spatiotemporal features with 3d convolutional networks. Proceedings of the IEEE international conference on computer vision. 4489–4497
He K, Zhang X, Ren S, Sun J. 2016 Deep residual learning for image recognition. Proceedings of the IEEE conference on computer vision and pattern recognition. 770–778
Hung CH, Lu SN, Wang JH et al (2003) Correlation between ultrasonographic and pathologic diagnoses of hepatitis B and C virus-related cirrhosis. J Gastroenterol 38(2):153–157
Clopper CJ, Pearson ES (1934) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26(4):404–413
Delong ER, Delong DM, Clarkepearson DI (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Selvaraju RR, Cogswell M, Das A et al (2020) Grad-CAM: visual explanations from deep networks via gradient-based localization. Int J Comput Vision 128(2):336–359
Sugimoto K, Shiraishi J, Moriyasu F et al (2010) Analysis of intrahepatic vascular morphological changes of chronic liver disease for assessment of liver fibrosis stages by micro-flow imaging with contrast-enhanced ultrasound: preliminary experience. Eur Radiol 20(11):2749–2757
Huang S, Liang P, Yu X et al (2018) The application of parametric micro-flow imaging in the evaluation of liver fibrosis. Ultrasound Q 34(3):148–155
Dietrich CF, Nolsøe CP, Barr RG et al (2020) Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver–update 2020–WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultraschall in der Medizin-European Journal of Ultrasound 41(05):562–585
Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD (2009) Liver biopsy. Hepatology 49(3):1017–1044
Neuberger J, Patel J, Caldwell H et al (2020) Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 69(8):1382–1403
Funding
This study has received funding supports from National Natural Science Foundation of China under Grants 81871429, 81971630, and 61901282, and Shenzhen Basic Science Research under Grant JCYJ20210324093006017.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The guarantor of this manuscript is Prof. Xin Chen, Head of School of Biomedical Engineering, Shenzhen University, 1066 Xueyuan Road, Nanshan District, Shenzhen, P. R. China (Email: chenxin@szu.edu.cn).
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Prof. Wei Wang (one of the authors of the manuscript, Email: wangw73@mail.sysu.edu.cn) who is an expert at the First Affiliated Hospital of Sun Yat-Sen University provided statistical advice for this manuscript.
Informed consent
Written informed consent from the patients was exempted due to the retrospective nature of the study.
Ethical approval
Institutional Review Board approval was obtained from the First Affiliated Hospital of Sun Yat-Sen University.
Methodology
• retrospective
• diagnostic study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: due to a typesetting mistake the equal contribution statement "Zhong Liu and Wei Li contibuted equally to this work" was missing from the original article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Z., Li, W., Zhu, Z. et al. A deep learning model with data integration of ultrasound contrast-enhanced micro-flow cines, B-mode images, and clinical parameters for diagnosing significant liver fibrosis in patients with chronic hepatitis B. Eur Radiol 33, 5871–5881 (2023). https://doi.org/10.1007/s00330-023-09436-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09436-z