Abstract
Objective
To determine whether the degree of parenchymal involvement on chest radiograph (CXR) at the time of COVID-19 diagnosis and its early radiologic evolution can predict adverse events including hospitalization, intubation, and death in patients with cancer.
Methods
Retrospective study of 627 COVID-19-positive patients between March and April 2020, of which 248 had baseline CXR within 72 h of diagnosis and 64 patients had follow-up wihtin72 h. CXRs were classified as abnormal (i.e., radiologic findings suggestive of COVID-19 infection were noted), normal, or indeterminate. Baseline and follow-up severity scores were calculated based on lung regions in abnormal CXRs. Statistical analysis was performed to determine associations between abnormal CXR or severity score with adverse events.
Results
Of 248 patients (median age = 65) with a baseline CXR, 172/248 (69%) had an abnormal baseline study, which was associated with hospitalization (p < 0.001), intubation (p = 0.001), and death (p = 0.005). For patients with solid neoplasms, when adjusted for stage, it was associated with hospitalization (p = 0.0002), intubation (p = 0.019), and death (p = 0.03). The median baseline severity score was 3 (range = 1–10); the greater the score, the higher the likelihood of adverse outcome (p < 0.003 for all). A baseline severity score > 9 predicted > 50% probability of intubation and a score of ≥ 10 predicted > 50% of probability of death. The baseline severity score was not correlated with cancer-related treatment. Early radiologic progression was not correlated with hospitalization, intubation, or death.
Conclusion
The degree of parenchymal involvement on CXR within 72 h of COVID-19 diagnosis is associated with adverse outcomes in patients with cancer.
Key Points
• In patients with cancer, the presence and severity of radiologic manifestation of COVID-19 on chest radiographs within 72 h of COVID-19 diagnosis are associated with hospitalization, intubation, and death.
• Early radiologic progression on chest radiographs is not correlated with adverse outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The SARS-CoV-2 virus (COVID-19) pandemic continues with the USA leading in the number of new cases as of September 2020. Patients who are immunocompromised, including patients with cancer, may have an increased risk of infection from COVID-19 as well as a higher risk of adverse events [1,2,3]. Recent evidence from China, Italy, and the USA indicate that patients with both cancer and COVID-19 infection have more severe symptoms and poorer outcomes [3,4,5]. Recently, published data at a tertiary cancer institution in New York City revealed that 20% of patients with cancer diagnosed with COVID-19 demonstrated severe disease with a mortality of 12%, whereas mortality was lower at 7% for a general population with COVID-19 infection who were admitted to an emergency department in the same geographic area [6].
Multiple recent reports have focused on the radiologic features of COVID-19, predominantly those seen on computed tomography (CT) in the general population, with limited information in patients with cancer. Given its wide availability, chest radiographs (CXRs) are the primary imaging modality utilized to evaluate COVID-19 patients. Recent studies have demonstrated that the degree of parenchymal involvement on CXR was predictive of adverse events in a general population of adults [7,8,9].
A recent publication indicated that patients with cancer deteriorated more rapidly than those without, justifying more intensive surveillance in this setting. Therefore, the aim of our study was to determine whether the degree of parenchymal involvement on CXR at the time of diagnosis and its early radiologic evolution can provide valuable information in predicting adverse events including hospitalization, intubation, and death in patients with cancer.
Methods
Study population
Our retrospective study was approved by the institutional review board and was compliant with the Health Insurance Portability and Accountability Act. The need for written informed consent was waived. A total of 745 patients had a confirmed positive test for SARS-CoV-2 by respiratory polymerase chain reaction (PCR) detection at a tertiary cancer center in an urban setting from March 11 to April 20, 2020. Patients without cancer diagnosis were excluded, resulting in 627 cancer patients (Fig. 1). When clinically indicated, patients with a suspected respiratory infection received CXR studies as part of their standard care. We reviewed patients who had a “baseline CXR” performed within 72 h of COVID-19 diagnosis, and similarly, we reviewed the first “follow-up CXR” within 72 h of the initial study. We considered this timeline based on the four stages of COVID-19 evolution on CT described by Pan et al [10], with stage 1 (i.e., early stage) beginning from day 0 and extending to day 4 from the onset of symptoms, and stage 2 (i.e., early progressive stage, with CT scans demonstrating a significant increase in the number of lesions) from day 5 to day 8. To our knowledge, no staging of the disease has been reported on CXR. Thus, based on their classification of stages, we wanted to include the initial radiologic assessment (within the early stage) and the follow-up assessment (within the late early stage or early progressive stage), with the aim of assessing if early changes on CXR in the early stages of COVID-19 disease are associated with adverse events.
Exclusion criteria included patients without a cancer diagnosis, patients with CXRs performed after 72 h of COVID-19 diagnosis, and patients with indeterminate CXRs due to extensive background disease (Fig. 1).
Some of the patients included in our study population were previously described in the supplemental material of a prior publication analyzing clinical determinants for the severity of COVID-19 in patients with cancer. Their study population included 423 patients, of whom 207 had a CXR; however, radiologic findings were not included in their outcome analysis [6].
Clinical parameters
The electronic medical record was reviewed up to 3 months after COVID-19 diagnosis. Patient age at diagnosis, sex, date of COVID-19 diagnosis, type of malignancy, and tumor stage were recorded. Cancer type was recorded according to the site of origin and cancers were classified into one of three groups: hematologic malignancies, solid tumors, or central nervous system neoplasms. For solid neoplasms, the tumor stage was recorded at the time of COVID-19 diagnosis. Tumor stages 0–3 were classified as early/locoregional disease stage and tumor stage 4 was classified as advanced metastatic disease stage. In addition to tumor stage, patients with no evidence of disease (NED) were also recorded. NED patients with stage 4 disease were grouped into the early/locoregional disease stage. For hospitalized patients, date of hospital admission, intubation, and death were recorded and determined to be adverse outcome parameters. In the case of non-hospitalized patients, we reviewed the electronic medical record to record only death as an outcome. Of note, some laboratory markers were not available for all patients on the dates of CXRs, especially for non-hospitalized patients, and were not included in our model.
Cancer-related treatments such as thoracic surgery (wedge resection, lobectomy, and pneumonectomy), systemic therapy within 6 months of COVID-19 diagnosis (cytotoxic chemotherapy, immunotherapy, and targeted therapy including tyrosine kinase inhibitors or targeted monoclonal antibodies), and radiotherapy to the chest were also recorded. Comorbidities including hypertension, diabetes, chronic obstructive pulmonary disease (COPD), asthma, and obstructive sleep apnea were recorded.
Imaging assessment
Consensus reads were performed on all portable semi-erect anteroposterior or posteroanterior and lateral CXRs (General Electric Carestream DRX) within 72 h of diagnosis and the next radiologic follow-up CXR within 72 h of the baseline CXR. Each CXR was reviewed by two of five thoracic radiologists (R.P.J., J.A.F., A.J.P., C.C.L., with 2–8 years of thoracic radiology experience) and then in consensus with a senior thoracic radiologist (M.G. with 24 years of experience) who adjusted any initial disagreement.
Baseline CXR studies were classified as normal, abnormal, or indeterminate. When available, baseline studies were compared with prior imaging studies to ensure that findings were new. If a patient’s prior CXR demonstrated findings, the case was classified as having background disease. If no findings were detected on the baseline CXR or no new findings were noted when compared to prior imaging, the baseline CXR was determined to be normal. Studies with extensive background metastases, large neoplasms, effusions, or post-treatment changes that would significantly obscure new findings were classified as indeterminate. A study was determined to be abnormal if airspace and/or reticulonodular opacities were noted. Airspace opacities were divided into patchy opacities and segmental or lobar consolidations. Findings were further classified as unilateral or bilateral and as predominantly in the inferior lobes or diffused (involving upper and lower lobes). In order to establish the degree of parenchymal involvement, an experimental scoring system was developed, in which each lung was divided into six zones (Fig. 2). Each zone was assigned a score for the absence (0 points) or presence of airspace or reticulonodular opacities (1 point). All zone scores were added to obtain a total score (minimum 0 to maximum 12). Other scoring systems as the Brixia score evaluated the lung parenchyma by dividing it into three zones (upper, middle, and lower zone) and each zone was scored from 0 to 3 based on the type of lung abnormality (1 point for interstitial infiltrates, 2 points for interstitial and alveolar with interstitial predominance and 3 points for interstitial and alveolar with alveolar predominance). In addition, the extent of lung abnormalities was then estimated visually estimating and averaging the percentage of lung involvement [11]. Our aim was to develop a more simplified score based on the presence or absence of any parenchymal abnormality within the zones of the lung parenchyma.
a Severity score assessment. Each lung is first divided into three zones: upper (from the apex to the trachea), middle (from the trachea to the inferior hilar markings), and inferior (from the inferior hilar markings to the costophrenic angles). The lungs are then divided into peripheral and medial sections divided by a plane extending from the lung apex to the diaphragmatic domes. Each zone is assigned 1 point if opacities were noted or 0 points if the parenchyma is normal. b A 69-year-old man with follow-up chest radiograph (CXR) at 48 h from baseline, with patchy airspace opacities in all zones bilaterally, with a total score of 11
In order to assess the radiological evolution, follow-up CXRs were scored similarly to baseline CXRs; delta scores were calculated by subtracting the follow-up score from the baseline score. Studies were classified as unchanged or improved if the delta score was 0 or negative. Studies with a delta score > 1 were deemed to have progressed.
Statistical analysis
Frequencies were used to aggregate clinical and demographic information among the COVID-19-positive patients. We used the chi-square test and the Wilcoxon rank-sum test to ascertain differences between normal and abnormal CXRs and other clinical and demographic information. We performed univariable and multivariable logistic regression to assess the predictiveness of abnormal CXR and the effect of the numeric severity score on clinical outcomes, which consisted of hospital admission, mechanical ventilation, and death. In our multivariable model, we adjusted for age, gender, type of primary cancer, and presence of comorbidities. In addition, solid tumors were adjusted for tumor stage (early/locoregional versus advanced metastatic disease). We also performed logistic regression before and after adjusting for age and type of primary cancer for the effect of early progression on clinical outcome and for the effect of treatment type on early progression. Simple linear regression and multivariable linear regression were used to analyze the effect of specific treatment types on the numeric severity score. Results were reported as odds ratios (ORs) for logistic regression models and coefficients for the linear regression model with 95% confidence intervals (CI). We tested to see if the mean severity score of the baseline CXR changed significantly from baseline to the follow-up timepoint using a paired t-test with 95% CI. A p value of < 0.05 was considered statistically significant. All analyses were conducted in SAS (Version 9.4).
Results
Patient demographics and clinical characteristics
Of the 627 diagnosed patients, 297 had a CXR performed from 9 days pre- to 17 days post-COVID-19 diagnosis. Of the 297 patients, 279 patients had a “baseline CXR” (performed within 72 h of COVID-19 diagnosis) and after excluding 31 patients with an indeterminate baseline CXR, a total of 248 patients remained with either a normal or abnormal baseline CXR. Similarly, in order to capture early radiologic evolution, we included the first follow-up CXR performed within 72 h after the baseline CXR, available in 64 patients.
Demographics and clinical characteristics of the study sample (n = 248 with normal or abnormal baseline CXR) are summarized in Table 1. Across the entire study sample, 90/248 (36%) patients had a hematologic malignancy, 1/248 (0.4%) had a central nervous system neoplasm, and 157/248 (63%) patients had a solid malignancy, the most common being hepatobiliary/gastrointestinal neoplasm (44/157, 28%), breast cancer (38/157, 24%), and lung cancer (29/157, 18%). Of the 157 patients who had solid tumor malignancy, 82/157 (52%) patients had early/locoregional disease and 75/157 (48%) patients had advanced metastatic disease. Of the 31 patients who had NED at the time of COVID-19 diagnosis, 29/31 (94%) patients had early/locoregional disease (20 stage 0–1 disease and 9 stage 3 disease), and 2/31 (6) patients had baseline stage 4. Most patients (73%, 181/248) had at least one comorbidity; 97/248 (39%) patients had two or more comorbidities. Hypertension was the most frequent comorbidity present (61%, 151/248), followed by diabetes (27%, 67/248), chronic obstructive pulmonary disease (13%, 32/248), asthma (12%, 31/248), and obstructive sleep apnea (12%, 29/248). Cytotoxic chemotherapy (26%, 65/248) was the most frequent therapy within 6 months of a COVID-19 diagnosis, followed by targeted therapy (14%, 32/248) and radiation therapy (14%, 35/248). Only 15 patients had history of lung resections, four of which were performed within 6 months of COVID-19 diagnosis.
Baseline chest radiograph findings and association with adverse outcomes
Most patients had an abnormal baseline CXR (69%), with incidence of hospitalization, intubation, and death detailed in Table 1. An abnormal baseline CXR was significantly associated with increased odds of hospital admission (OR = 3.56, 95% CI = 1.95–6.52, p < 0.0001), intubation (OR = 10.91, 95% CI = 2.54–46.82, p = 0.0013), and death (OR = 4.56, 95% CI: 1.55–13.46, p = 0.0058) (Table 2). At baseline, the median severity score was 3 (range = 1–10). In the subset of patients with solid tumor, after adjusting the model for tumor stage, an abnormal baseline CXR was significantly associated with hospital admission (p = 0.0002), intubation (p = 0.0196), and death (p = 0.0348) (Table 3). Increased baseline severity scores were significantly associated with increased odds of hospital admission (OR = 1.39, 95% CI: 1.19–1.63; p < 0.001) intubation (OR = 1.29, 95% CI: 1.14–1.47; p < 0.001), and death (OR = 1.27, 95% CI: 1.11–1.44; p < 0.001) (Table 4). Similarly, in the subset of patients with solid tumors, after adjusting for tumor stage, increased baseline severity scores were significantly associated with hospital admission (p = 0.0027), intubation (p = 0.0015), and death (p = 0.0011) (Table 5).
The receiver operating characteristic (ROC) curve of the baseline severity score against adverse outcome parameters demonstrated that the severity score had acceptable discrimination regarding hospitalization (area under the curve (AUC) = 0.74), intubation (AUC = 0.73), and death AUC = 0.73).
Probability plots show that a baseline severity score of > 9 increased the probability of intubation by ≥ 50% while a score of > 10 increased the probability of death by ≥ 50% (Fig. 3). A severity cutoff for hospitalization was difficult to establish given that 53% of the patients with normal and 81% of patients with abnormal CXR were hospitalized, and regardless of the radiologic severity, just by being diagnosed with COVID-19, patients had a probability of hospitalization of > 50%.
The baseline severity score was not associated with any prior cancer-related treatments. While there was a trend towards negative regression between the severity score and some prior treatments (surgery, targeted therapy, and immunotherapy), none of these associations was statistically significant (p > 0.05).
Of the 68 non-hospitalized patients, 4 patients died (6%) within the 3-month follow-up period post-COVID-19 diagnosis. One patient had a normal baseline CXR, and per the hospice note, the patient died of stage 4 breast cancer complications. Another lymphoma patient had an abnormal baseline CXR with a score of 3, and per the outside hospital note, the patient died of bacteria-related sepsis from a urinary tract infection. The third patient had lymphoma and an abnormal baseline CXR with a score of 5; per the outside hospital note, the patient died of COVID-19-related complications. The last patient had metastatic melanoma and an abnormal baseline CXR with a score of 7; this patient was transferred to another institution, and unfortunately, the cause of death was not entered into our electronic medical record.
Early evolution on chest radiograph and association with adverse outcomes
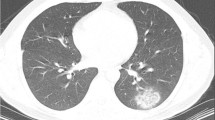
Out of the 248 patients, 64/248 (26%) patients had early follow-up CXR, all of whom were hospitalized. There was a significant difference between the baseline average score and the one at follow-up: 3.72 (± 2.75) versus 5.16 (± 2.89), p < 0.001 (Fig. 4). Of the 76 patients who had a normal baseline CXR, nine also had a follow-up CXR, among whom seven showed new radiologic manifestations of the disease with an average severity score at follow-up of 3.7 (range = 1–10). All seven patients with new radiologic manifestations were hospitalized; one patient who was hospitalized also died. Of the 172 patients who had an abnormal baseline CXR, 55 also had a follow-up CXR, among whom 24 improved or remained stable (11 intubated, 7 died) and 31 had increased findings with an average score of 5.1 and a delta average score of 1.3 (16 intubated and 12 died) (Figs. 5 and 6).
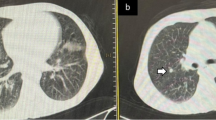
An 81-year-old man with chronic lymphocytic leukemia, without diagnosed comorbidities, treated with ibrutinib, with a baseline chest radiograph (CXR) with a severity score of 3 (a). Within 48 h, the patient deteriorated clinically, necessitating mechanical intubation. b On follow-up CXR, the severity score increased to 10. The patient passed away on day 38 of hospitalization
A 69-year-old man with a history of renal cell carcinoma and hypertension, not on systemic therapy, who presented to urgent care with fever. a The baseline chest radiograph (CXR) had a severity score of 7. The patient suffered clinical deterioration within 24 h, for which he required mechanical ventilation. b On follow-up CXR, the severity score decreased to 2. The patient was discharged after 51 days of hospitalization
Worsening early radiologic progression on CXR was not found to be associated with intubation, death, or prior cancer treatment (p > 0.05) (Table 6).
Discussion
As the COVID-19 pandemic continues to affect patients worldwide, the aim of our study was to determine if the severity of the radiologic manifestations of the disease and its evolution can help predict adverse outcomes in patients with cancer as hospitalization, intubation, and death. Our study determined that baseline parenchymal involvement on CXR but not early radiologic progression is associated with adverse outcomes.
Immunosuppression from malignant disease or its treatment renders many susceptible to infection, and moreover, studies have demonstrated that patients with cancer who have COVID-19 have worse outcomes compared with the general population [3,4,5, 12]. It has been also been demonstrated in viral etiologies that the extent of radiologic involvement is associated with a worse outcome [12, 13]. Despite the lower reported sensitivity of CXR (69%) versus CT (97–98%), CXR is the primary imaging modality used for the clinical management of COVID-19 patients. Few studies have demonstrated that baseline CXR can predict a worse outcome in COVID-19 patients. To our knowledge, this is the first study to address whether the extent of the radiologic manifestation of COVID-19 and its early radiologic evolution can help predict outcomes in patients with cancer [7,8,9]. We found that while the degree of parenchymal involvement on chest radiograph within 72 h of COVID-19 diagnosis is associated with adverse outcomes including hospitalization, intubation, and death in patients with cancer, worsening early radiologic progression on chest radiograph was not associated with these same adverse outcomes.
Most patients (69%) in our study had an abnormal baseline CXR (within 72 h of COVID-19 diagnosis), which is in agreement with the 50–69% range reported in the general population [7, 14]. The presence of radiologic findings on baseline CXR was significantly associated with an increased risk of hospitalization, mechanical ventilation, and death in our study, similar to prior reports in the general population [7, 15]. We had a higher incidence of hospitalization (73%) when compared with a prior report from a tertiary cancer institution where 40% of the oncologic population was hospitalized; this discrepancy might be explained by a selection bias within our study sample, since patients included in this study had to have both a positive RT-PCR and CXR within 72 h of diagnosis and the indication for the CXR close to the date of diagnosis might have been related to worst clinical presentation [6]. In addition, known immunosuppression and added comorbidities may have prompted the clinical teams to have a lower threshold for hospitalization.
The severity of parenchymal involvement was significantly associated with hospitalization, mechanical ventilation, and death, which is in agreement with prior reports in the general population. Toussie et al scored the severity of parenchymal involvement on CXR based on lung zones and demonstrated that the severity score was independently predictive of hospitalization and intubation; due to the small number of cases and short follow-up period, they were not able to statistically assess the relationship between the severity score and death [7]. Balbi et al developed a scoring system, based on anatomical location and the type of lung abnormality, as well as estimating the percentage of parenchymal involvement. They found that the higher the Brixia score, the more significantly it was associated with death. The main predictor of the need for ventilatory support was the higher percentage of lung parenchyma involved [11]. Yang et al developed a severity score on chest CT based on the involvement of lung segments and found that higher scores were significantly associated with severe versus mild clinical presentation of the disease; however, they had very few cases of mechanical ventilation [16]. Yuan et al scored involvement on CT based on the density of the findings (score = 1, normal parenchyma; 2, ground glass; and 3, consolidation) as well as the extent of the involvement per lung zones (upper, middle, and lower lung) and found that a certain cutoff of the score was predictive of mortality (sensitivity 85.6%, specificity 84.5%) [15]. Park et al demonstrated regarding volumetric assessment on CT that a higher volume of normally aerated parenchyma was significantly associated with lower rates of intubation and death [17].
The probability plots in our study demonstrated that a baseline score of > 9 and ≥ 10 was predictive of increased risk of intubation and death, respectively. We also found in our study that further parenchymal involvement (the equivalent of over four parenchymal zones) correlated with intubation, similar to Toussie et al who reported that parenchymal involvement of over three lung zones (approximate equivalent to a score of ≥ 6 on our severity scale) was predictive of intubation [7]. No studies have established a cutoff for parenchymal involvement and death on CXR.
We found no significant association between the baseline score and any cancer-related treatment. We believe this is related to the small sample size for each treatment type. A larger study by Robilotti et al found that recent systemic treatment was not associated with a higher risk of complications [6].
While follow-up severity scores within 72 h after baseline CXR were overall significantly increased from the baseline in our study, early radiologic evolution was not found to be significantly associated with any adverse outcome or any cancer-related treatment, probably due to the small number of follow-up CXRs included in our study (only 26%). Other studies have reviewed the progression of COVID-19 patients on CT. Pan et al reported that 86% of patients had progressive findings on CT 3–14 days after diagnosis [18]. Li et al reviewed follow-up CTs in 24 patients approximately 5 days post-baseline scan, noticing progression of findings in 75% of patients [19]. In our study, only 59% of patients progressed radiologically. This disparity is probably due to the lower sensitivity on CXR compared with CT.
Wong et al evaluated the evolution of the severity score over time on CXR and found significant differences between different times points including baseline (0–3 days from symptoms onset) and follow-up at 4–6 days which is similar to our results. However, they did not perform any statistical analysis on the evolution of CXR with clinical outcome [14]. Interestingly, Park et al demonstrated that a marked increase in CT scores in a short period of time was associated with increased mortality and that stability of the parenchymal involvement was associated with survival; however, the follow-up time was not specified [17].
There are several limitations in our study. First of all, it is important to emphasize that our study was designed during the first COVID-19 outbreak, a fast-evolving wave between March and April 2020 deeply marked by challenges such as testing capacity, laboratory test delays, and limited health resources that overwhelmed hospitals worldwide and, therefore, may have been prone to various forms of bias. Considering that our study population was varied in terms of primary cancer and treatments that were either cancer-related or COVID-19 related, it is possible that patients in our heterogenous sample have confounding effects from other factors that were not fully evaluable in our population, thus limiting the generalizability of our results. Furthermore, our assessment was based on the date of COVID diagnosis and not on the onset of symptoms as was done in Pan et al [18]. This was decided due to the fact that within our study sample, many patients were symptomatic prior to infection due to their cancer diagnosis, active treatment, or recent surgery. We also did not correlate the severity score with the clinical severity of the disease based on symptoms or vital signs. Imaging classification was determined by consensus, and therefore, inter-reader agreement was not evaluated. Finally, the small number of follow-up patients probably influenced the absence of association between early evolution and outcome. Future prospective studies with a greater sample, adequate adjustment for different comorbidities, laboratory correlations, and longer follow-up are needed to establish more meaningful associations.
In summary, the degree of parenchymal involvement at baseline is associated with adverse outcomes in patients with both cancer and COVID-19. Baseline radiological assessment of severity using an objective scoring system as presented in this study may help triage patients with cancer who may need more aggressive treatment and closer monitoring with hospitalization.
Abbreviations
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- COVID-19:
-
SARS-CoV-2 virus
- CT:
-
Computed tomography
- CXR:
-
Chest radiograph
- OR:
-
Odds ratio
- PCR:
-
Polymerase chain reaction
- ROC:
-
Receiver operating characteristic
References
Verity R, Okell LC, Dorigatti I et al (2020) Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 20:669–677
Yu J, Ouyang W, Chua MLK, Xie C (2020) SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 6:1108–1110
Liang W, Guan W, Chen R et al (2020) Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 21:335–337
Stroppa EM, Toscani I, Citterio C et al (2020) Coronavirus disease-2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy). Future Oncol 16:1425–1432
Mehta V, Goel S, Kabarriti R et al (2020) Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov 10:935–941
Robilotti EV, Babady NE, Mead PA et al (2020) Determinants of COVID-19 disease severity in patients with cancer. Nat Med 26:1218–1223
Toussie D, Voutsinas N, Finkelstein M et al (2020) Clinical and chest radiography features determine patient outcomes in young and middle-aged adults with COVID-19. Radiology 297:E197-e206
Liang W, Liang H, Ou L et al (2020) Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med 180:1081–1089
Hui TCH, Khoo HW, Young BE et al (2020) Clinical utility of chest radiography for severe COVID-19. Quant Imaging Med Surg 10:1540–1550
Pan F, Ye T, Sun P et al (2020) Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 295:715–721
Balbi M, Caroli A, Corsi A et al (2021) Chest X-ray for predicting mortality and the need for ventilatory support in COVID-19 patients presenting to the emergency department. Eur Radiol 31:1999–2012
Kamboj M, Sepkowitz KA (2009) Nosocomial infections in patients with cancer. Lancet Oncol 10:589–597
Hui DS, Wong KT, Antonio GE et al (2004) Severe acute respiratory syndrome: correlation between clinical outcome and radiologic features. Radiology 233:579–585
Wong HYF, Lam HYS, Fong AH et al (2020) Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology 296:E72-e78
Yuan M, Yin W, Tao Z, Tan W, Hu Y (2020) Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One 15:e0230548
Yang R, Li X, Liu H et al (2020) Chest CT severity score an imaging tool for assessing severe COVID-19. Radiologys: Cardiothoracic Imaging 2:e200047
Park B, Park J, Lim JK et al (2020) Prognostic implication of volumetric quantitative CT analysis in patients with COVID-19: a multicenter study in Daegu, Korea. Korean J Radiol 21:1256–1264
Pan Y, Guan H, Zhou S et al (2020) Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 30:3306–3309
Li Y, Xia L (2020) Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol 214:1280–1286
Acknowledgements
The authors would like to thank Joanne Chin, MFA, ELS, for assisting with the editing of the manuscript.
Funding
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Rocio Perez-Johnston, MD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Natalie Gangai, MPH) has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some patients in our study sample were described in the supplemental material: “Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, Bogler Y, Caldararo M, Figueroa CJ, Glickman MS, Joanow A, Kaltsas A, Lee YJ, Lucca A, Mariano A, Morjaria S, Nawar T, Papanicolaou GA, Predmore J, Redelman-Sidi G, Schmidt E, Seo SK, Sepkowitz K, Shah MK, Wolchok JD, Hohl TM, Taur Y, Kamboj M. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020 Aug;26(8):1218-1223. https://doi.org/10.1038/s41591-020-0979-0. Epub 2020 Jun 24. PMID: 32581323.”
The time period of inclusion of patients was different (March 10th to April 7th). The radiologic data was not used in their analysis of outcome but only described in the supplemental material including both chest radiograph and computed tomography without establishing a cutoff period between the diagnosis and the time of the imaging studies. While the previous study clinically followed patients for a 30-day period, they did not include and radiologic follow-up.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Perez-Johnston, R., Araujo-Filho, J., Mckenney, A.S. et al. COVID-19 in patients with cancer: can baseline radiologic severity and early evolution predict clinical outcomes?. Eur Radiol 32, 2661–2671 (2022). https://doi.org/10.1007/s00330-021-08341-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08341-7