Abstract
Objectives
To quantify intra-tumor heterogeneity (ITH) in non-small cell lung cancer (NSCLC) from computed tomography (CT) images.
Methods
We developed a quantitative ITH measurement—ITHscore—by integrating local radiomic features and global pixel distribution patterns. The associations of ITHscore with tumor phenotypes, genotypes, and patient’s prognosis were examined on six patient cohorts (n = 1399) to validate its effectiveness in characterizing ITH.
Results
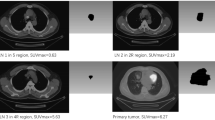
For stage I NSCLC, ITHscore was consistent with tumor progression from stage IA1 to IA3 (p < 0.001) and captured key pathological change in terms of malignancy (p < 0.001). ITHscore distinguished the presence of lymphovascular invasion (p = 0.003) and pleural invasion (p = 0.001) in tumors. ITHscore also separated patient groups with different overall survival (p = 0.004) and disease-free survival conditions (p = 0.005). Radiogenomic analysis showed that the level of ITHscore in stage I and stage II NSCLC is correlated with heterogeneity-related pathways. In addition, ITHscore was proved to be a stable measurement and can be applied to ITH quantification in head-and-neck cancer (HNC).
Conclusions
ITH in NSCLC can be quantified from CT images by ITHscore, which is an indicator for tumor phenotypes and patient’s prognosis.
Key Points
• ITHscore provides a radiomic quantification of intra-tumor heterogeneity in NSCLC.
• ITHscore is an indicator for tumor phenotypes and patient’s prognosis.
• ITHscore has the potential to be generalized to other cancer types such as HNC.





Similar content being viewed by others
Abbreviations
- AAH:
-
Atypical adenomatous hyperplasia
- AIS:
-
Adenocarcinoma in situ
- CPH:
-
Cox proportional hazard
- DE:
-
Differentially expressed
- DFS:
-
Disease-free survival
- DMFS:
-
Distant metastasis-free survival
- GDPH:
-
Guangdong Provincial Peoples’ Hospital
- GSEA:
-
Gene set enrichment analysis
- HNC:
-
Head-and-neck cancer
- HR:
-
Hazard ratio
- IAC:
-
Invasive adenocarcinoma
- ITH:
-
Intra-tumor heterogeneity
- K-M:
-
Kaplan-Meier
- LRFS:
-
Local recurrence-free survival
- LUAD:
-
Lung adenocarcinoma
- LVI:
-
Lymphovascular invasion
- MIA:
-
Minimally invasive adenocarcinoma
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PI:
-
Pleural invasion
- TCIA:
-
The Cancer Imaging Archive
References
Herbst RS, Morgensztern D, Boshoff C (2018) The biology and management of non-small cell lung cancer. Nature 553:446–454. https://doi.org/10.1038/nature25183
Marusyk A, Almendro V, Polyak K (2012) Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer 12:323–334. https://doi.org/10.1038/nrc3261
Jamal-Hanjani M, Quezada SA, Larkin J, Swanton C (2015) Translational implications of tumor heterogeneity. Clin Cancer Res 21:1258–1266. https://doi.org/10.1158/1078-0432.CCR-14-1429
Hammerman PS, Hayes DN, Grandis JR (2015) Therapeutic insights from genomic studies of head and neck squamous cell carcinomas. Cancer Discov 5:239–244. https://doi.org/10.1158/2159-8290.CD-14-1205
O’Sullivan B, Huang SH, Su J et al (2016) Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol 17:440–451. https://doi.org/10.1016/S1470-2045(15)00560-4
McGranahan N, Swanton C (2017) Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168:613–628. https://doi.org/10.1016/j.cell.2017.01.018
Chen Z, Fillmore CM, Hammerman PS et al (2014) Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 14:535–546. https://doi.org/10.1038/nrc3775
Ang KK, Harris J, Wheeler R et al (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35. https://doi.org/10.1056/NEJMoa0912217
Dagogo-Jack I, Shaw AT (2018) Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 15:81–94. https://doi.org/10.1038/nrclinonc.2017.166
de Sousa VML, Carvalho L (2018) Heterogeneity in lung cancer. Pathobiology 85:96–107. https://doi.org/10.1159/000487440
Reck M, Rabe KF (2017) Precision diagnosis and treatment for advanced non–small-cell lung cancer. N Engl J Med 377:849–861. https://doi.org/10.1056/NEJMra1703413
Lee G, Bak SH, Lee HY (2018) CT radiomics in thoracic oncology: technique and clinical applications. Nucl Med Mol Imaging 52:91–98. https://doi.org/10.1007/s13139-017-0506-5
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762. https://doi.org/10.1038/nrclinonc.2017.141
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577. https://doi.org/10.1148/radiol.2015151169
O’Connor JPB, Rose CJ, Waterton JC et al (2015) Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res 21:249–257. https://doi.org/10.1158/1078-0432.CCR-14-0990
Wu J, Aguilera T, Shultz D et al (2016) Early-stage non–small cell lung cancer: quantitative imaging characteristics of 18 F fluorodeoxyglucose PET/CT allow prediction of distant metastasis. Radiology 281:270–278. https://doi.org/10.1148/radiol.2016151829
Song J, Shi J, Dong D et al (2018) A new approach to predict progression-free survival in stage IV EGFR-mutant NSCLC patients with EGFR-TKI therapy. Clin Cancer Res 24:3583–3592. https://doi.org/10.1158/1078-0432.CCR-17-2507
Wu J, Cao G, Sun X et al (2018) Intratumoral spatial heterogeneity at perfusion mr imaging predicts recurrence-free survival in locally advanced breast cancer treated with neoadjuvant chemotherapy. Radiology 288:26–35. https://doi.org/10.1148/radiol.2018172462
Xie C, Yang P, Zhang X et al (2019) Sub-region based radiomics analysis for survival prediction in oesophageal tumours treated by definitive concurrent chemoradiotherapy. EBioMedicine 44:289–297. https://doi.org/10.1016/j.ebiom.2019.05.023
Wu J, Gensheimer MF, Dong X et al (2016) Robust intratumor partitioning to identify high-risk subregions in lung cancer: a pilot study. Int J Radiat Oncol Biol Phys 95:1504–1512. https://doi.org/10.1016/j.ijrobp.2016.03.018
Li J, Lu H, Fang X et al (2019) Pixel-level clustering reveals intra-tumor heterogeneity in non-small cell lung cancer. In: 2019 IEEE international conference on bioinformatics and biomedicine (BIBM). IEEE, San Diego, CA, USA, pp 1536–1539
Aerts HJWL, Velazquez ER, Leijenaar RTH et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006. https://doi.org/10.1038/ncomms5006
Bakr S, Gevaert O, Echegaray S et al (2018) A radiogenomic dataset of non-small cell lung cancer. Sci Data 5:180202. https://doi.org/10.1038/sdata.2018.202
Zhao B, James LP, Moskowitz CS et al (2009) Evaluating variability in tumor measurements from same-day repeat CT scans of patients with non–small cell lung cancer. Radiology 252:263–272. https://doi.org/10.1148/radiol.2522081593
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77:e104–e107. https://doi.org/10.1158/0008-5472.CAN-17-0339
Subramanian A, Tamayo P, Mootha VK et al (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci 102:15545–15550. https://doi.org/10.1073/pnas.0506580102
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. https://doi.org/10.1093/bioinformatics/btp616
Wu T, Hu E, Xu S et al (2021) clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation (Camb) 2:100141. https://doi.org/10.1016/j.xinn.2021.100141
Liberzon A, Birger C, Thorvaldsdóttir H et al (2015) The molecular signatures database hallmark gene set collection. Cell Syst 1:417–425. https://doi.org/10.1016/j.cels.2015.12.004
Travis WD, Brambilla E, Noguchi M et al (2011) International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 6:244–285. https://doi.org/10.1097/JTO.0b013e318206a221
Zhang C, Zhang J, Xu F-P et al (2019) Genomic landscape and immune microenvironment features of preinvasive and early invasive lung adenocarcinoma. J Thorac Oncol 14:1912–1923. https://doi.org/10.1016/j.jtho.2019.07.031
Bedard PL, Hansen AR, Ratain MJ, Siu LL (2013) Tumour heterogeneity in the clinic. Nature 501:355–364. https://doi.org/10.1038/nature12627
Niikawa H, Suzuki T, Miki Y et al (2008) Intratumoral estrogens and estrogen receptors in human non–small cell lung carcinoma. Clin Cancer Res 14:4417–4426. https://doi.org/10.1158/1078-0432.CCR-07-1950
Nieto MA, Huang RY-J, Jackson RA, Thiery JP (2016) EMT: 2016. Cell 166:21–45. https://doi.org/10.1016/j.cell.2016.06.028
Nogueira V, Hay N (2013) Molecular pathways: reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin Cancer Res 19:4309–4314. https://doi.org/10.1158/1078-0432.CCR-12-1424
Napel S, Mu W, Jardim-Perassi BV et al (2018) Quantitative imaging of cancer in the postgenomic era: radio(geno)mics, deep learning, and habitats. Cancer 124:4633–4649. https://doi.org/10.1002/cncr.31630
Traverso A, Wee L, Dekker A, Gillies R (2018) Repeatability and reproducibility of radiomic features: a systematic review. International Journal of Radiation Oncology*Biology*Physics 102:1143–1158. https://doi.org/10.1016/j.ijrobp.2018.05.053
Vaidya P, Bera K, Gupta A et al (2020) CT derived radiomic score for predicting the added benefit of adjuvant chemotherapy following surgery in stage I, II resectable non-small cell lung cancer: a retrospective multicohort study for outcome prediction. Lancet Digit Health 2:e116–e128. https://doi.org/10.1016/S2589-7500(20)30002-9
Shiradkar R, Panda A, Leo P et al (2021) T1 and T2 MR fingerprinting measurements of prostate cancer and prostatitis correlate with deep learning–derived estimates of epithelium, lumen, and stromal composition on corresponding whole mount histopathology. Eur Radiol 31:1336–1346. https://doi.org/10.1007/s00330-020-07214-9
Funding
This work was supported by the National Science Foundation of China (Grant Nos. 61721003 and 81872510), National Key R&D Program of China (2021YFF1200901), High-level Hospital Construction Project (DFJH201801), Guangdong Basic and Applied Basic Research Foundation (No. 2019B1515130002), and Tsinghua-Fuzhou Institute of Data Technology Project (TFIDT2021005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Xuegong Zhang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained. This study was approved by the ethics committee of Guangdong Provincial Peoples’ Hospital (No. GDRHEC2018115H) and was conducted in accordance with ethical standards of the 1964 Helsinki Declaration and its later amendments.
Study subjects or cohorts overlap
This study includes some public patient cohorts available on The Cancer Imaging Archive (TCIA):
• LUNG1 (https://wiki.cancerimagingarchive.net/display/Public/NSCLC-Radiomics)
• R01 (https://wiki.cancerimagingarchive.net/display/Public/NSCLC+Radiogenomics)
• HN1 (https://wiki.cancerimagingarchive.net/display/Public/Head-Neck-Radiomics-HN1)
• RIDER (https://wiki.cancerimagingarchive.net/display/Public/RIDER+Lung+CT).
This study also includes a patient cohort collected from Guangdong Provincial Peoples’ Hospital which was not included in other studies.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 526 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Qiu, Z., Zhang, C. et al. ITHscore: comprehensive quantification of intra-tumor heterogeneity in NSCLC by multi-scale radiomic features. Eur Radiol 33, 893–903 (2023). https://doi.org/10.1007/s00330-022-09055-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09055-0